Abstract
An enantioselective transesterification in non-aqueous organic solvent was developed by utilizing a lipase-displaying yeast whole cell biocatalyst constructed in our previous study. As a model reaction, optical resolution of (RS)-1-phenylethanol, which serves as one of chiral building blocks, was carried out by enantioselective transesterification with vinyl acetate. Recombinant Rhizopus oryzae lipase displayed on the yeast cell surface retained its activity in hexane, heptane, cyclohexane and octane. The effective amount of whole-cell biocatalyst in the reaction mixture was 10 mg/ml solvent. In a reaction mixture incubated for 36 h with molecular sieves 4A, the concentration of (R)-1-phenylethyl acetate reached 39.8 mM (97.3% yield) with high enantiomeric excess (93.3%ee). In contrast, a reaction mixture incubated without molecular sieves 4A produced little (R)- and (S)-1-phenylethyl acetate. The results obtained in this study demonstrate the applicability of the lipase-displaying yeast whole cell biocatalyst to bioconversion processes in non-aqueous organic solvents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, enzymatic transformation of organic compounds has been developed for the preparation of optically active products from racemic substrates (Wong and Whitesides 1994; Otsubo 2000; Berglund 2001). Since enzymes can catalyze a variety of reactions with high specificity and under mild conditions such as low temperature and atmospheric pressure, biotransformation is looked to as a way of resolving the environmental problems involved in chemical procedures (Koeller and Wong 2001). Further, regioselective synthesis is difficult to achieve by chemical procedures, but appears to be amenable to an enzymatic approach.
Lipase-catalyzed biotransformation is one of the most popular and practical of these enzymatic procedures. Lipases (triacylglycerol acylhydrolases, EC 3.1.1.3) are enzymes that catalyze a broad spectrum of reactions, such as hydrolysis of ester bonds and transesterification and ester synthesis, at the interface between substrate and water or in non-aqueous organic solvents (Hiol et al. 2000) and are therefore used in a wide range of industrial processes including food, chemical, pharmaceutical and detergent production. In pharmaceutical production using lipases, previous reports have demonstrated regioselective transesterifications such as kinetic resolution of 1-phenylethanol (Schöfer et al. 2001) or α-methylene β-lactams (Adam et al. 2000) and the enantiodivergent synthesis of the antitumor antibiotic fredericamycin A analog (Akai et al. 2001). In many cases, however, lipases have been used in immobilized form, which is cost- and time-consuming, and can be a barrier to widespread use of enzymatic processes.
To improve cost-efficiency, the present study attempted to achieve enantioselective transesterification using a lipase-displaying yeast in the form of a whole-cell biocatalyst. With the recent development of cell-surface display technology, functional proteins such as enzymes can be genetically immobilized on the yeast cell surface via anchor proteins (Murai et al. 1997). For industrial bioconversion processes, the utilization of surface-displayed lipase in the form of whole-cell biocatalysts is more cost-effective and advantageous because the enzymes are displayed on the cell surface spontaneously, regarded as immobilized enzyme, and can be separated easily. We previously reported successful yeast cell-surface display of the active form of ProROL, consisting of pro- and mature-region of Rhizopus oryzae lipase (ROL), via the flocculation functional domain of Flo1 protein (Flo1p) (Matsumoto et al. 2002), a yeast flocculin, which is a lectin-like protein and causes cell flocculation by attachment to cell wall mannan (Watari et al. 1994). Truncated Flo1p was fused as an anchor protein to the N-terminus of ProROL. Since this lipase-displaying yeast had been found to efficiently catalyze a methanolysis reaction in a solvent-free and water-containing system (Kaieda et al. 1999), the present study undertook an investigation of the applicability of this whole-cell biocatalyst to non-aqueous enantioselective transesterification.
Materials and methods
Materials
(RS)-1-Phenylethanol (Wako Pure Chemical Industries, Osaka, Japan) and vinyl acetate (Nacalai Tesque, Kyoto, Japan) were used as reaction substrates in the enantioselective transesterification experiment. The reaction solvents hexane, heptane, octane and cyclohexane and the dehydrating reagent molecular sieves 4A (∅2 mm) were obtained from Nacalai Tesque. (R)-, (S)-1-Phenylethanol (Wako Pure Chemical Industries) and (RS)-1-phenylethyl acetate (Aldrich, Milwaukee, Wis.) were used as standards in HPLC analysis.
Strains and media
The ProROL-displaying yeast strain was obtained by transformation of Saccharomyces cerevisiae MT8–1 (MATa ade his3 leu2 trp1 ura3) with plasmid pWIFSProROL, constructed in a previous study (Matsumoto et al. 2002). To prepare the ProROL-displaying yeast, an enriched selective medium (SDC: 0.67% yeast nitrogen base supplemented with appropriate amino acids and nucleotides, 0.5% glucose, and 2% casamino acids) was used.
Preparation of ProROL-displaying yeast
To prepare the ProROL-displaying yeast as a whole-cell biocatalyst, the transformant described above was cultivated in 400 ml SDC medium in a 1-l baffled-flask at 30°C with 150-rpm rotation. After 194-h cultivation, at which point the lipase activity on the yeast transformant peaks (Matsumoto et al. 2002), the cells were collected, washed with distilled water, lyophilized with FreeZone FZ-1 (Labconco, Kansas, Mo.) for 36 h, and sieved to homogenize cell particle size with a 40-mesh sieve.
Measurement of lipase activity on yeast cells
The hydrolytic activity of lipase in culture broth and yeast cells was measured using Lipase Kit S (Dainippon Pharmaceutical, Osaka, Japan) according to the protocol specified by the supplier, and the resulting values were expressed in international units (IU). One unit of lipase activity was defined as the amount of enzyme catalyzing the formation of 1 μmol 2,3-dimercaptopropan-1-ol from 2,3-dimercaptopropan-1-ol tributyl ester per minute. The detailed procedure of lipase activity measurement on the yeast cell surface has been described previously (Matsumoto et al. 2002).
Enantioselective transesterification
In order to confirm the performance of the ProROL-displaying yeast whole-cell biocatalyst, the optical resolution of (RS)-1-phenylethanol was carried out by enantioselective transesterification with vinyl acetate (Fig. 1). The reaction mixture, consisting (unless otherwise noted) of 30 mg (RS)-1-phenylethanol, 21.2 mg vinyl acetate, 30 mg lyophilized cells, 0.3 g molecular sieves 4A and 3 ml heptane was incubated at 30°C with shaking at 150 rpm. The concentration of (RS)-1-phenylethanol and vinyl acetate was 80 mM. Aliquots of 50 μl withdrawn and diluted in 950 μl of the mobile phase of HPLC (described below) were used to determine the amount and the enantiomeric excess of the ester formed during the reaction. Solvents for the reaction experiments were dehydrated with molecular sieves 4A for 24 h before use.
High-performance liquid chromatography analysis
In the enantioselective transesterification of (RS)-1-phenylethanol, the yield and the enantiomeric excess of the reaction product, 1-phenylethyl acetate, were determined by HPLC using a Chiralcel OB-H column (∅ 0.46 mm, 25 cm, Daicel Chemical Industries, Tokyo, Japan). The mobile phase was a mixture of hexane:isopropanol (90:10, v/v) at a flow rate of 0.3 ml/min on LC10A (Shimadzu, Kyoto, Japan). Quantification of the reaction products and substrates was performed by measuring absorbance at 210 nm on an SPD10A UV detector (Shimadzu). The retention times for (R)- and (S)-1-phenylethanol, and for (R)- and (S)-1-phenylethyl acetate were 21, 28, 16.5 and 17 min, respectively. Enantiomeric excess (%ee) was calculated as:
where R and S are the concentration of (R)- and (S)-1-phenylethyl acetate, respectively.
Results
Preparation of ProROL-displaying yeast
ProROL-displaying yeast whole-cell biocatalyst was cultivated in SDC medium according to the method reported previously (Matsumoto et al. 2002), and lyophilized for enantioselective transesterification in organic solvent, which was inhibited by the presence of water. Interestingly, lyophilized ProROL-displaying cells exhibited approximately four times higher lipase activity (265 IU/g dry cell weight) than fresh cells (61.3 IU/g dry cell weight). Since this phenomenon was not observed in the case of soluble ROL enzyme (data not shown), this activity enhancement might be unique to the surface-displayed enzyme.
Effect of water content in reaction mixture
In order to investigate the effect of water content in the reaction mixture, transesterification was carried out with and without molecular sieves 4A (Table 1). In the reaction mixture incubated with molecular sieves 4A, the reaction yield of (R)-1-phenylethyl acetate reached 63.2% after a 24-h reaction and enantiomeric excess was 93.3%ee, but without molecular sieves 4A, neither (R)- nor (S)-products were produced in significant quantity.
Activities in various solvents
A comparison of activity on ProROL-displaying yeast in various organic solvents is shown in Table 2. ProROL displayed on the yeast-cell surface could efficiently catalyze an enantioselective reaction in hexane, heptane, cyclohexane and octane. Enantioselective transesterification was the most efficiently catalyzed in the case of heptane, although the other solvents also showed good reaction rates. Enantiomeric excess recorded a high level (over 93%ee) in all cases.
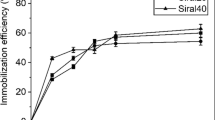
Effect of amount of cells in reaction mixture
Figure 2 shows the effect of the amount of cells in the reaction mixture on the reaction yield of each enantiomeric product. A higher (R)-1-phenylethyl acetate yield was obtained by increasing the amount of cells added to the reaction mixture. However, linearity was not retained above 10 mg/ml solvent and the yield seemed to reach a plateau. From this result, 10 mg/ml lipase-displaying yeast cells was used in subsequent experiments. (S)-Product yield remained low under all conditions while enantiomeric excess remained over 90%ee throughout.
Time-course of reaction
Figure 3 shows the time-course of reaction during enantioselective transesterification. When the reaction began, the reaction substrates, (R)-1-phenylethanol and vinyl acetate were immediately consumed and (R)-1-phenylethyl acetate was produced. After 36-h reaction, the concentration of (R)-1-phenylethyl acetate reached 39.8 mM, equivalent to 97.3% reaction yield with high enantiomeric excess (93.3%ee). The concentrations of (S)-1-phenylethanol and (S)-1-phenylethyl acetate meanwhile remained unchanged from the beginning of the reaction (data not shown).
Discussion
As a model reaction for enantioselective transesterification, optical resolution of (RS)-1-phenylethanol, which serves as a chiral building blocks, was examined. The substrate reacted in an esterification process using vinyl-acetate as acyl donor (Fig. 1).
In the present study, ProROL displayed on the yeast cell surface could catalyze transesterification only under non-aqueous conditions with dehydration by molecular sieves 4A (Table 1). Kyotani et al. (1988a, 1988b) found that lipase-catalyzed transesterification in organic solvent was inhibited by the presence of water in excess of the amount required to maintain the structure of the lipase molecule in organic solvent. Our result also indicates that water content in the reaction mixture has a critical effect on the transesterification yield. The lyophilization procedure, necessary for removal of water from yeast cells, enhanced the performance of the ProROL-displaying yeast whole-cell biocatalyst. Although the mechanism of this activation was unclear, accessibility between displayed enzyme and substrate might be improved by partial destruction of cell wall components by freezing. Since yeast cells lyophilized for 36 h contain approximately 5 wt% water (data not shown), a dehydrating reagent was needed to catalyze this reaction.
The results shown in Fig. 2 suggest that the effective amount of cells in the reaction mixture is 10 mg/ml solvent. This result suggests that fairly small amount of ProROL-displaying yeast cells, approximately the same as immobilized lipase catalysts previously used by Cardenas et al. (2001) and Salunkhe and Nair (2001), is needed for complete conversion of substrate. In a Michaelis-Menten-type reaction, a higher reaction rate is commonly obtained by increasing the amount of enzyme in soluble form. In the present reaction, however, at amounts greater than 10 mg/ml solvent, it appeared that the interfacial area between the solution and cell surface became rate-limiting, with the presence of excess cells causing a reduction of the solution-cell surface interfacial area.
As shown in Fig. 3, rather higher levels of yield and enantiomeric excess were obtained in the present study than in the reports of Cardenas et al. (2001) (with 240-h incubation, 41% yield, 62%ee) and Schöfer et al. (2000) (72-h, 50% yield, 98%ee). Moreover, native ROL enzyme (F-AP15, Amano Enzyme, Nagoya, Japan) could not catalyze enantioselective transesterification in organic solvent, although rather higher hydrolytic activity (30,000 IU/g) was evident than in ProROL-displaying yeast. From these results, displaying ProROL on yeast cell surfaces by the fusion of truncated Flo1p to its N-terminus might affect the properties of the enzyme molecule. Alternatively, properties of the yeast cell surface, such as hydropathy and charges, might support the accessibility and affinity between enzymes and substrates in organic solvent. One of the advantages of enzyme-displaying whole-cell biocatalysts is that enzymes are immobilized on the yeast cell surface by genetically modified anchor systems, which not only minimizes the risk of inactivation and inhibition, but also improves the performance of displayed enzyme. In addition, this latter features be partially influenced by the function of the pro-sequence of ROL in modulating the folding process, secretion pathway, or thermostability of ROL (Beer et al. 1996).
The ProROL-displaying yeast whole-cell biocatalyst studied here can catalyze enantioselective transesterification in non-aqueous organic solvent, suggesting its potential as a cost-effective whole-cell biocatalyst for applications in pharmaceutical production.
References
Adam W, Groer P, Humpf HU, Saha-Möller CR (2000) Synthesis of optically active α-methylene β-lactams through lipase-catalyzed kinetic resolution. J Org Chem 65:4919–4922
Akai S, Tsujino T, Fukuda N, Iio K, Takeda Y, Kawaguchi K, Naka T, Higuchi K, Kita Y (2001) Enantiodivergent synthesis of either enantiomer of ABCDE-ring analogue of antitumor antibiotic fredericamycin A via intramolecular [4+2] cycloaddition approach. Org Lett 3:4015–4018
Beer HD, Wohlfahrt G, Schmid RD, McCarthy JE (1996) The folding and activity of the extracellular lipase of Rhizopus oryzae are modulated by a prosequence. Biochem J 15351–359
Berglund P (2001) Controlling lipase enantioselectivity for organic synthesis. Biomol Eng 18:13–22
Cardenas F, de Castro MS, Sanchez-Montero JM, Sinisterra JV, Valmaseda M, Elson SW, Alvarez E (2001) Novel microbial lipases: catalytic activity in reactions in organic media. Enzyme Microb Technol 28:145–154
Hiol A, Jonzo MD, Rugani N, Druet D, Sarda L, Comeau LC (2000) Purification and characterization of an extracellular lipase from a thermophilic Rhizopus oryzae strain isolated from palm fruit. Enzyme Microb Technol 26:421–430
Kaieda M, Samukawa T, Matsumoto T, Ban K, Kondo A, Shimada Y, Noda H, Nomoto F, Ohtuka K, Izumoto E, Fukuda H (1999) Biodiesel fuel production from plant oil catalyzed by Rhizopus oryzae lipase in a water-containing system without an organic solvent. J Biosci Bioeng 88:627–631
Koeller KM, Wong CH (2001) Enzymes for chemical synthesis. Nature 409:232–240
Kyotani S, Fukuda H, Morikawa H, Yamane T (1988a) Interesterification of fats and oils by immobilized fungus at constant water concentration. J Ferment Technol 66:71–83
Kyotani S, Fukuda H, Nojima Y, Yamane T (1988b) Kinetic studies on the interesterification of fats and oils using dried cells of fungus. J Ferment Technol 66:567–575
Matsumoto T, Fukuda H, Ueda M, Tanaka A, Kondo A (2002) Construction of yeast strains with high cell surface lipase activity by using novel display systems based on the Flo1p flocculation functional domain. Appl Environ Microbiol 68:4517–4522
Murai T, Ueda M, Yamamura M, Atomi H, Shibasaki Y, Kamasawa N, Osumi M, Amachi T, Tanaka A (1997) Construction of a starch-utilizing yeast by cell surface engineering. Appl Environ Microbiol 63:1362–1366
Otsubo K (2000) Studies on the efficient syntheses of the drug metabolites. Yakugaku Zasshi 120:1135–1147
Salunkhe MM, Nair RV (2001) Novel route for the resolution of both enantiomers of dropropizine by using oxime esters and supported lipases of Pseudomonas cepacia. Enzyme Microb Technol 28:333–338
Schöfer SH, Kaftzik N, Wasserscheid P, Kragl U (2001) Enzyme catalysis in ionic liquids: lipase catalysed kinetic resolution of 1-phenylethanol with improved enantioselectivity. Chem Commun 425–426
Watari J, Takata Y, Ogawa M, Sahara H, Koshino S, Onnela ML, Airaksinen U, Jaatinen R, Penttila M, Keranen S (1994) Molecular cloning and analysis of the yeast flocculation gene FLO1. Yeast 10:211–225
Wong CH, Whitesides GM (1994) Enzymes in synthetic organic chemistry. Pergamon, Oxford
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsumoto, T., Ito, M., Fukuda, H. et al. Enantioselective transesterification using lipase-displaying yeast whole-cell biocatalyst. Appl Microbiol Biotechnol 64, 481–485 (2004). https://doi.org/10.1007/s00253-003-1486-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1486-1