Abstract
Phosphorus (P)-accumulating microbial granules were developed at different substrate P/chemical oxygen demand (COD) ratios in the range of 1/100 to 10/100 by weight in sequencing batch reactors. The soluble COD and PO4-P profiles showed that the granules had typical P-accumulating characteristics, with concomitant uptake of soluble organic carbon and the release of phosphate in the anaerobic stage, followed by rapid phosphate uptake in the aerobic stage. The size of P-accumulating granules exhibited a decreasing trend with the increase in substrate P/COD ratio, while the structure of the granules became more compact and denser as the substrate P/COD ratio increased. The P uptake by granules fell within the range of 1.9% to 9.3% by weight, which is comparable with uptake obtained in conventional enhanced biological phosphorus removal (EBPR) processes. It was further found that low aerobic respirometric activity of granules in terms of specific oxygen utilization rate favors P uptake by granules. The results presented would be useful for the further development of a novel granule-based EBPR technology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
At present, environmental regulations in many countries require a reduction of phosphorus (P) concentrations in wastewater to levels of 0.5–2 mg l−1 before discharge into water resources. The enhanced biological phosphorus removal (EBPR) process had been developed for efficient P removal without the use of chemical precipitation and is an economical and reliable option for the removal of phosphorus from wastewater (Barnard et al. 1985; EPA 1987; Daigger and Polson 1991). The EBPR process operates on the basis of alternating anaerobic and aerobic conditions, with substrate supply in the anaerobic stage (Comeau et al. 1986; Wentzel et al. 1988). It must be pointed out that most EBPR processes have been based on suspended biomass cultures, which require a large reactor volume. Although full-scale experiments showed the strong potential of EBPR, difficulties in assuring stable and reliable operation have also been recognized. Process failure of biological P removal not only in the laboratory, but also in full-scale plants often occurred without understanding of the exact reasons (Barnard et al. 1985; Fukase et al. 1985; Pitman et al. 1988).
During the past a few years, aerobic granules for organic carbon removal have been developed in aerobic sequencing batch reactors (SBR) (Beun et al. 1999; Tay et al. 2001; Moy et al. 2002). Compared to conventional activated sludge flocs, aerobic granular sludge has a regular, dense and strong physical structure, good settling ability, high biomass retention, and the ability to withstand shock-loading rate. So far, little information is currently available on the development of P-accumulating granules in SBR. If P-accumulating granules could form, problems encountered in the suspended growth P-removal system, such as sludge bulking, large treatment plant space, secondary P release in a clarifier, higher production of waste sludge, would be overcome by applying a more compact and efficient granule-based biotechnology. Therefore, the aim of this study is to look into the feasibility of the formation of P-accumulating granules and, further, to investigate the characteristics of the P-accumulating granules developed at different substrate P/COD ratios.
Materials and methods
Experimental set-up and operation
Five columns (120 cm in height and 5 cm in diameter) with a working volume of 2.5 l were used as SBR as described by Tay et al. (2002). They were operated with a 6-h cycle, consisting of 5 min feeding, 120 min anaerobic reaction, 226 min aerobic reaction, 5 min for sludge settling and 4 min for effluent discharge. A hydraulic retention time of 12 h was maintained as 1.25 l treated liquor was discharged from the middle port of the column. The experiments were conducted in a temperature-controlled room at 25°C. In order to provide mixing in the anaerobic stage, pure nitrogen gas was introduced. In the aerobic stage, air was supplied at a flow rate of 3.0 l min−1, which gave a dissolved oxygen concentration of above 50% saturation. The sequential operations of the reactors were controlled by electronic timers, peristaltic pumps and gas valve solenoids.
Media
The seed sludge used was taken from a local municipal wastewater treatment plant. The synthetic wastewater consisted of acetate as the main carbon source and other micronutrients and had a pH value of 7.8. Details on the micronutrients can be found elsewhere (Tay et al. 2001). Phosphates in forms of KH2PO4 and Na2HPO4 were added to the medium in a 1:1 molar ratio. The substrate organic carbon concentration in terms of chemical oxygen demand (COD) was kept constant at 500 mg l−1, while substrate P concentration was 5, 12.5, 25, 37.5 and 50 mg P l−1 in five separate reactors. This gave corresponding substrate P/COD ratios of 1/100, 2.5/100, 5/100, 7.5/100 and 10/100 in the five reactors (R1–R5).
Analytical methods
In order to determine phosphate concentration in solution, samples taken from the reactors were filtered through 0.2 μm Whatman cellulose membrane, and then the filtrate was used to analyze phosphate concentration by the ascorbic acid method (APHA 1998). Sludge samples taken from the reactors were digested first by potassium persulfate (APHA 1998), and the supernatant recovered by high-speed centrifugation was used to determine P content in granules by the ascorbic acid method. The mixed-liquor suspended solids (MLSS), mixed-liquor volatile suspended solids (MLVSS), COD, suspended solids (SS), specific oxygen utilization rate (SOUR), sludge volumetric index (SVI) and specific gravity were measured using standard methods (APHA 1998). To determine SOUR, a certain amount of granule sample taken at the end of the aerobic stage was carefully washed with tap water, and placed in a pre-cleaned biological oxygen demand (BOD) bottle. The BOD bottle was fully filled with the pre-aerated substrate and nutrient solution, and the dissolved oxygen probe with stirring mechanism was immediately inserted into the BOD bottle. The decrease in dissolved oxygen concentration was recorded at time intervals of 15 s. SOUR was then calculated according to the change in dissolved oxygen concentration recorded over time and the MLVSS in the BOD bottle. The temperature was fixed at 25°C. The size of the sludge was quantified by a laser particle size analysis system (Malvern Mastersizer Series 2600, Malvern Instruments, Worcestershire, UK) or image analyzer (Quantimnet 500 Image Analyzer, Leica Cambridge Instruments, Cambridge, UK).
Results
Formation of P-accumulating microbial granules
After 2 months of operation, microbial granules with a respective mean diameter of 1.65, 1.22, 1.03, 0.69 and 0.42 mm stabilized in reactors R1–R5 operated at different substrate P/COD ratios (Fig. 1), and were present in all five reactors. These results seem to indicate that smaller microbial granules developed at higher substrate P/COD ratios. In contrast with the seed sludge, which had a very loose and irregular structure with a mean size of 0.11 mm, the granules developed in R1–R5 show a compact and regular structure with a clear outer shape (Fig. 2).
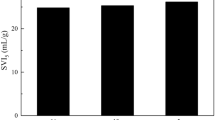
Effect of substrate P/COD ratio on SVI of microbial granules
The effect of substrate P/COD ratio on the SVI of mature granules is shown in Fig. 3.The SVI of microbial granules decreased with the increase in substrate P/COD ratio. The lowest SVI of 12 ml g−1 was recorded at the highest substrate P/COD ratio of 10/100. These results reveal that the substrate P/COD ratio has a profound effect on the structure of microbial granules, and a more compact microbial structure could be expected at high substrate P/COD ratios. Compared to the seed sludge, with a SVI of 270 ml g−1, the settling ability of microbial granules was improved significantly, while it has been reported that non P-accumulating aerobic granules have SVI in the range of 50–100 ml g−1 (Beun et al. 1999; Tay et al. 2001; Moy et al. 2002).
Effect of substrate P/COD ratio on specific gravity of microbial granules
Figure 3 shows the effect of substrate P/COD ratio on the specific gravity of microbial granules. It appears that the specific gravity of granules is proportional to the substrate P/COD ratio. In fact, this result is consistent with the SVI trend obtained at different substrate P/COD ratios as shown in Fig. 3. In the environmental engineering literature, specific gravity of sludge had been commonly used to describe how compact or dense a microbial structure is. Compared to the seed sludge, with a specific gravity of 1.002, the granules have a much denser and more compact microbial structure.
Profiles of soluble organic carbon and P in one cycle operation
Figure 4 shows typical soluble COD and P profiles in one cycle operation of R5 fed with a substrate P/COD of 10/100. Similar curves were also obtained in the other four reactors. These results indicate that the microbial granules would release phosphate with concomitant uptake of organic carbon in the anaerobic stage, and then assimilate phosphate rapidly in the aerobic stage. In fact, the COD and P profiles in Fig. 4 are similar to those obtained in typical EBPR processes (Comeau et al. 1986; Wentzel et al. 1988).
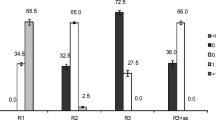
Effect of substrate P/COD ratio on the P content in microbial granules
In EBPR processes, P removed from wastewater is accumulated in the form of polyphosphate (poly-P) in microorganisms. The P content of dry biomass in aerobic granules developed at different P/COD ratios is shown in Fig. 5. If the seed sludge, with a P content of 0.0085 g P (g dry weight)−1 is taken as reference, the P content of granular sludge is 2.2- to 11-times higher than that in the seed sludge. It is most likely that the increased substrate P/COD ratio highly stimulates the P-accumulating ability of aerobic granules. This seems to indicate that the microbial granules developed under alternative anaerobic and aerobic conditions with different substrate P/COD ratios have a high P-accumulating ability.
Effect of substrate P/COD ratio on microbial activity of granules
Microbial activity can be characterized by SOUR. The effect of substrate P/COD ratio on the SOUR of granules is shown in Fig. 6. It appears that the activity of microbial granules is very sensitive to changes in the substrate P/COD ratio, i.e., SOUR decreased from 100 to 20 mg O2 g−1 VSS h−1 as the substrate P/COD ratio increased from 1/100 to 10/100. Figure 7 further reveals that high P content in granules was inversely correlated to low SOUR, i.e., low respirometric activity of granules favors P accumulation in granules.
Discussion
Our results show that microbial granules can be successfully developed in alternative anaerobic and aerobic SBR operated at different substrate P/COD ratios, while the characteristics of microbial granules are closely related to the substrate P/COD ratios in the range studied (Figs. 1, 2, 3, 4). It appears from Fig. 3 that the specific gravity of microbial granules was proportional to the substrate P/COD ratio, while Fig. 5 indicates that P-accumulating organisms were enriched as the substrate P/COD ratio was increased. Figure 8 further shows the relationship between the specific gravity and P content of microbial granules developed at different substrate P/COD ratios. The microbial granules with high P content are much denser than those with low P content. This is due to the fact that the poly-P accumulated in the cells is of higher density than a typical bacterial cell. This in turn implies that the density of the P-accumulating organisms is higher than that of non-P-accumulating bacteria. It should be pointed out that such differences in density have been used to separate P-accumulating organisms from suspended EBPR processes (Schuler et al. 2002). The curves of COD removal and P release in anaerobic phase and uptake in subsequent aerobic phase as shown in Fig. 4 are very similar to those observed in conventional EBPR processes using suspended activated sludge (Hiraishi et al. 1989; Jeon and Park 2000). It is highly likely that the P accumulation in microbial granules is subject to mechanisms similar to those of P accumulation in suspended culture.
The P content in the microbial granules was in the range of 1.9 to 9.3% by weight, which was 2.2- to 11-times higher than that in the seed sludge taken from a local wastewater treatment plant (Fig. 5). It should be pointed out that the P content was typically in the range of 1.5–2% by dry weight in conventional activated sludge processes without EBPR (Droste 1997), and 4–15% in EBPR processes (Crocetti et al. 2000; Panswad et al. 2003). Figures 4 and 5 seem to suggest that the microbial granules developed in this study have a great ability to accumulate P. It appears from Fig. 7 that high P uptake in the aerobic phase is associated with low SOUR. In EBPR processes, carbon sequestration by P-accumulating organisms in the anaerobic zone should be as high as possible so as to result in a lower aerobic activity in terms of SOUR in the aerobic phase. These results seem to indicate that P-accumulating organisms kinetically compete with non-P-accumulating organisms. A high respirometric activity may imply that the oxidation of external carbon source would be a dominant metabolic pathway for bacteria to obtain energy instead of utilizing the stored carbon source. This would lead to reduced biological P uptake as shown in Fig. 7. Chang et al. (1996) also observed that higher P accumulation in the aerobic zone was related to a lower specific substrate utilization rate in the oxic zone in conventional suspended activated sludge process. It has been commonly accepted that P-accumulating organisms are capable of assimilating simple soluble organic carbon, which is converted to internal storage products under anaerobic conditions. However, under aerobic conditions the storage products are further metabolized to provide carbon and energy for microbial growth (Satoh et al. 1992). It is only in this way that P-accumulating organisms can compete with other kinds of organisms in the aerobic famine stage and become dominant. Thus, the concentration of soluble organic carbon in the anaerobic stage is very important to P-accumulating organisms. This and previous research suggest that high metabolic activity of microorganisms in the aerobic phase may be responsible for the eventual failure of EBPR processes.
References
APHA (1998) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington, D.C.
Barnard JL, Stevens GM, Leslie PL (1985) Design strategies for nutrient removal plants. Water Sci Technol 17:233–242
Beun JJ, Hendriks A, Loosdrecht MCM van, Morgenroth E, Wilderer PA, Heijnen JJ (1999) Aerobic granulation in a sequencing batch reactor. Water Res 33:2283–2290
Chang WC, Chiou RJ, Ouyang CF (1996) The effect of residual substrate utilization on sludge settling in an enhanced biological phosphorus removal process. Water Sci Technol 34:425–430
Comeau Y, Hall KJ, Hancock REW, Oldham WK (1986) Biochemical model for biological enhanced phosphorus removal. Water Res 20:1511–1521
Crocetti GR, Hugenholtz P, Bond PL, Schuler A, Keller J, Jenkins D, Blackall LL (2000) Identification of polyphosphate accumulating organisms and design of 16S rRNA-directed probes for their detection and quantitation. Appl Environ Microbiol 66:1175–1182
Daigger GT, Polson SR (1991) Design and operation of biological phosphorus removal facilities. In: Sedlak RI (ed) Phosphorus and nitrogen removal from municipal wastewater. Lewis, New York, pp 167–202
Droste RL (1997) Theory and practice of water and wastewater treatment. Wiley, New York
EPA (1987) Design manual: phosphorus removal. Environmental Protection Agency, Washington, D.C.
Fukase T, Shibata M, Miyaji Y (1985) The role of an anaerobic stage on the biological phosphorus removal. Water Sci Technol 17:69–80
Hiraishi A, Ueda Y, Ishihara J (1989) Characterization of the bacterial population structure in an anaerobic-aerobic activated sludge system on the basis of respiratory quinine profiles. Appl Environ Microbiol 55:897–901
Jeon CO, Park JM (2000) Enhanced biological phosphorus removal in a sequencing batch reactor supplied with glucose as sole carbon source. Water Res 34:2160–2170
Moy BYP, Tay JH, Toh SK, Liu Y, Tay STL (2002) High organic loading influences the physical characteristics of aerobic granules. Letts Appl Microbiol 34:407–412
Panswad T, Doungchai A, Anotai J (2003) Temperature effect on microbial community of enhanced biological phosphorus removal system. Water Res 37:409–415
Pitman AR, Trim BC, van Dalsen L (1988) Operating experience with biological nutrient removal at the Hohannesburg Bushkoppie works. Water Sci Technol 20:51–61
Satoh H, Mino T, Matsuo T (1992) Uptake of organic substrates and accumulation of polyhydroxyalkanoates linked with glycolysis of intracellular carbohydrates under anaerobic conditions in the biological excess phosphate removal process. Water Sci Technol 26:933–942
Schuler AJ, Onuki M, Satoh H, Mino T (2002) Density separation and molecular methods to characterize enhanced biological phosphorus removal system populations. Water Sci Technol 46:195–198
Tay JH, Liu QS, Liu Y (2001) The effects of shear force on the formation, structure and metabolism of aerobic granules. Appl Microbiol Biotechnol 57:227–233
Tay JH, Yang SF, Liu Y (2002) Hydraulic selection pressure-induced nitrifying granulation in sequencing batch reactors. Appl Microbiol Biotechnol 59:332–337
Wentzel MC, Loewenthal RE, Ekama GA, Marais GVR (1988) Enhanced polyphosphate organism cultures in activated sludge system. Water SA 14:81–92
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, YM., Liu, Y. & Tay, JH. Development and characteristics of phosphorus-accumulating microbial granules in sequencing batch reactors. Appl Microbiol Biotechnol 62, 430–435 (2003). https://doi.org/10.1007/s00253-003-1359-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1359-7