Abstract
The tumor environment contributes importantly to tumor cell behavior and cancer progression. Aside from biochemical constituents, physical factors of the environment also influence the tumor. Growing evidence suggests that mechanics [e.g., tumor (stroma) elasticity, tissue pressure] are critical players of cancer progression. Underlying mechanobiological mechanisms involve among others the regulation of focal adhesion molecules, cytoskeletal modifications, and mechanosensitive (MS) ion channels of cancer- and tumor-associated cells. After reviewing the current concepts of cancer mechanobiology, we will focus on the canonical transient receptor potential 1 (TRPC1) channel and its role in mechano-signaling in tumor-associated pancreatic stellate cells (PSCs). PSCs are key players of pancreatic fibrosis, especially in cases of pancreatic ductal adenocarcinoma (PDAC). PDAC is characterized by the formation of a dense fibrotic stroma (desmoplasia), primarily formed by activated PSCs. Desmoplasia contributes to high pancreatic tissue pressure, which in turn activates PSCs, thereby perpetuating matrix deposition. Here, we investigated the role of the putatively mechanosensitive TRPC1 channels in murine PSCs exposed to elevated ambient pressure. Pressurization leads to inhibition of mRNA expression of MS ion channels. Migration of PSCs representing a readout of their activation is enhanced in pressurized PSCs. Knockout of TRPC1 leads to an attenuated phenotype. While TRPC1-mediated calcium influx is increased in wild-type PSCs after pressure incubation, loss of TRPC1 abolishes this effect. Our findings provide mechanistic insight how pressure, an important factor of the PDAC environment, contributes to PSC activation. TRPC1-mediated activation could be a potential target to disrupt the positive feedback of PSC activation and PDAC progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mechanotransduction, the conversion of physical stimuli into biochemical signals, plays an important role in many physiological and pathophysiological processes. Examples include proprioception, touch, hearing, sensation of blood flow, and cell migration (Lee et al. 1999; Martinac 2004; Lombardi et al. 2008). In addition to these physiological processes, biomechanics have been recognized to play a role in tumor pathophysiology. Tumor tissues are considerably stiffer than healthy tissues, which allows them to be detected by palpation when examining a patient, and which correlates with tumor cell survival and proliferation (Lu et al. 2012). Tumors are frequently characterized by an abnormal deposition of extracellular matrix (ECM) mediated by cancer cells, stromal fibroblasts, and immune cells (Bhowmick et al. 2004; Orimo et al. 2005; Vonlaufen et al. 2008a; Quante et al. 2011; Apte et al. 2013). Increasing evidence suggests that altered tumor mechanics is an important hallmark of cancer (Suresh 2007; Levental et al. 2009; Nagelkerke et al. 2015). In this paper, we will review current aspects of tumor mechanics with special emphasis on the role of mechanosensitive ion channels in tumor mechanosensing. We will focus on PDAC to accentuate the relevance of biophysics in cancer biology and highlight the role of TRPC1 channels in this system.
Matrix rigidity in cancer and fibroblast activation
The physical environment of tumors and their surrounding tumor microenvironment have become the subject of intense research (Jain et al. 2014; Wei and Yang 2015; Nagelkerke et al. 2015; Ivey et al. 2015). The increased deposition of ECM proteins and the compression of the interstitium arising from proliferating cancer cells and tumor-associated fibroblasts lead to an increased stiffness of the tumor. Matrix stiffening is not only the resulting outcome of tumorigenesis, it is one of the contributing factors for tissue fibrosis and tumor formation (Levental et al. 2009). The physical properties of the tumor environment have a major impact on tumor cells and contribute to their malignant transformation. Culturing of A549 lung carcinoma cells on stiff substrates provoked an enhanced transition to a mesenchymal phenotype as opposed to growth on softer substrates (Tilghman et al. 2010). Another example was shown in hepatocellular carcinoma cells, which proliferate faster and are more chemotherapy-resistant when cultured on a stiffer matrix (Schrader et al. 2011).
In addition to tumor cells, the surrounding stromal cells also sense the altered mechanics. A correlation of matrix rigidity and myofibroblast differentiation could be shown for fibroblasts of various organs, e.g., lungs, heart, and liver (Li et al. 2007; Quinlan and Billiar 2012; Rahaman et al. 2014; Yong et al. 2015). Thus, hepatic stellate cells, which represent the main hepatic myofibroblasts, are activated in response to the rigidity of the matrix (Olsen et al. 2011; Guvendiren et al. 2014). Sensing of substrate rigidity involves actin stress fiber formation, integrin-mediated signaling, and mechanosensitive ion channels signaling at the focal adhesions (Koo et al. 2002; Kobayashi and Sokabe 2010; Jansen et al. 2015). In lung myofibroblast, the matrix stiffness-dependent differentiation involves the transient receptor potential vanilloid 4 (TRPV4) channel, a known mechanosensitive ion channel of the TRP family (Rahaman et al. 2014).
Pancreatic ductal adenocarcinoma
The starring role of the biomechanical microenvironment is particularly evident in pancreatic ductal adenocarcinoma (PDAC). Tumors of the exocrine pancreas account for over 95 % of all pancreatic cancers. PDAC is by far the most frequent cancer of the exocrine pancreas (Matthaios et al. 2011). It is a highly aggressive malignancy with one of the worst prognoses of all cancer types (Malvezzi et al. 2015). Its lethal nature is in part due to the late onset of clinical symptoms, the aggressive growth, and the rapid expansion of distant metastases (Hidalgo 2010; Oettle 2014). PDAC is characterized by abundant deposition of connective tissue surrounding the tumor, a process called “desmoplasia” (Apte et al. 2004; Vonlaufen et al. 2008b). The stroma can form up to 90 % of the total tumor mass and contributes to the marked chemo-resistance and to an extraordinarily high tissue pressure (Erkan et al. 2012; Stylianopoulos et al. 2012; Schober et al. 2014; Xie and Xie 2015). ECM production in PDAC is mainly accomplished by pancreatic stellate cells (PSCs). The ECM production of the PSCs contributes in combination with other fibroblasts, macrophages and immune cells to the formation of a so-called tumor microenvironment (TME), which plays a critical role in metastasis and tumor progression (Joyce and Pollard 2009; Hanahan and Weinberg 2011). Tumor vessel compression as well as solid stress and high interstitial fluid pressure promote hypoxia and oxidative stress in the PDAC and the surrounding TME (Koong et al. 2000; Asaumi et al. 2007; Longo et al. 2016). Hypoxia, together with paracrine stimulation and increased tissue pressure, is a typical PDAC feature and promotes cancer cell proliferation, survival and metastasis (Niizeki et al. 2002; Yokoi and Fidler 2004; Büchler et al. 2004; Cohen et al. 2015). The physical and biochemical properties of the TME (pressure, ECM components, stiffness, acidity, hypoxia, growth factors, and cytokines) in turn also contribute to PSC activation (Watanabe et al. 2004; Feig et al. 2012). However, there are also recent reports showing a protective role of the abundant stroma against PDAC progression (Özdemir et al. 2014; Rhim et al. 2014). It is not yet clear, whether the desmoplastic reaction is part of a defense mechanism by providing a barrier limiting the metastasis of the tumor cells or whether the stroma drives and stimulates the aggressive behavior and progression of pancreatic cancer (Vonlaufen et al. 2008a; Apte et al. 2013; Gore and Korc 2014). Stroma-targeted therapies have not yet been successful (Neesse et al. 2015).
Pancreatic stellate cells
Pancreatic stellate cells (PSCs) reside in the periacinar and periductal space of the pancreas and comprise ~4–7 % of all pancreatic cells (Apte et al. 1998; Omary et al. 2007). They are often compared with hepatic stellate cells (HSCs), the major hepatic myofibroblast, mediating among others the fibrotic reaction in liver injury. PSCs and HSCs share many morphological and functional characteristics (Buchholz et al. 2005; Moreira 2007; Kordes et al. 2009; Puche et al. 2013). PSCs exhibit two states, a quiescent and an activated state (Bachem et al. 1998). Quiescent PSCs contain numerous fat droplets with vitamin A and express desmin and glial fibrillary acidic protein (Apte et al. 1998; Masamune and Shimosegawa 2009). In healthy pancreas, PSCs mainly contribute to tissue integrity and homeostasis by regulating the turnover of the extracellular matrix via secretion of metalloproteinases and their inhibitors (Phillips et al. 2003). Under pathological conditions, such as in PDAC or chronic pancreatitis, PSCs convert to an activated, myofibroblast-like phenotype. Activated PSCs lose their vitamin A droplets and show an enhanced alpha smooth muscle actin (αSMA) expression. The activation leads to an increase in proliferative and migratory activity (Masamune and Shimosegawa 2009). One characteristic property of activated PSCs is the abundant production and secretion of extracellular matrix components (Bachem et al. 2005; Masamune and Shimosegawa 2009). The activation process is mediated by a variety of soluble factors, like cytokines (Interleukin-1, Interleukin-6, TNFα), growth factors (PDGF, TGF-β, Activin A), ethanol and oxidative stress (Kikuta et al. 2006; Masamune and Shimosegawa 2009; Feig et al. 2012). Potential sources of these factors can be pancreatic cancer cells as well as activated macrophages, platelets, acinar cells, endothelial cells, and PSCs themselves (Bachem et al. 2005; Vonlaufen et al. 2008a). These paracrine and autocrine interactions produce positive feedback of cross-stimulation resulting in a perpetuation of PSCs activation and disease progression (Apte et al. 1999; Mews et al. 2002).
Pressure in chronic pancreatitis and PDAC
The tissue pressure in desmoplastic areas of the chronically inflamed pancreas as well as in PDAC is significantly higher than in healthy pancreas (Jalleh et al. 1991; Provenzano et al. 2012; Stylianopoulos et al. 2012). Values for chronic pancreatitis are in the range of 30 mmHg, while those for the healthy pancreas are ~10 mmHg (Madsen and Winkler 1982; Bradley 1982; Manes et al. 1994). It was proposed that desmoplasia and a fibrotic capsule ensheathing the gland lead to a misbalance of main pancreatic duct pressure (Karanjia et al. 1992). In murine PDAC models, extraordinarily high pressures of ~100 mmHg (interstitial fluid pressure, IFP) were measured within the tumors. IFP measurements were performed with a piezoelectric pressure transducer in pancreata of normal mice and in a genetic autochthonous PDAC mouse model (Provenzano et al. 2012). In addition to elevated IFP within the tumor, growth-induced solid stress from proliferating cancer cells and cancer-associated fibroblasts contributes to the mechanical microenvironment (Stylianopoulos et al. 2012). High tissue pressures in PDAC were either ascribed to elevated interstitial fluid pressure (IFP) or to growth-induced solid stress (Provenzano et al. 2012; Stylianopoulos et al. 2012). While the cause of the high-pressure in PDAC is discussed controversially (Provenzano et al. 2012; Chauhan et al. 2014; DelGiorno et al. 2014) its presence is not questioned and has important implications for therapy resistance of PDAC. The elevated pressure in PDAC precludes its effective perfusion and thereby greatly diminishes its accessibility by chemotherapeutic drugs.
PSCs can be directly activated by pressure, and on the other hand increased tissue pressure is one of the predominant factors promoting the fibrotic response of PSCs (Watanabe et al. 2004). The exact mechanisms of pressure-induced PSC activation and pressure sensation are largely unknown. In general, two mechanisms of pressure-induced PSC activation are discussed: (1) pressure-mediated activation of p38 MAPK signal transduction leads to an increased proliferation of PSCs (Watanabe et al. 2004). Interestingly, risk factors of pancreatic cancer, like ethanol and acetaldehyde, also activate p38 MAPK signaling pathways mediating cell growth and differentiation (Masamune et al. 2002; Kikuta et al. 2004; Masamune and Shimosegawa 2009). p38 MAPK activity is responsible for αSMA expression in PSCs, a prominent marker for activation, and could also be shown in hepatic stellate cells (Reeves et al. 2000; Watanabe et al. 2004; Novo et al. 2014). (2) Pressure also stimulates TGF-β secretion of PSCs, which is in line with findings in hepatic stellate cells and consistent with an autocrine pressure-mediated PSC activation (Sakata et al. 2004; Watanabe et al. 2004). Increased TGF-β levels could also be detected in patients with chronic pancreatitis (Sri Manjari et al. 2012). Nevertheless, the mechanisms of mechanosensing in PSCs are still elusive.
Mechanosensing
Sensing of the mechanical changes of the microenvironments involves intra- as well as extracellular components. The mechanosensing is based on (1) integrins, and (2) their interaction with the ECM, (3) the intracellular cytoskeleton and (4) mechanosensitive ion channels, which are all integrated in various intracellular signaling cascades (Hytönen and Wehrle-Haller 2015; Jansen et al. 2015). Being mechanosensitive switches, MS ion channels convert mechanical stimuli attaining the cell membrane (pressure, stretch, shear) into electrical and biochemical signals, which affect the cellular and physiological reactions. Several members of the transient receptor potential (TRP) family have been identified to mediate a variety of mechanosensory processes (Tobin et al. 2002; Sidi et al. 2003; Christensen and Corey 2007).
TRPC channels
Channels of the canonical transient receptor potential (TRPC) family have been implicated in a variety of sensory systems, including mechano-signaling (Lin and Corey 2005; Christensen and Corey 2007; Gottlieb et al. 2008). TRP channels have the molecular architecture of voltage-gated cation channels, with a tetrameric organization, each subunit having six transmembrane domains. In addition to chemical parameters (pH, osmolarity, ligand interaction) which affect TRPC channel activity, the family of TRPC ion channels was also attributed to mechano-signaling functions (Minke and Cook 2002; Maroto et al. 2005; Lin and Corey 2005; Spassova et al. 2006; Christensen and Corey 2007; Gottlieb et al. 2008). The mechanical force can activate the TRPC channels directly by sensory stimuli or indirectly via a variety of second messengers (Liu and Montell 2015). TRPC6 channels were proposed to be activated by mechanically and osmotically induced membrane stretch (Spassova et al. 2006). In parallel, TRPC6 is also activated via Phospholipase C and diacylglycerol (Vazquez et al. 2004; Dietrich and Gudermann 2014).
TRPC1 channels
TRPC1 was the first identified member of the mammalian TRPC family, forming a cation channel (Zitt et al. 1996). TRPC1 can function as a homotetrameric cation channel (Minke and Cook 2002) and is also able to heteromultimerize with TRPC3, TRPC4, TRPC5, and TRPC6 as well as TRPP2 and TRPV4 (Hofmann et al. 2002; Gottlieb et al. 2008; Patel et al. 2010; Ma et al. 2011). The role of TRPC1 as mechano-sensitive channel has been the subject of controversial debates (Beech et al. 2003; Vazquez et al. 2004; Maroto et al. 2005). Initially, it was reported that overexpression of TRPC1 in frog oocytes increased the number of stretch-activated ion channels in patch-clamp recordings (Maroto et al. 2005). The same authors showed that TRPC1 anti-sense treatment was able to reduce the stretch-induced channel activity. However, subsequent studies were not able to reproduce these findings. Dietrich and colleagues showed that pressure-induced constriction of cerebral arteries (Bayliss effect) was not altered in TRPC1-deficient mice (Dietrich et al. 2007). Additionally, the effect on MS ion channel activity after overexpression of human TRPC1 in COS cells was remarkably lower (tenfold increase compared to mock control) than the 1.000-fold increase achieved with an activation of the mechanosensitive K2P channel (Gottlieb et al. 2008). Interestingly, the same authors showed a different localization pattern of TRPC1 in different cell lines and systems. TRPC1 was barely detectable in the plasma membrane of COS and CHO cells, but instead accumulated in the membrane of the endoplasmic reticulum (Hofmann et al. 2002; Gottlieb et al. 2008). In comparison, TRPC1 expression in oocytes (with a TRPC1-mediated response to mechanical force) was located in the surface membrane (Gottlieb et al. 2008).
On the other hand, numerous studies performed in different organ systems and cell types clearly showed that TRPC1 channels are part of mechano-signaling cascades. Thus, TRPC1 was proposed to be a critical component of (1) biomechanical signaling in the heart underlying the development of pressure-induced heart failure and hypertrophy (Seth et al. 2009; Eder and Molkentin 2011), (2) mechanotransduction in primary afferent sensory neurons (Garrison et al. 2012) as well as (3) in dorsal root ganglion neurons (Staaf et al. 2009), and (4) stretch-activated ion channels in the larval bullfrog skin (Hillyard et al. 2010). (5) A role of TRPC1 in mechano-signaling during cell migration could be shown by Fabian and colleagues. The migration of siTRPC1 Madin-Darby canine kidney focus cells (MDCK-F) and synoviocytes from TRPC1−/− mice has a reduced directionality and attenuated calcium transients following mechanical stretch (Fabian et al. 2012). (6) TRPC1 links the outgrowth of spinal axons to substrate rigidity (Kerstein et al. 2013). The reasons underlying the apparent discrepancies with respect to the mechanical regulation of TRPC1 are not yet known. Possibly, a varying subunit composition depending on the background of the respective cell type may account for the differential results of studies addressing the putative mechano-sensitivity of TRPC1 channels.
In our study, we show that TRPC1 channels are part of the mechanisms causing pressure-induced activation of murine PSCs. Our findings suggest that TRPC1 channels, by mediating the pressure-induced activation of PSCs, contribute to the perpetuation of PSC activation in PDAC.
Results and discussion
Altered mRNA expression of different mechanosensitive ion channels in response to exposure to elevated pressure
To test the effect of increased pressure on ion channel expression levels in PSCs, cells were pre-incubated for 24 h under elevated pressure (100 mmHg) inside a humidified pressure chamber. mRNA expression of different putatively mechanosensitive ion channels (TRPM7, TREK1, TRPV4, PIEZO1, and TRPC1) were analyzed by means of qPCR under control conditions and following pressurization in WT and TRPC1-KO cells. TRPM7 is a known mechanosensitive member of the TRP family, which has been linked to mediating the calcium (and magnesium) influx into fibroblasts in response to increased membrane tension (Wei et al. 2009; Ryazanova et al. 2010; Kuipers et al. 2012) and to pressure loading in mesenchymal stem cells (Xiao et al. 2015). TREK1 is a member of the two-pore domain (K2P) K+ channel family and is one of the first molecularly identified stretch-activated mechanosensitive channel in mammals (Patel et al. 1998; Enyedi and Czirják 2010). It is directly sensitive to membrane tension (Berrier et al. 2013). TRPV4 was shown to be activated by osmotic stimuli causing cell swelling and to participate in sensation of pressure and nociception (Liedtke et al. 2000; Suzuki et al. 2003). Likewise, mechanically activated TRPV4 mediates calcium influx at focal adhesions by indirectly interacting with β1 integrins (Matthews et al. 2010). In tumor vessels, the downregulation of TRPV4 channels was shown to result in aberrant mechano-signaling of tumor endothelial cells in response to the extracellular matrix. The resulting abnormal angiogenesis was linked to tumor progression (Adapala et al. 2015). PIEZO1 has been identified as a member of a novel class of mechanosensitive channels, albeit it is still unknown whether Piezo1 proteins are pore-forming ion channels or modulators of neighboring ion channels (Coste et al. 2012; Volkers et al. 2015).
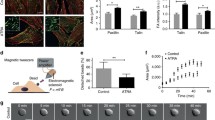
All of these channels (TRPM7, TREK1, TRPV4, PIEZO1, and TRPC1) are expressed in primary murine PSCs with PIEZO1, TRPM7, and TRPC1 being the most abundant ones when normalized to HPRT expression (see Fig. 1a). TRPC1-KO leads to marked changes of their mRNA expression when compared to WT PSCs (see Fig. 1b–d). Expression of Trpm7, Trpv4, and Trek1 is significantly increased in TRPC1-KO cells under control conditions. Thus, it appears as if knock-out of TRPC1 is compensated for by elevated expression levels of other mechanosensitive ion channels. Expression coupling between different TRP channels was also observed in vascular smooth muscle cells (VSMCs) in which siRNA-mediated downregulation of TRPC1 is accompanied by increased TRPC6 expression (Selli et al. 2009). Conversely, in VSMCs of TRPC6-deficient mice TRPC3 channels are upregulated (Dietrich et al. 2005). Therefore, it is not unexpected that TRPC1 deficiency leads to altered expression of alternative mechanosensitive ion channels in PSCs.
mRNA analysis of mechanosensitive ion channels in WT and TRPC1-KO PSCs. mRNA expression of different putatively mechanosensitive ion channels (TRPM7, TREK1, TRPV4, PIEZO1, TRPC1) was evaluated in PSCs from WT and TRPC1-KO mice. a All of these channels are expressed in murine PSCs. b–e Under control conditions (white panel) TRPC1-KO leads to an increased mRNA expression of Trek1 (b), Trpm7 (c), and Trpv4 (d) when compared to WT PSCs. After pressure incubation (grey panel), expression of Trpm7, Trpv4, Piezo1, and Trpc1 is reduced in WT. In TRPC1-KO, only mRNA expression of Trpv4 is significantly reduced after pressure incubation. Expression levels were normalized to WT controls and are shown relative to mRNA expression of the housekeeping gene Hprt (n = 4; *p < 0.05 vs. WT control)
Pressurization of PSCs causes a drastic inhibition of the ion channel mRNA expression in both genotypes (except for Trek1 in WT and Piezo1 in TRPC1-KO). In WT cells, expression of Trpc1 mRNA is reduced after pressure incubation (see Fig. 1f). Similarly, the upregulation of Trpv4, Trpm7, and Trek1 is reversed in TRPC1-KO PSCs. It can be speculated that the presumed activation of mechanosensitive ion channels by elevated pressure induces negative feedback on their mRNA expression levels to reduce the number of activated ion channels in the plasma membrane. To test this hypothetical scenario, future studies will have to combine pressure incubation with simultaneous dampening of, e.g., intracellular calcium signaling in PSCs, for instance by using the calcium chelator BAPTA. Alternatively, channel blockers could be applied that would also impair TRP channel-mediated monovalent cation influx into PSCs.
Pressure leads to enhanced migration of PSCs
Activation of quiescent PSCs in chronic pancreatitis or PDAC leads to an increased recruitment and migration of PSCs. Therefore, migration represents a suitable parameter to analyze pressure-induced activation of PSCs and the role of TRPC1 played therein. The role of mechanosensitive calcium channels in PSC activation and migration is still elusive. However, there is accumulating evidence for an involvement of mechano-sensitive ion channels in cell migration obtained in other cell types. Thus, TRPC1 knockdown and knock-out lead to an impaired directionality of migrating MDCK-F cells and synoviocytes, respectively (Fabian et al. 2012). Likewise, reduced TRPV4 expression in tumor-derived endothelial cells from transgenic adenocarcinoma mice lead to an abnormally high migration velocity and impaired substrate mechanosensitivity (Adapala et al. 2015).
We analyzed the impact of TRPC1 knockout in PSCs after pressure incubation on cell migration. Figure 2a–d depicts trajectories of individual WT and TRPC1-KO PSCs. Under control conditions, translocation of WT and TRPC1-KO PSCs is not different (Fig. 2a, b; WT: 43.4 ± 3.1 vs. KO: 44.8 ± 4.9 µm). Pressurization accelerates migration of WT PSCs by 29 % (Fig. 2c; mean translocation WT: 55.6 ± 6.8 µm). This stimulatory effect of the pressurization is strongly attenuated in TRPC1-KO PSCs. Translocation of pressurized TRPC1-KO PSCs is not different from that of TRPC1-KO control cells (Fig. 2d; mean translocation TRPC1-KO: 51.8 ± 6.8 µm). In Fig. 2e, we analyzed the time course of migration speed following pressurization. The speed increases in both genotypes, however, it is significantly higher in WT compared to TRPC1-KO PSCs, which is particularly evident 2–6 h after pressurization (see Fig. 2e).
Migration of PSCs from WT and TRPC1-KO mice after pressure incubation. a–d Singe cell trajectories are normalized to common starting points and mean translocation is indicated by the radius of a circle. Under control conditions, translocation of WT and TRPC1-KO PSCs does not differ (a and b). Pressure incubation significantly increases the translocation only of WT PSCs (c and d). e Additionally, migration velocity is significantly higher in WT than in TRPC1-KO after pressurization, most notably 2–6 h after pressure incubation (N = 5, n = 20; *p < 0.05 WT vs. KO after pressure)
Effects of pressurization could also be shown in VSMCs, where pressure pre-incubation (72 h, 180 mmHg) leads to a significant increase of VSMCs migration on different matrices (Onoue et al. 2008). It was also shown that exposure to pressure leads to an increase of the intracellular calcium concentration in VSMCs (Hishikawa et al. 1994). Lung cancer cells also respond with increased migration to pressurization (Kao et al. 2014). The authors postulated that cancer cells must cope with the increased interstitial fluid pressure within tumors and therefore adapt to the increasing pressure for initial invasion steps. Pressure-induced lung cancer cell migration was proposed to be accelerated because of an increased expression of aquaporin 1, which has been linked to cell migration in different cell types (Papadopoulos et al. 2008; Schwab et al. 2012; Kao et al. 2014). In PSCs, we can link the effect of accelerated migration after exposure to enhanced pressure to TRPC1 channels. Loss of TRPC1 leads to an attenuated reaction to enhanced pressure.
Loss of TRPC1 channels leads to reduced calcium influx after pressure incubation
Based on the attenuated increase of migration of pressurized TRPC1-KO PSCs (see Fig. 2) and the known importance of calcium in this process (Schwab et al. 2012), we tested whether pressurization induces calcium influx into PSCs and whether this is TRPC1-dependent. Calcium influx was measured by employing the Mn2+ quench method. The slope of the Mn2+-induced decrease of the Fura-2 fluorescence was taken as a surrogate of calcium influx (Merritt et al. 1989; Lindemann et al. 2013). Figure 3a, b shows original recordings from individual WT and TRPC1-KO PSCs. While calcium influx is increased in pressure incubated WT PSCs (Fig. 3c), this is not the case in TRPC1-KO PSCs.
Calcium influx in WT and TRPC1-KO PSCs after pressure incubation. a, b Representative graphs showing the relative fluorescence intensity (F 365[%]) of Fura-2 AM loaded PSCs. After addition of Mn2+ (dashed line), quenching of the Fura-2 AM signal could be observed. The corresponding mean slope Δm(F 365/t) was calculated, with m1 representing the slope under control conditions and m2 that after Mn2+ application. The Mn2+ quenching was performed in control cells (grey line) and in pressurized PSCs (black line). c Pressure incubation leads to an increase of calcium influx into WT PSCs. In contrast, calcium influx of TRPC1-KO is not affected by pressurization (b). Mean change of the slope was normalized to control conditions (HEPES/Ringer solution; N = 4, n = 29–49; p < 0.05)
Conclusion and model
Elevated pressure leads to a significant activation of murine PSCs. This could be validated by quantifying different activation parameters (migration, calcium signaling). Pressure incubation was performed at Δ + 100 mmHg above atmospheric pressure, representing an elevation of only ~13 %. Accordingly, pH changes caused by pressure-induced alterations of pCO2 were negligible. The fact that PSCs respond to these small changes points to the sensitivity of the mechanosensation in PSCs. Nevertheless, the question is how this isotropic force can lead to mechano-signaling in nearly uncompressible cells. The effect of isotropic hydrostatic pressure can be explained in different ways: (1) pressure within a constant (cell) volume will lead to changes in thermodynamics. According to the model of cellular tensegrity, it can affect the cytoskeleton and impair the balance between contractile filaments and extracellular tethering sites to the ECM, leading to changes in mechano-signaling (Myers et al. 2007; Ingber et al. 2014). (2) Elevated pressure leads to changes in the lipid bilayer membrane (e.g., increased membrane bending rigidity, increase of gel-like states of the membrane, stronger lipid chain ordering) and can directly affect the membrane-bound proteins like ion channels or transporters (Scarlata 2005; Skanes et al. 2006; Purushothaman et al. 2015). The influence of pressure on the plasma membrane could be shown in different high-pressure approaches (+80 MPa) (Brooks 2014; Winter 2015; Purushothaman et al. 2015), as well as under physiologic, isotropic pressurization (+310 mmHg) (Nirmalanandhan et al. 2015).
Our results are consistent with the idea, that pressure contributes to activation of TRPC1 channels and leads to calcium influx into PSCs. This in turn affects the activation of PSCs and their cell behavior such as migration. Therefore, we propose that TRPC1 channels contribute to pressure-induced activation of PSCs. Calcium-dependent differentiation of cardiac fibroblast was linked to TRPM7 or TRPV4 channel activation (Du et al. 2010; Adapala et al. 2013). By forming heteromers with TRPV4 channels TRPC1 also participates in calcium signaling of vascular endothelial cells (Ma et al. 2011). Homomeric TRPC1 channels did not seem to be functional, so that their role in functional heteromeric channel complexes with other members of the TRPC family or TRPV4/6 is more likely (Storch et al. 2012; Dietrich et al. 2014). Along these lines, TRPC1 could be viewed as a pressure-activated subunit of heteromeric mechanosensitive ion channels. To elucidate the details of the TRPC1-mediated pressure sensing and subsequent signal transduction, will be the aim of future studies.
The activation of WT PSCs after pressurization may be linked to TRPC1-dependent cytokine signaling in these cells. In TRPC1-deficient mice cytokine production (e.g., KC) in both alveolar macrophages and lung epithelial cells is reduced in response to bacterial infections (Zhou et al. 2015). A similar mechanism is also conceivable for the activation of pressurized PSCs, since it is well known that they are an abundant source of growth factors and cytokines causing an autocrine activation (Shek et al. 2002; Bachem et al. 2005). Along these lines the impaired activation of pressurized TRPC1-KO PSCs would be a consequence of reduced cytokine production (Bachem et al. 2006; Apte and Wilson 2012). TGF-β potently stimulates matrix secretion by PSCs (Schneider et al. 2001; Shek et al. 2002). Interestingly, both TRPV4 as well as TRPC1 channels are involved in TGF-β signaling pathways (Dong et al. 2010; Song et al. 2014). These studies and our findings are consistent with our idea proposed earlier that TRP channels in tumor stroma cells fulfil important sensor and effector functions in the tumor microenvironment (Nielsen et al. 2014). The contribution of TRPC1 channels to pressure sensing in PSCs combined with a potential role in cytokine secretion could thereby make them a target to disrupt the positive feedback induced by pressure-induced activation of PSCs.
Materials and methods
Animals
Primary pancreatic stellate cells were used for all experiments. They were isolated from 8 to 12-week-old male/female 129Sv/C57BL/6J WT and TRPC1-KO mice (Dietrich et al. 2007). Experimental protocols were approved by the local committee for animal care.
Reagents
Chemicals were obtained from the following sources: DMEM/F12, RPMI 1640 and laminin were purchased from Sigma Aldrich (Steinheim, Germany). FCS Gold from was purchased from GE-Healthcare (Little Chalfont, UK), collagen I and penicillin/streptomycin were from Biochrom (Berlin; Germany). Gey’s balanced salt solution (GBSS) was from Pan-Biotech GmbH (Aidenbach, Germany). Collagen III, collagen IV and fibronectin were purchased from BD Biosciences (Franklin Lakes, US), collagenase P, Reverse Transcriptase Assay and LightCycler® 480 SYBR Green I master mix were from Roche Applied Science (Mannheim, Germany). Fura-2 AM and DNase was purchased from Thermo Fisher Scientific (Waltham, USA) and TRI-reagent from Gibco (Darmstadt, Germany). All cell culture dishes and flasks were obtained from Corning (New York, USA).
Isolation of murine PSCs
Isolation of primary PSCs from murine pancreata was performed in a modified procedure as described previously (Haanes et al. 2012). Mice were sacrificed by isoflurane treatment and subsequent cervical dislocation. The pancreas was removed and briefly washed in cold balanced salt solution (GBSS) before homogenization. Pancreas homogenates were then transferred to 3 ml GBSS with 0.1 % collagenase P and incubated at 37 °C for 25 min on a shaker. After resuspending and adding GBSS to a final volume of 8 ml cells were centrifuged (8 min, 220 × g), resuspend in cell culture media (DMEM/F12, 10 % FCS-Gold, 1 % penicillin/streptomycin, supplemented with 14.3 mM NaHCO3, pH 7.2) and seeded on to a pre-coated tissue culture dish (coated with FCS-Gold). Cells were incubated for 2 h in the incubator, with subsequent forceful washing steps with warm culture media. After each washing step cells were observed under the microscope to ensure optimal purity. Freshly isolated PSCs were incubated for 5–6 days until first passaging. Cells were used for experiments after two passages.
Pressure-incubation
Incubation of PSCs was performed in a custom-made pressure chamber for 24 h at 100 mmHg above ambient atmospheric pressure (~760 mmHg). To this end, a Plexiglas chamber was used fitting into a standard cell culture incubator. Pressurization with the humidified incubator atmosphere containing 5 % CO2 was achieved with an air pump system and was monitored continuously with a digital barometer. Pressure controls were also incubated in the pressure chamber, but at atmospheric pressure (~760 mmHg). Increasing the partial pressure of CO2 in the chamber by 13 % is theoretically expected to decrease pH by Δ0.06 (14.3 mmol/l NaHCO3 in cell culture media, 5 % CO2 with \(\alpha_{{{\text{CO}}_{ 2} }} = 0.0 30 7\;{\text{mmol}}/{\text{L}}*{\text{mmHg}}\) (Burnett and Noonan 1974)). Experimentally, we found no significant differences (control: pH = 7.16 ± 0.04; pressure: pH = 7.23 ± 0.05; n = 8).
Quantitative real-time PCR (qPCR)
Total RNA was isolated from control cells and pressure-incubated PSCs from wild-type and TRPC1-KO mice using TRI reagent and subsequent DNase incubation. Total RNA (1 µg) was used for reverse transcription with the Reverse Transcriptase Assay. Isolated cDNA was diluted to a total volume of 100 µl. For qPCR analysis, 2 µl cDNA template was used in combination with the LightCycler® 480 SYBR Green I Master Mix in a LightCycler® 480 System. Sequences of the used primer pairs (10 pM) are listed in Table 1. qPCR reaction was performed at 60 °C annealing temperature for 45 cycles. qPCR experiments were done in cooperation with the Institute of Pharmacology and Toxicology (University of Münster, Germany).
Migration experiments
The migration of PSCs was recorded with time-lapse video microscopy, as described previously (Schwab et al. 2005). PSCs were seeded in tissue culture flasks (12.5 cm2, ~27.000 cells per flask) coated with a matrix whose composition mimics desmoplastic regions in PDAC. Its composition is shown in Table 2. Polymerization of the desmoplastic matrix was done overnight in the tissue culture incubator prior to seeding PSCs. Pressure incubation and control treatments were performed with PSCs already seeded in matrix-coated tissue culture flasks. Flasks were sealed immediately after incubation and transferred to preheated (37 °C) microscopy chambers. Cell migration was recorded for 6 h at 5-min intervals using time-lapse video microscopy. The outlines of migrating PSCs were segmented with the Amira Imaging Software Version 2.2 (Template Graphics Software, Mercury Communication Systems, Carlsbad, CA, USA), a self-made Java program and the National Institutes of Health ImageJ Software (http://rsb.info.nih.gov/ij/). Based on segmented cell contours, we calculated parameters such as migratory velocity (in µm/min) and translocation (in µm). Migration was defined as the movement of the cell center as a function of time. Mean translocation represents the net distance covered during the whole experiment.
Measurements of calcium influx into PSCs
For measurements of calcium influx, PSCs were pre-incubated for 30 min with DMEM/F12 containing 3 µM of the calcium-sensitive dye Fura-2 AM in the incubator. After dye loading, medium was changed to Ringer solution (122.5 mM NaCl, 5.4 mM KCl, 1.2 mM CaCl2, 0.8 mM MgCl2, 5.5 mM d-glucose and 10.0 mM HEPES, pH 7.4) and incubated for another 10 min at 37 °C. Experiments were carried out at 37 °C. Fluorescence emission was recorded at 500 nm after exciting the cells at a wavelength of 365 nm. Images were acquired at 5-s intervals. The experimental setup (monochromator, camera, and data acquisition) was controlled by Metaflour software (Visitron Systems, Puchheim, Germany).
Calcium influx into PSCs was indirectly measured by means of the manganese (Mn2+) quenching method (Merritt et al. 1989; Fabian et al. 2011). Mn2+ enters the cell lumen via calcium channels and binds to the calcium-sensitive dye Fura-2 AM with higher affinity than calcium. This leads to a decrease of the Fura-2 fluorescence intensity. The decrease in intensity over time can be used for indirect estimation of calcium influx. Fluorescence intensity was measured over the whole cell area and was corrected for background fluorescence. During measurements, a 5-min control period with Ringer solution was followed by a 5-min perfusion of Mn2+-Ringer solution (see above, plus 200 µM Mn2+). For data analysis the intensity was normalized to the values of the control period. Afterwards, regression analysis of the calcium-dependent Fura-2 AM fluorescence intensity over time allowed the determination of the change of fluorescence quenching. The corresponding mean change of the slope (Δm F 365/t = m2 − m1) was calculated (m1 = slope during control conditions; m2 = initial slope during Mn2+ perfusion). A more negative value of Δm indicates a higher calcium influx into the cells.
Statistical analysis
All experiments were repeated with PSCs from at least N = 3 mice. The number of cells (n) analyzed is indicated in the figure captions. Values are reported as mean values ± SEM. All data were tested for normality prior to further statistical analysis. For normally distributed data, the Student’s t test was used. For mRNA data analysis, the Software REST V2.0.13 (Relative Expression Software Tool, QIAGEN GmbH, Hilden, Germany) was used. Expression data were normalized to expression of the housekeeping gene Hprt and random statistical analysis was performed with 10,000 iterations. Differences between experimental groups reaching p values <0.05 were considered significant.
Abbreviations
- ECM:
-
Extracellular matrix
- IFP:
-
Interstitial fluid pressure
- MDCK-F:
-
Madin–Darby canine kidney focus cells
- MS ion channels:
-
Mechanosensitive ion channels
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PSCs:
-
Pancreatic stellate cells
- TME:
-
Tumor microenvironment
- TRPC:
-
Canonical transient receptor potential
- TRPV4:
-
Transient receptor potential vanilloid 4
- VSMC:
-
Vascular smooth muscle cells
- αSMA:
-
α-Smooth muscle actin
References
Adapala RK, Thoppil R, Luther DJ et al (2013) TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J Mol Cell Cardiol 54:45–52. doi:10.1016/j.yjmcc.2012.10.016
Adapala RK, Thoppil RJ, Ghosh K et al (2015) Activation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapy. Oncogene. doi:10.1038/onc.2015.83
Apte MV, Wilson JS (2012) Dangerous liaisons: pancreatic stellate cells and pancreatic cancer cells. J Gastroenterol Hepatol 27(Suppl 2):69–74. doi:10.1111/j.1440-1746.2011.07000.x
Apte MV, Haber PS, Applegate TL et al (1998) Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 43:128–133
Apte MV, Haber PS, Darby SJ et al (1999) Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut 44:534–541
Apte MV, Park S, Phillips PA et al (2004) Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas 29:179–187
Apte MV, Wilson JS, Lugea A, Pandol SJ (2013) A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology 144:1210–1219. doi:10.1053/j.gastro.2012.11.037
Asaumi H, Watanabe S, Taguchi M et al (2007) Externally applied pressure activates pancreatic stellate cells through the generation of intracellular reactive oxygen species. Am J Physiol Gastrointest Liver Physiol 293:G972–G978. doi:10.1152/ajpgi.00018.2007
Bachem MG, Schneider E, Gross H et al (1998) Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 115:421–432
Bachem MG, Schünemann M, Ramadani M et al (2005) Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 128:907–921
Bachem MG, Zhou Z, Zhou S, Siech M (2006) Role of stellate cells in pancreatic fibrogenesis associated with acute and chronic pancreatitis. J Gastroenterol Hepatol 21(Suppl 3):S92–S96. doi:10.1111/j.1440-1746.2006.04592.x
Beech DJ, Xu SZ, McHugh D, Flemming R (2003) TRPC1 store-operated cationic channel subunit. Cell Calcium 33:433–440
Berrier C, Pozza A, de Lavalette ADL et al (2013) The purified mechanosensitive channel TREK-1 is directly sensitive to membrane tension. J Biol Chem 288:27307–27314. doi:10.1074/jbc.M113.478321
Bhowmick NA, Neilson EG, Moses HL (2004) Stromal fibroblasts in cancer initiation and progression. Nature 432:332–337. doi:10.1038/nature03096
Bradley EL (1982) Pancreatic duct pressure in chronic pancreatitis. Am J Surg 144:313–316
Brooks NJ (2014) Pressure effects on lipids and bio-membrane assemblies. IUCrJ 1:470–477. doi:10.1107/S2052252514019551
Buchholz M, Kestler HA, Holzmann K et al (2005) Transcriptome analysis of human hepatic and pancreatic stellate cells: organ-specific variations of a common transcriptional phenotype. J Mol Med Berl Ger 83:795–805. doi:10.1007/s00109-005-0680-2
Büchler P, Reber HA, Lavey RS et al (2004) Tumor hypoxia correlates with metastatic tumor growth of pancreatic cancer in an orthotopic murine model. J Surg Res 120:295–303. doi:10.1016/j.jss.2004.02.014
Burnett RW, Noonan DC (1974) Calculations and correction factors used in determination of blood pH and blood gases. Clin Chem 20:1499–1506
Chauhan VP, Boucher Y, Ferrone CR et al (2014) Compression of pancreatic tumor blood vessels by hyaluronan is caused by solid stress and not interstitial fluid pressure. Cancer Cell 26:14–15. doi:10.1016/j.ccr.2014.06.003
Christensen AP, Corey DP (2007) TRP channels in mechanosensation: direct or indirect activation? Nat Rev Neurosci 8:510–521. doi:10.1038/nrn2149
Cohen R, Neuzillet C, Tijeras-Raballand A et al (2015) Targeting cancer cell metabolism in pancreatic adenocarcinoma. Oncotarget 6:16832–16847. doi:10.18632/oncotarget.4160
Coste B, Xiao B, Santos JS et al (2012) Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483:176–181. doi:10.1038/nature10812
DelGiorno KE, Carlson MA, Osgood R et al (2014) Response to Chauhan et al.: interstitial pressure and vascular collapse in pancreas cancer-fluids and solids, measurement and meaning. Cancer Cell 26:16–17. doi:10.1016/j.ccr.2014.06.004
Dietrich A, Gudermann T (2014) TRPC6: physiological function and pathophysiological relevance. Handb Exp Pharmacol 222:157–188. doi:10.1007/978-3-642-54215-2_7
Dietrich A, y Schnitzler MM, Gollasch M et al (2005) Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol 25:6980–6989. doi:10.1128/MCB.25.16.6980-6989.2005
Dietrich A, Kalwa H, Storch U et al (2007) Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflüg Arch Eur J Physiol 455:465–477. doi:10.1007/s00424-007-0314-3
Dietrich A, Fahlbusch M, Gudermann T (2014) Classical transient receptor potential 1 (TRPC1): channel or channel regulator? Cells 3:939–962. doi:10.3390/cells3040939
Dong H, Shim K-N, Li JMJ et al (2010) Molecular mechanisms underlying Ca2+-mediated motility of human pancreatic duct cells. Am J Physiol Cell Physiol 299:C1493–C1503. doi:10.1152/ajpcell.00242.2010
Du J, Xie J, Zhang Z et al (2010) TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res 106:992–1003. doi:10.1161/CIRCRESAHA.109.206771
Eder P, Molkentin JD (2011) TRPC channels as effectors of cardiac hypertrophy. Circ Res 108:265–272. doi:10.1161/CIRCRESAHA.110.225888
Enyedi P, Czirják G (2010) Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev 90:559–605. doi:10.1152/physrev.00029.2009
Erkan M, Reiser-Erkan C, Michalski CW et al (2012) The impact of the activated stroma on pancreatic ductal adenocarcinoma biology and therapy resistance. Curr Mol Med 12:288–303
Fabian A, Fortmann T, Bulk E et al (2011) Chemotaxis of MDCK-F cells toward fibroblast growth factor-2 depends on transient receptor potential canonical channel 1. Pflüg Arch Eur J Physiol 461:295–306. doi:10.1007/s00424-010-0901-6
Fabian A, Bertrand J, Lindemann O et al (2012) Transient receptor potential canonical channel 1 impacts on mechanosignaling during cell migration. Pflüg Arch Eur J Physiol 464:623–630. doi:10.1007/s00424-012-1169-9
Feig C, Gopinathan A, Neesse A et al (2012) The pancreas cancer microenvironment. Clin Cancer Res Off J Am Assoc Cancer Res 18:4266–4276. doi:10.1158/1078-0432.CCR-11-3114
Garrison SR, Dietrich A, Stucky CL (2012) TRPC1 contributes to light-touch sensation and mechanical responses in low-threshold cutaneous sensory neurons. J Neurophysiol 107:913–922. doi:10.1152/jn.00658.2011
Gore J, Korc M (2014) Pancreatic cancer stroma: friend or foe? Cancer Cell 25:711–712. doi:10.1016/j.ccr.2014.05.026
Gottlieb P, Folgering J, Maroto R et al (2008) Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflüg Arch Eur J Physiol 455:1097–1103. doi:10.1007/s00424-007-0359-3
Guvendiren M, Perepelyuk M, Wells RG, Burdick JA (2014) Hydrogels with differential and patterned mechanics to study stiffness-mediated myofibroblastic differentiation of hepatic stellate cells. J Mech Behav Biomed Mater 38:198–208. doi:10.1016/j.jmbbm.2013.11.008
Haanes KA, Schwab A, Novak I (2012) The P2X7 receptor supports both life and death in fibrogenic pancreatic stellate cells. PLoS One. doi:10.1371/journal.pone.0051164
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. doi:10.1016/j.cell.2011.02.013
Hidalgo M (2010) Pancreatic cancer. N Engl J Med 362:1605–1617. doi:10.1056/NEJMra0901557
Hillyard SD, Willumsen NJ, Marrero MB (2010) Stretch-activated cation channel from larval bullfrog skin. J Exp Biol 213:1782–1787. doi:10.1242/jeb.040568
Hishikawa K, Nakaki T, Marumo T et al (1994) Pressure promotes DNA synthesis in rat cultured vascular smooth muscle cells. J Clin Invest 93:1975–1980. doi:10.1172/JCI117189
Hofmann T, Schaefer M, Schultz G, Gudermann T (2002) Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci USA 99:7461–7466. doi:10.1073/pnas.102596199
Hytönen VP, Wehrle-Haller B (2015) Mechanosensing in cell-matrix adhesions—converting tension into chemical signals. Exp Cell Res. doi:10.1016/j.yexcr.2015.10.027
Ingber DE, Wang N, Stamenovic D (2014) Tensegrity, cellular biophysics, and the mechanics of living systems. Rep Prog Phys Phys Soc G B 77:46603. doi:10.1088/0034-4885/77/4/046603
Ivey JW, Bonakdar M, Kanitkar A et al (2015) Improving cancer therapies by targeting the physical and chemical hallmarks of the tumor microenvironment. Cancer Lett. doi:10.1016/j.canlet.2015.12.019
Jain RK, Martin JD, Stylianopoulos T (2014) The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng 16:321–346. doi:10.1146/annurev-bioeng-071813-105259
Jalleh RP, Aslam M, Williamson RC (1991) Pancreatic tissue and ductal pressures in chronic pancreatitis. Br J Surg 78:1235–1237
Jansen KA, Donato DM, Balcioglu HE et al (2015) A guide to mechanobiology: where biology and physics meet. Biochim Biophys Acta 1853:3043–3052. doi:10.1016/j.bbamcr.2015.05.007
Joyce JA, Pollard JW (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9:239–252. doi:10.1038/nrc2618
Kao Y-C, Lee C-H, Kuo P-L (2014) Increased hydrostatic pressure enhances motility of lung cancer cells. Conf Proc IEEE Eng Med Biol Soc 2014:2928–2931. doi:10.1109/EMBC.2014.6944236
Karanjia ND, Singh SM, Widdison AL et al (1992) Pancreatic ductal and interstitial pressures in cats with chronic pancreatitis. Dig Dis Sci 37:268–273
Kerstein PC, Jacques-Fricke BT, Rengifo J et al (2013) Mechanosensitive TRPC1 channels promote calpain proteolysis of talin to regulate spinal axon outgrowth. J Neurosci Off J Soc Neurosci 33:273–285. doi:10.1523/JNEUROSCI.2142-12.2013
Kikuta K, Masamune A, Satoh M et al (2004) 4-hydroxy-2,3-nonenal activates activator protein-1 and mitogen-activated protein kinases in rat pancreatic stellate cells. World J Gastroenterol 10:2344–2351
Kikuta K, Masamune A, Satoh M et al (2006) Hydrogen peroxide activates activator protein-1 and mitogen-activated protein kinases in pancreatic stellate cells. Mol Cell Biochem 291:11–20. doi:10.1007/s11010-006-9189-4
Kobayashi T, Sokabe M (2010) Sensing substrate rigidity by mechanosensitive ion channels with stress fibers and focal adhesions. Curr Opin Cell Biol 22:669–676. doi:10.1016/j.ceb.2010.08.023
Koo LY, Irvine DJ, Mayes AM et al (2002) Co-regulation of cell adhesion by nanoscale RGD organization and mechanical stimulus. J Cell Sci 115:1423–1433
Koong AC, Mehta VK, Le QT et al (2000) Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys 48:919–922
Kordes C, Sawitza I, Häussinger D (2009) Hepatic and pancreatic stellate cells in focus. Biol Chem 390:1003–1012. doi:10.1515/BC.2009.121
Kuipers AJ, Middelbeek J, van Leeuwen FN (2012) Mechanoregulation of cytoskeletal dynamics by TRP channels. Eur J Cell Biol 91:834–846. doi:10.1016/j.ejcb.2012.05.006
Lee J, Ishihara A, Oxford G et al (1999) Regulation of cell movement is mediated by stretch-activated calcium channels. Nature 400:382–386. doi:10.1038/22578
Levental KR, Yu H, Kass L et al (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139:891–906. doi:10.1016/j.cell.2009.10.027
Li Z, Dranoff JA, Chan EP et al (2007) Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatol Baltim Md 46:1246–1256. doi:10.1002/hep.21792
Liedtke W, Choe Y, Martí-Renom MA et al (2000) Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103:525–535
Lin S-Y, Corey DP (2005) TRP channels in mechanosensation. Curr Opin Neurobiol 15:350–357. doi:10.1016/j.conb.2005.05.012
Lindemann O, Umlauf D, Frank S et al (2013) TRPC6 regulates CXCR2-mediated chemotaxis of murine neutrophils. J Immunol Baltim Md 1950 190:5496–5505. doi:10.4049/jimmunol.1201502
Liu C, Montell C (2015) Forcing open TRP channels: mechanical gating as a unifying activation mechanism. Biochem Biophys Res Commun 460:22–25. doi:10.1016/j.bbrc.2015.02.067
Liu X, Cheng KT, Bandyopadhyay BC et al (2007) Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(−/−) mice. Proc Natl Acad Sci U S A 104:17542–17547. doi:10.1073/pnas.0701254104
Lombardi ML, Knecht DA, Lee J (2008) Mechano-chemical signaling maintains the rapid movement of dictyostelium cells. Exp Cell Res 314:1850–1859. doi:10.1016/j.yexcr.2008.02.001
Longo V, Brunetti O, Gnoni A et al (2016) Angiogenesis in pancreatic ductal adenocarcinoma: a controversial issue. Oncotarget 7(36):58649–58658. doi:10.18632/oncotarget.10765
Lu P, Weaver VM, Werb Z (2012) The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 196:395–406. doi:10.1083/jcb.201102147
Ma X, Cheng K-T, Wong C-O et al (2011) Heteromeric TRPV4-C1 channels contribute to store-operated Ca(2+) entry in vascular endothelial cells. Cell Calcium 50:502–509. doi:10.1016/j.ceca.2011.08.006
Madsen P, Winkler K (1982) The intraductal pancreatic pressure in chronic obstructive pancreatitis. Scand J Gastroenterol 17:553–554
Malvezzi M, Bertuccio P, Rosso T et al (2015) European cancer mortality predictions for the year 2015: does lung cancer have the highest death rate in EU women? Ann Oncol. doi:10.1093/annonc/mdv001
Manes G, Büchler M, Pieramico O et al (1994) Is increased pancreatic pressure related to pain in chronic pancreatitis? Int J Pancreatol Off J Int Assoc Pancreatol 15:113–117
Maroto R, Raso A, Wood TG et al (2005) TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol 7:179–185. doi:10.1038/ncb1218
Martinac B (2004) Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci 117:2449–2460. doi:10.1242/jcs.01232
Masamune A, Shimosegawa T (2009) Signal transduction in pancreatic stellate cells. J Gastroenterol 44:249–260. doi:10.1007/s00535-009-0013-2
Masamune A, Kikuta K, Satoh M et al (2002) Alcohol activates activator protein-1 and mitogen-activated protein kinases in rat pancreatic stellate cells. J Pharmacol Exp Ther 302:36–42
Matthaios D, Zarogoulidis P, Balgouranidou I et al (2011) Molecular pathogenesis of pancreatic cancer and clinical perspectives. Oncology 81:259–272. doi:10.1159/000334449
Matthews BD, Thodeti CK, Tytell JD et al (2010) Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr Biol Quant Biosci Nano Macro 2:435–442. doi:10.1039/c0ib00034e
Merritt JE, Jacob R, Hallam TJ (1989) Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J Biol Chem 264:1522–1527
Mews P, Phillips P, Fahmy R et al (2002) Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut 50:535–541
Minke B, Cook B (2002) TRP channel proteins and signal transduction. Physiol Rev 82:429–472. doi:10.1152/physrev.00001.2002
Moreira RK (2007) Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med 131:1728–1734. doi:10.1043/1543-2165(2007)131[1728:HSCALF]2.0.CO;2
Myers KA, Rattner JB, Shrive NG, Hart DA (2007) Hydrostatic pressure sensation in cells: integration into the tensegrity model. Biochem Cell Biol Biochim Biol Cell 85:543–551. doi:10.1139/o07-108
Nagelkerke A, Bussink J, Rowan AE, Span PN (2015) The mechanical microenvironment in cancer: how physics affects tumours. Semin Cancer Biol 35:62–70. doi:10.1016/j.semcancer.2015.09.001
Neesse A, Algül H, Tuveson DA, Gress TM (2015) Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut 64:1476–1484. doi:10.1136/gutjnl-2015-309304
Nielsen N, Lindemann O, Schwab A (2014) TRP channels and STIM/ORAI proteins: sensors and effectors of cancer and stroma cell migration. Br J Pharmacol 171:5524–5540. doi:10.1111/bph.12721
Niizeki H, Kobayashi M, Horiuchi I et al (2002) Hypoxia enhances the expression of autocrine motility factor and the motility of human pancreatic cancer cells. Br J Cancer 86:1914–1919. doi:10.1038/sj.bjc.6600331
Nirmalanandhan VS, Hurren R, Cameron WD et al (2015) Increased pressure alters plasma membrane dynamics and renders acute myeloid leukemia cells resistant to daunorubicin. Haematologica 100:e406–e408. doi:10.3324/haematol.2015.129866
Novo E, Cannito S, Paternostro C et al (2014) Cellular and molecular mechanisms in liver fibrogenesis. Arch Biochem Biophys 548:20–37. doi:10.1016/j.abb.2014.02.015
Oettle H (2014) Progress in the knowledge and treatment of advanced pancreatic cancer: from benchside to bedside. Cancer Treat Rev 40:1039–1047. doi:10.1016/j.ctrv.2014.07.003
Olsen AL, Bloomer SA, Chan EP et al (2011) Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol 301:G110–G118. doi:10.1152/ajpgi.00412.2010
Omary MB, Lugea A, Lowe AW, Pandol SJ (2007) The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest 117:50–59. doi:10.1172/JCI30082
Onoue N, Nawata J, Tada T et al (2008) Increased static pressure promotes migration of vascular smooth muscle cells: involvement of the Rho-kinase pathway. J Cardiovasc Pharmacol 51:55–61. doi:10.1097/FJC.0b013e31815b9d26
Orimo A, Gupta PB, Sgroi DC et al (2005) Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121:335–348. doi:10.1016/j.cell.2005.02.034
Özdemir BC, Pentcheva-Hoang T, Carstens JL et al (2014) Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25:719–734. doi:10.1016/j.ccr.2014.04.005
Papadopoulos MC, Saadoun S, Verkman AS (2008) Aquaporins and cell migration. Pflüg Arch Eur J Physiol 456:693–700. doi:10.1007/s00424-007-0357-5
Patel AJ, Honoré E, Maingret F et al (1998) A mammalian two pore domain mechano-gated S-like K + channel. EMBO J 17:4283–4290. doi:10.1093/emboj/17.15.4283
Patel A, Sharif-Naeini R, Folgering JRH et al (2010) Canonical TRP channels and mechanotransduction: from physiology to disease states. Pflüg Arch Eur J Physiol 460:571–581. doi:10.1007/s00424-010-0847-8
Phillips PA, McCarroll JA, Park S et al (2003) Rat pancreatic stellate cells secrete matrix metalloproteinases: implications for extracellular matrix turnover. Gut 52:275–282
Provenzano PP, Cuevas C, Chang AE et al (2012) Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21:418–429. doi:10.1016/j.ccr.2012.01.007
Puche JE, Saiman Y, Friedman SL (2013) Hepatic stellate cells and liver fibrosis. Compr Physiol 3:1473–1492. doi:10.1002/cphy.c120035
Purushothaman S, Cicuta P, Ces O, Brooks NJ (2015) Influence of high pressure on the bending rigidity of model membranes. J Phys Chem B 119:9805–9810. doi:10.1021/acs.jpcb.5b05272
Quante M, Tu SP, Tomita H et al (2011) Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 19:257–272. doi:10.1016/j.ccr.2011.01.020
Quinlan AMT, Billiar KL (2012) Investigating the role of substrate stiffness in the persistence of valvular interstitial cell activation. J Biomed Mater Res A 100:2474–2482. doi:10.1002/jbm.a.34162
Rahaman SO, Grove LM, Paruchuri S et al (2014) TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J Clin Invest 124:5225–5238. doi:10.1172/JCI75331
Reeves HL, Dack CL, Peak M et al (2000) Stress-activated protein kinases in the activation of rat hepatic stellate cells in culture. J Hepatol 32:465–472
Rhim AD, Oberstein PE, Thomas DH et al (2014) Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25:735–747. doi:10.1016/j.ccr.2014.04.021
Ryazanova LV, Rondon LJ, Zierler S et al (2010) TRPM7 is essential for Mg(2+) homeostasis in mammals. Nat Commun 1:109. doi:10.1038/ncomms1108
Sakata R, Ueno T, Nakamura T et al (2004) Mechanical stretch induces TGF-beta synthesis in hepatic stellate cells. Eur J Clin Invest 34:129–136
Scarlata S (2005) The effect of hydrostatic pressure on membrane-bound proteins. Braz J Med Biol Res Rev Bras Pesqui Médicas E Biológicas Soc Bras Biofísica Al 38:1203–1208. doi:10.1590/S0100-879X2005000800007
Schneider E, Schmid-Kotsas A, Zhao J et al (2001) Identification of mediators stimulating proliferation and matrix synthesis of rat pancreatic stellate cells. Am J Physiol Cell Physiol 281:C532–C543
Schober M, Jesenofsky R, Faissner R et al (2014) Desmoplasia and chemoresistance in pancreatic cancer. Cancers 6:2137–2154. doi:10.3390/cancers6042137
Schrader J, Gordon-Walker TT, Aucott RL et al (2011) Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatol Baltim Md 53:1192–1205. doi:10.1002/hep.24108
Schwab A, Rossmann H, Klein M et al (2005) Functional role of Na+–HCO3− cotransport in migration of transformed renal epithelial cells. J Physiol 568:445–458. doi:10.1113/jphysiol.2005.092957
Schwab A, Fabian A, Hanley PJ, Stock C (2012) Role of ion channels and transporters in cell migration. Physiol Rev 92:1865–1913. doi:10.1152/physrev.00018.2011
Selli C, Erac Y, Kosova B, Tosun M (2009) Post-transcriptional silencing of TRPC1 ion channel gene by RNA interference upregulates TRPC6 expression and store-operated Ca2+ entry in A7r5 vascular smooth muscle cells. Vascul Pharmacol 51:96–100. doi:10.1016/j.vph.2009.04.001
Seth M, Zhang Z-S, Mao L et al (2009) TRPC1 channels are critical for hypertrophic signaling in the heart. Circ Res 105:1023–1030. doi:10.1161/CIRCRESAHA.109.206581
Shek FW-T, Benyon RC, Walker FM et al (2002) Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol 160:1787–1798
Sidi S, Friedrich RW, Nicolson T (2003) NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science 301:96–99. doi:10.1126/science.1084370
Skanes ID, Stewart J, Keough KMW, Morrow MR (2006) Effect of chain unsaturation on bilayer response to pressure. Phys Rev E Stat Nonlin Soft Matter Phys 74:51913. doi:10.1103/PhysRevE.74.051913
Song Y, Zhan L, Yu M et al (2014) TRPV4 channel inhibits TGF-β1-induced proliferation of hepatic stellate cells. PLoS One 9:e101179. doi:10.1371/journal.pone.0101179
Spassova MA, Hewavitharana T, Xu W et al (2006) A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA 103:16586–16591. doi:10.1073/pnas.0606894103
Sri Manjari K, Nallari P, Vidyasagar A et al (2012) Plasma TGF-β1, MMP-1 and MMP-3 levels in chronic pancreatitis. Indian J Clin Biochem IJCB 27:152–156. doi:10.1007/s12291-011-0167-6
Staaf S, Maxvall I, Lind U et al (2009) Down regulation of TRPC1 by shRNA reduces mechanosensitivity in mouse dorsal root ganglion neurons in vitro. Neurosci Lett 457:3–7. doi:10.1016/j.neulet.2009.03.082
Storch U, Forst A-L, Philipp M et al (2012) Transient receptor potential channel 1 (TRPC1) reduces calcium permeability in heteromeric channel complexes. J Biol Chem 287:3530–3540. doi:10.1074/jbc.M111.283218
Stylianopoulos T, Martin JD, Chauhan VP et al (2012) Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc Natl Acad Sci USA 109:15101–15108. doi:10.1073/pnas.1213353109
Suresh S (2007) Biomechanics and biophysics of cancer cells. Acta Biomater 3:413–438. doi:10.1016/j.actbio.2007.04.002
Suzuki M, Mizuno A, Kodaira K, Imai M (2003) Impaired pressure sensation in mice lacking TRPV4. J Biol Chem 278:22664–22668. doi:10.1074/jbc.M302561200
Tilghman RW, Cowan CR, Mih JD et al (2010) Matrix rigidity regulates cancer cell growth and cellular phenotype. PLoS One 5:e12905. doi:10.1371/journal.pone.0012905
Tobin DM, Madsen DM, Kahn-Kirby A et al (2002) Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron 35:307–318
Vazquez G, Wedel BJ, Aziz O et al (2004) The mammalian TRPC cation channels. Biochim Biophys Acta 1742:21–36. doi:10.1016/j.bbamcr.2004.08.015
Volkers L, Mechioukhi Y, Coste B (2015) Piezo channels: from structure to function. Pflüg Arch Eur J Physiol 467:95–99. doi:10.1007/s00424-014-1578-z
Vonlaufen A, Joshi S, Qu C et al (2008a) Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res 68:2085–2093. doi:10.1158/0008-5472.CAN-07-2477
Vonlaufen A, Phillips PA, Xu Z et al (2008b) Pancreatic stellate cells and pancreatic cancer cells: an unholy alliance. Cancer Res 68:7707–7710. doi:10.1158/0008-5472.CAN-08-1132
Watanabe S, Nagashio Y, Asaumi H et al (2004) Pressure activates rat pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol 287:G1175–G1181. doi:10.1152/ajpgi.00339.2004
Wei SC, Yang J (2015) Forcing through tumor metastasis: the interplay between tissue rigidity and epithelial–mesenchymal transition. Trends Cell Biol. doi:10.1016/j.tcb.2015.09.009
Wei C, Wang X, Chen M et al (2009) Calcium flickers steer cell migration. Nature 457:901–905. doi:10.1038/nature07577
Winter R (2015) Pressure effects on artificial and cellular membranes. Subcell Biochem 72:345–370. doi:10.1007/978-94-017-9918-8_17
Xiao E, Yang HQ, Gan Y-H et al (2015) Brief reports: TRPM7 senses mechanical stimulation inducing osteogenesis in human bone marrow mesenchymal stem cells. Stem Cells (Dayt Ohio) 33:615–621. doi:10.1002/stem.1858
Xie D, Xie K (2015) Pancreatic cancer stromal biology and therapy. Genes Dis 2:133–143. doi:10.1016/j.gendis.2015.01.002
Yokoi K, Fidler IJ (2004) Hypoxia increases resistance of human pancreatic cancer cells to apoptosis induced by gemcitabine. Clin Cancer Res Off J Am Assoc Cancer Res 10:2299–2306
Yong KW, Li Y, Huang G et al (2015) Mechanoregulation of cardiac myofibroblast differentiation: implications for cardiac fibrosis and therapy. Am J Physiol Heart Circ Physiol 309:H532–H542. doi:10.1152/ajpheart.00299.2015
Zhou X, Ye Y, Sun Y et al (2015) Transient receptor potential channel 1 deficiency impairs host defense and proinflammatory responses to bacterial infection by regulating protein kinase Cα signaling. Mol Cell Biol 35:2729–2739. doi:10.1128/MCB.00256-15
Zitt C, Zobel A, Obukhov AG et al (1996) Cloning and functional expression of a human Ca2+-permeable cation channel activated by calcium store depletion. Neuron 16:1189–1196
Acknowledgments
We thank Alexander Dietrich’s group (LMU Munich) for providing us the TRPC1-KO mice and Frank Ulrich Müller’s group (WWU Muenster) for providing the LightCycler® 480 System (Roche Applied Science, Mannheim, Germany). We are grateful to Sarah Sargin and Jana Welzig for their excellent technical assistance. This work was supported by the Marie Curie Initial Training Network IonTraC (FP7-PEOPLE-2011-ITN Grant Agreement No. 289648).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Special Issue: Ion Channels, Transporters and Cancer.
An erratum to this article can be found at http://dx.doi.org/10.1007/s00249-016-1184-4.
Rights and permissions
About this article
Cite this article
Fels, B., Nielsen, N. & Schwab, A. Role of TRPC1 channels in pressure-mediated activation of murine pancreatic stellate cells. Eur Biophys J 45, 657–670 (2016). https://doi.org/10.1007/s00249-016-1176-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-016-1176-4