Abstract
Harmful blooms of Prorocentrum donghaiense occur annually in the phosphorus-scarce coastal waters of the East China Sea (ECS). The enzymatic activities of alkaline phosphatase (AP) and its regulation by external phosphorus were studied during a P. donghaiense bloom in this area. The AP characteristics of P. donghaiense was further compared with Prorocentrum minimum and Prorocentrum micans in monocultures with both bulk and single-cell enzyme-labeled fluorescence AP assays. Concentrations of dissolved inorganic phosphorus (DIP) varied between 0.04 and 0.73 μmol l−1, with more than half recording stations registering concentrations below 0.10 μmol l−1. Concentrations of dissolved organic phosphorus (DOP) were comparable or even higher than those of DIP. P. donghaiense suffered phosphorus stress and expressed abundant AP, especially when DIP was lower than 0.10 μmol l−1. The AP activities showed a negative correlation with DIP but a positive correlation with DOP. The AP activities were also regulated by internal phosphorus pool. The sharp increase in AP activities was observed until cellular phosphorus was exhausted. Most AP of P. donghaiense was located on the cell surface and some were released into the water with time. Compared with P. minimum and P. micans, P. donghaiense showed a higher AP affinity for organic phosphorus substrates, a more efficient and energy-saving AP expression quantity as a response to phosphorus deficiency. The unique AP characteristic of P. donghaiense suggests that it benefits from the efficient utilization of DOP, and outcompete other species in the phosphorus-scarce ECS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Prorocentrum donghaiense is one of the most well-known harmful algal bloom species in the Changjiang River Estuary and its coastal waters of the East China Sea (ECS) [1, 2]. P. donghaiense blooms have been recorded annually in spring since 2000, at times lasting for more than 1 month and encompassing several thousand square kilometers [1, 3, 4]. The notorious blooms have caused serious damage to the regional ecosystem, marine fisheries, and public health [5, 6]. The estimated economic losses due to P. donghaiense blooms range from hundreds of thousands to millions of US dollars every year [7]. Many studies have been carried out to study the mechanism of P. donghaiense blooms in this area, among which the importance of phosphorus dynamics has been noted [2, 8,9,10]. The nutrient structure in the coastal waters of the Changjiang River Estuary has changed greatly in the past 60 years [10]. The concentrations of nitrate and phosphate have been increased significantly with the dissolved inorganic N:P ratios increasing sharply from 40 in the 1960s to 200 in the 2010s due to a much more abundant nitrate discharge from the Changjiang River compared with phosphate [10, 11]. Phytoplankton suffer from phosphorus stress or limitation during spring to summer times in this area [6, 12, 13]. The dominant species of Skeletonema costatum was replaced by P. donghaiense during spring when phosphate was scarce in this area [2, 8]. Furthermore, P. donghaiense blooms often persist for a long period under phosphorus-deficient conditions and only collapse as nitrate is depleted [2, 12]. All these findings suggest that P. donghaiense is adapted to the low-phosphate environment and must possess some advantageous strategies in competition and utilization of phosphorus.
Phytoplankton have developed adaptive strategies to deal with phosphorus deficiency, e.g., inducing high-affinity phosphate transporters, changing phosphorus allocation through polyphosphate production, decreasing cellular phosphorus demand by substituting phospholipids with sulfolipids and/or nitrolipids, and efficiently utilizing organic phosphorus compounds by phosphatases [14,15,16]. Among the phosphatases, alkaline phosphatase (AP), which hydrolyzes a broad spectrum of organic phosphorus compounds (mostly mono-phosphoester) into inorganic phosphate, is one of the best studied [17,18,19,20]. AP plays a prominent role in the regeneration of organic phosphorus in the marine environment [19, 21]. AP has been widely used to study the phosphorus status of microorganisms or as an indicator of phosphorus deficiency or limitation [13, 17, 19]. Traditionally, bulk AP activity (APA) is quantitatively measured by adding some artificial chromogenic or fluorescent substrate [18, 22]. The origin of AP (heterotrophic bacteria or phytoplankton) can be roughly analyzed through size fractionation of water samples [18]. A qualitative cell-specific assay using enzyme-labeled fluorescence (ELF) made it possible to accurately tag cells that exhibit APA [23]. The combination of bulk AP and single-cell ELF assays are recommended to better explain the phosphorus status of phytoplankton in both natural environments and laboratory studies [24,25,26]. Transcriptomic and proteomic techniques have also been used to analyze the molecular responses of AP to the variation of phosphorus in recent years [15, 27,28,29]. Diverse AP characteristics among phytoplankton species have been observed in marine ecosystems. Algal AP might be constitutive or inducible [19, 23]. Some phytoplankton may not even express AP [18]. Algal AP might differ in gene sequence types [27, 30], subcellular localization [27, 31], response threshold value to phosphorus stress [20, 32], and affinity for organic phosphorus substrates [25] etc. These AP differences among microorganisms influence the hydrolysis efficiency in organic phosphorus compounds and thus determine inter-specific competition capability in marine ecosystems, especially when phosphate is scarce.
The regeneration of organic phosphorus compounds cannot be ignored in the coastal waters of ECS, especially during P. donghaiense blooms [33, 34]. The dissolved organic phosphorus (DOP) concentrations sometimes are higher than phosphate concentrations and contribute 5–70% of the total dissolved phosphorus (TDP) in these areas [33, 35]. Inducible expression is observed in P. donghaiense as it suffers severe phosphorus stress and expresses high APA during blooms [13]. A turnover time of ~ 10 h for organic phosphorus compounds was calculated during P. donghaiense blooms in situ by an AP kinetic assay [13]. AP seems to play a prominent role in the regeneration of organic phosphorus during P. donghaiense blooms. Previous laboratory studies indicated that P. donghaiense was more competitive in growth with organic phosphorus substrates [36, 37]. Shi et al. [9] suggested that P. donghaiense enhanced DOP utilization to cope with phosphorus deficiency. P. donghaiense showed a higher AP affinity for organic phosphorus substrate compared with S. costatum [38]. Thus, we hypothesize that due to their ability to efficiently utilize DOP by AP, P. donghaiense is able to outcompete other species and dominate phytoplankton communities. To explain the mechanism of P. donghaiense blooms, it is important to comprehensively understand AP characteristics of P. donghaiense and its efficiency in utilizing DOP.
Two other species of P. minimum and P. micans in the family of Prorocentraceae often coexist with P. donghaiense in the ECS [3]. Of special interest is that P. minimum is the cause of harmful blooms in many estuarine and coastal waters [11, 39, 40]. We ask why the bloom species in the ECS is P. donghaiense instead of P. minimum or P. micans? Might the difference in the efficiency in utilizing DOP by phosphatase among Prorocentrum species help determine the fates in inter-specific competition? In this study, the differences in AP characteristics and the efficiency in hydrolyzing organic phosphorus compounds among three Prorocentrum species were compared with the bulk and single-cell ELF assays in both the field and the laboratory. The objectives of this study were to investigate the phosphorus status of phytoplankton and the AP regulation capability during P. donghaiense blooms to better understand the adaptive strategies of P. donghaiense to phosphorus deficiency and the importance of DOP regeneration through AP hydrolysis during blooms. The inter-specific differences in AP distribution among three Prorocentrum species were also investigated.
Materials and Methods
Study of AP Activities During a P. donghaiense Bloom in the Coastal Waters of ECS
A field cruise was carried out in the coastal waters of ECS during May 7–24, 2010 using the RV “Science 3.” A total of 27 stations were sampled (Fig. 1). Water samples were taken from the surface layer (1-m depth) using 2.5-l Niskin bottles. During the cruise, a P. donghaiense bloom with the density higher than > 106 cells l−1 were observed in the study area, especially along transects A and B. Cells of S. costatum, P. minimum, and P. micans were observed to coexist with P. donghaiense in most stations.
Two replicate seawater samples were filtered through pre-combusted Whatman GF/F filters (450 °C, 2 h) for measurement of nutrient. The samples were frozen at − 20 °C until analysis in the laboratory in one month. Nitrate + nitrite, ammonium, reactive silicate, and DIP were determined spectrophotometrically using a flow injection analyzer (Technicon AA II autoanalyzer)[41]. Dissolved inorganic nitrogen (DIN) was the sum of nitrate, nitrite, and ammonium. Total dissolved nitrogen (TDN) and TDP were measured after wet oxidation with alkaline K2S2O8 [41]. Dissolved organic nitrogen (DON) was calculated by subtracting DIN from TDN whereas DOP was calculated by subtracting DIP from TDP.
Approximately 10 l of seawater was concentrated using a 10 μm mesh and centrifuged for 5 min at 4000 g. The concentrated cell pellets were processed for the analysis of single-cell APA with ELF reagent (2-(5′-chloro-2′-phosphoryloxyphenyl)-6-chloro-4-(3H)-quinazolinone, Molecular Probes, Inc., OR) according to the method of Ou et al. [25]. The pellets were labeled with ELF for 30 min at room temperature in the dark and then was rinsed 3 times using sterile seawater. The samples were stored in 50–100 μl sterile seawater in the dark at 4 °C until analysis in the laboratory in one month. For analysis, the samples were observed under an epifluorescence microscope (OLYMPUS, X61) with a DAPI filter set. The ELF of three Prorocentrum species and S. costatum were observed. When the density was high, at least 200 cells of the observed species were counted. When the density was relatively low, all cells of the species in the sample were counted. The observed cells were divided into three groups based on the intensity of fluorescence: absence of fluorescence; medium fluorescence, where ELF coverd < 30% of the cell bodies; strong fluorescence, where ELF covered > 30% of the cell bodies. Percentages of ELF for a given species were calculated as the fraction of ELF labeled cells in one group divided by the total number of cells counted.
Study of AP Characteristics of Three Prorocentrum Species in the Laboratory
Culture and Growth Conditions
Cultures of P. donghaiense (MEL 203), P. minimum (MEL 310), and P. micans (MEL 56) were maintained in the Algal Collection, Research Center of Harmful Algae and Marine Biology, Jinan University, China. The cultures were inoculated at 21 ± 1 °C in a light: dark cycle of 12: 12 h with irradiation of 100 μmol m−2 s−1.
Prior to the experiment, the cultures were re-inoculated 3 times during the exponential phase in the Aquil* artificial seawater medium (enriched with f/2 except for PO43−)[42] to allow the algae to acclimatize to the subsequent experimental conditions. The initial concentrations of PO43− were decreased to ~ 18, 9 and 5 μmol l−1 gradually at each inoculation. Antibiotics (penicillin G, 3 g l−1, and streptomycin sulfate, 5 g l−1) were used to eliminate bacterial contamination 48 h before the next inoculation [43]. The cultures were checked for bacterial contamination with 4′,6-diamidino-2-phenylindole (DAPI)(Sigma) stain at regular intervals by microscopic inspection.
Given the differences in cell volumes among three Prorocentrum species, monocultures of P. donghaiense, P. minimum, and P. micans were inoculated in triplicate with densities as shown in Table 1. The cell volumes of three species were calculated according to the method of Hillebrand et al. [44]. The initial concentrations of PO43−were adjusted to ~ 3 μmol l−1 based on the f/2 medium. When the observed DIP concentration in media did not decrease anymore (lower than 0.1–0.2 μmol l−1) and the cells of Prorocentrum species stopped growing for 3 days, the cultures were considered as phosphorus-starved cells. ~ 5 μmol l−1 of PO43− was replenished into each treatment on day 11 (for P. donghaiense) or day 12 (for P. minimum and P. micans) just before sampling. Surprisingly, DIP in each media decrease sharply to lower than 0.5 μmol l−1 in the next day; another two times of 5 and 10 μmol l−1 of PO43− were added separately into each treatment in the next 2 days after the measurement of DIP. Samples for analysis were taken each day at 12:00.
Measurement of Parameters
Cells were fixed in a 2% acid Lugol’s solution and the densities were counted using a light microscope (Olympus BX61). The specific growth rates (μ) of each Prorocentrum species were calculated according to the equation μ = (ln N2 – ln N1)/(t2 − t1), where N2 and N1 were the cell densities at respective times, t2 and t1. For Chl a analysis, cells were filtered onto Whatman GF/F filters and were stored at − 20 °C. Chl a was extracted with 90% acetone in the dark for 24 h and were analyzed using a spectrofluorometer (Hitachi U-2810, USA) [45].
The seawater was filtered through pre-combusted (450 °C, 2 h) GF/F filters. The filtrate was used for the analysis of DIP with the method of Valderrama [46]. The filters were used for the analysis of particulate phosphorus (PP) according to Solórzano and Sharp [47].
Bulk APA was determined by fluorescence as the release of 4-Methylumbelliferone (MUF, Sigma) from 4-methylumbelliferyl phosphate (MUP, Sigma) according to Hoppe [48]. The seawater was first filtered through a 120-μm filter to remove zooplankton. The assay was run in the dark and at 30 °C for 1 h with the final substrate 4-MUP concentration of 25 μmol l−1. The fluorescence was measured using the spectrofluorometer (Ex = 435 nm, Em = 520 nm). Sterile seawater was used as control blanks. AP in the 0.2-μm filtrate (filtered through 0.22 μm polycarbonate filters under < 100 mmHg pressure) was regarded as free AP. AP in unfractionated samples was regarded as total AP. Bulk APA was expressed as fmol of MUP released cell−1 h−1.
Single-cell APA of three Prorocentrum species was determined as in the field study. At least 200 cells of each species were counted in one sample. To analyze the location sites of AP, samples labeled with ELF were observed using a confocal epifluorescence microscope (Zeiss, LSM 510 META) to scan in ~ 0.50-μm section through cells.
Study of AP Hydrolytic Kinetics of Three Prorocentrum Species in the Laboratory
Monocultures of three Prorocentrum species were transferred four times with the initial concentrations of PO43− reducing gradually to 18, 9, 4, and 2 μmol l−1 at each inoculation. The P-starved cells were used for the study. Substrate MUP was added with the final concentrations of 0, 0.1, 0.2, 0.5, 2, 5, 10, 20, 50 μmol l−1 in triplicate. Sample fluorescence was measured in the dark and at 30 °C for 1 h. The hydrolytic kinetic constants (Ks and Vmax) which followed the Michaelis-Menten equation were calculated using the non-linear least squares method.
Data Analysis
A one-way ANOVA with Tukey’s test was performed to compare the differences among the treatments of each test parameter. A p value < 0.05 was regarded as significant. Prior to analysis, data were tested for normality and homogeneity of variance. A Log 10 or square-root transformation of the data was performed prior to any statistical test when necessary. All tests were performed using the SPSS 19.0 software (SPSS Inc., USA).
Results
Nutrients and APA in the Coastal Waters of ECS
DIN, DON, DIP, and SiO32− decreased gradually from inshore to offshore. DIN and DON varied between 0.93–28.54 and 2.97–54.81 μmol l−1, with the averages of 12.31 ± 7.39 and 13.65 ± 9.76 μmol l−1, respectively (Table 2). DIP changed from 0.04 to 0.73 μmol l−1, with most stations in the southern part of the Changjiang River Estuary (latitude < 30°) lower than 0.10 μmol l−1. DOP varied between 0.07–0.58 μmol l−1 and the high concentrations were observed close to the estuary. The high values of DIN/DIP and TDN/TDP suggested that most stations suffered P stress.
Phytoplankton in the studied stations were divided into three groups according to the DIP concentrations in situ. Measured cellular fluorescences showed that single-cell APA strengthened with the decrease of DIP, particularly at levels lower than 0.10 μmol l−1 (Table 3). When DIP was higher than 0.10 μmol l−1, most S. costatum cells were not labeled with fluorescence whereas 65–86 % of Prorocentrum cells were labeled with medium or even strong fluorescence (Fig. 2). When DIP decreased to lower than 0.10 μmol l−1, higher percentages of strong ELF were found in the sampled species, especially S. costatum. The percentages of ELF of P. donghaiense decreased with DIP but increased with DOP (p < 0.05 for both) (Fig. 3). No obvious difference was found in single-cell APA of three Prorocentrum species.
Transects A and B were in the central zone of P. donghaiense bloom. The densities of P. donghaiense in the nearshore stations were all higher than 106 cells l−1, with the maximum of 4.5 × 106 cells l−1 in Stn A2 (Fig. 4). The morphology of P. donghaiense, abundant observed green fluorescent debris in ELF samples, together with decomposing odors in the air in situ, suggested that the bloom along transect B, in particular in Stns B1 and B2, was during the declining phase. DIP was all higher than 0.10 μmol l−1 and was similar with DOP along transect A. However, DIP decreased to lower than 0.10 μmol l−1 and DOP contributed most to TDP pool along transect B. Most Prorocentrum cells were labeled with strong or medium fluorescence whereas only 0–30% of S. costatum cells were labeled with fluorescence along transect A (Fig. 5). The percentages of strong and medium fluorescing cells increased greatly along transect B, especially in Stn B1. When compared among three Prorocentrum species, it seemed that the fluorescences of P. donghaiense and P. micans were stronger than that of P. minimum.
Physiological and Growth Response of three Prorocentrum to the Variation of Phosphorus in the Laboratory
Variations of DIP, PP, and Cell Densities
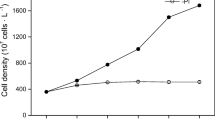
DIP in the treatment of P. donghaiense decreased quickly to ~0.2 μmol l−1 compared with that in P. minimum or P. micans (Fig. 6a). DIP decreased sharply to lower than 0.5 μmol l−1 in all treatments on the other day after the first two doses of 5 and 10 μmol l−1 of PO43− were re-added separately. 3–6 μmol l−1 of DIP were observed in all treatments at the end of the study.
PP decreased gradually after the first days (Fig. 6b). The lowest PP of P. donghaiense, P. minimum, and P. micans were 0.07, 0.10, and 0.72 pmol cell−1 respectively. PP increased sharply when PO43− were replenished in all treatments.
The cell densities of all three Prorocentrum species stopped increasing when PP became the lowest in all treatment (Fig. 6c). The maximum growth rate of P. donghaiense, P. minimum, and P. micans were 0.51, 0.38, and 0.35 d−1 respectively. All three Prorocentrum species continued to grow after PO43− were replenished and P. donghaiense grew the fastest.
Variation of APA
The bulk APA in all treatments were undetectable at the beginning. APA of P. minimum and P. micans increased sharply after day 9 when PP was depleted and decreased gradually when PO43− was replenished in the media (Fig. 7). The maximum APA of P. minimum and P. micans were 43.28 ± 6.44 and 485.84 ± 46.09 fmol cell−1 h−1, respectively. Variation of APA in P. donghaiense was quite different. The APA of P. donghaiense increased sharply when phosphate decreased to ~ 0.2 μmol l−1 and the maximum value of 3.40 ± 0.33 fmol cell−1 h−1 was one to two orders of magnitude lower than those of P. minimum and P. micans (p < 0.01). Some AP of all three species was released into the media and the free AP showed similar variation trends with total AP. Free APA contributed 33–60% to total bulk APA at the peak.
Most sites with APA of P. donghaiense and P. minimum were located on or near cell surface whereas those of P. micans were located evenly inside the whole cell body (Fig. 8). The ELF was not observed for all three species on day 0 but became apparent with time (Fig. 9). Single-cell APA did not show an obvious immediate decrease after PO43− were replenished in media. Higher percentages of medium and Strong ELF cells were found in P. donghaiense and P. micans compared with those in P. minimum. However, the percentages of ELF cells didn’t increase with the bulk APA in all cultures.
Variations in the percentages of enzyme-labeled fluorescence (ELF) of aP. donghaiense, bP. minimum, c and P. micans. The observed cells are divided into three groups based on the intensity of fluorescence: absence of fluorescence; medium fluorescence, where ELF covered < 30% of the cell bodies; strong fluorescence, where ELF covered > 30% of the cell bodies. Percentages of ELF for a given species are calculated as the fraction of ELF labeled cells in one group divided by the total number of cells counted.
AP Hydrolytic Kinetics of Three Prorocentrum Species in the Laboratory
AP hydrolytic kinetics of three Prorocentrum species followed the Michaelis-Menten equation (Fig. 10). The Vmax of P. donghaiense (2.24 fmol cell−1 h−1) was much lower than those of P. minimum (17.21 fmol cell−1 h−1) and P. micans (738.83 fmol cell−1 h−1). The Ks of P. donghaiense (0.28 μmol l−1) was comparable with that of P. minimum (0.35 μmol l−1) but was much lower than that of P. micans (1.86 μmol l−1).
Discussions
Phytoplankton in the coastal waters of ECS suffer from phosphorus stress or limitation, especially during spring and summer when phytoplankton bloom occurs [6, 7, 10]. During the P. donghaiense bloom in the spring of 2010, phosphate concentrations in more than half of the studied stations were lower than 0.10 μmol l−1 which is considered as the phosphorus threshold limit value of primary production [13, 35]. DOP concentrations were comparable or even higher than phosphate concentrations in this area. AP, which is one of the most important phosphatases, plays a prominent role in the regeneration of organic phosphorus compounds. The phosphorus status of P. donghaiense and the AP regulation by external phosphorus during the bloom were analyzed by the single-cell ELF assay. Because three Prorocentrum species coexisted in the water, it was possible to compare the different AP characteristics of phytoplankton that belonged to the same genus but different species in natural environments. Moreover, both the bulk and single-cell AP assays were used in the laboratory to further clarify the regulation mechanisms of AP by phosphorus in monocultures of three Prorocentrum species.
The observed three Prorocentrum species and S. costatum all expressed AP in situ which suggested phytoplankton suffered phosphorus stress during the bloom period. AP has been widely used as an indicator of phosphorus stress or limitation as studies have proven APA are highly correlated with external phosphate concentrations [17, 20, 49]. Our findings were similar in the field study. The phosphorus stress status of three Prorocentrum species strengthened with the decrease of phosphate. The AP intensity of P. donghaiense was inversely correlated with phosphate concentration. Furthermore, our results suggested the APA of P. donghaiense was positively correlated with DOP concentrations in situ. The existence of available DOP might induce phytoplankton to express more AP [50, 51]. In contrast, a negative correlation between APA and DOP were found by Bogé et al. [52, 53] and refractory organic phosphorus compounds interfering with phosphate for the control of AP synthesis was suggested. Thus, we consider the bioavailability of DOP in seawater determines AP regulation. Following initial phosphate depletion, phytoplankton turn to utilize DOP, as the abundantly available DOP induce phytoplankton to upregulate AP expression. However, after the easily hydrolyzable organic phosphorus compounds have been utilized, the rest stable DOP might inhibit AP expression in return. Furthermore, phytoplankton APA is also regulated by the internal phosphorus pool [18, 54, 55]. Our laboratory results showed that it was not until cellular phosphorus pool was exhausted that APA of two Prorocentrum species rapidly increased by two to three orders of magnitude when external phosphate initially depleted. In summary, AP expression and its activities are determined by both the external available phosphorus and the internal phosphorus pool, and the internal phosphorus pool which determines the phosphorus status of phytoplankton might play a more important role.
Although AP expression might be constitutive in some species [19, 23], most agree that AP is an inducible enzyme and is upregulated when phosphate is lower than a threshold limit value [17, 18]. However, the phosphate threshold limit value we found was quite different from previous studies, varying from 0.05 to 1 μmol l−1 [50, 56,57,58]. Nausch [59] found an inverse correlation between APA and phosphate at the range of 0.2–1 μmol l−1 and an obvious increase in APA at phosphate concentrations lower than 0.2 μmol l−1. Furthermore, certain amounts of APA were observed in nutrient-repleted conditions, thus some studies suggest AP is not a good indicator of phosphorus limitation [20, 32, 60]. The phosphate concentrations in our field studies varied from below the detection limit (0.02 μmol l−1) to 0.73 μmol l−1, mostly lower than 0.2 μmol l−1. The studied species expressed low levels of APA when phosphate was higher than 0.2 μmol l−1. The APA expression did not change much in the phosphate range of 0.1–0.2 μmol l−1. However, the APA expression of the studied species increased significantly when phosphate was lower than 0.1 μmol l−1. Our laboratory findings were consistent with those in the field study. Small amounts of APA were found in all three Prorocentrum species when external phosphate was in the range of 0.2~3 μmol l−1 and the APA increased significantly until cellular P pool was exhausted. Meseck et al. [32] observed ten species of phytoplankton express AP in response to high phosphate concentrations (10.1–16.4 μmol l−1), especially P. minimum. Fuentes et al. [61] suggested that AP could be stimulated by stressors other than phosphate deficiency, such as silicon deficiency. The physiological status other than phosphorus stress might also induce phytoplankton to express AP. Thus, from our results, we still considered AP a good indicator of phosphorus limitation and the phosphorus threshold limit value is 0.10 μmol l−1 in situ. However, this indicator should be more cautiously used, in particular when only detecting low levels of APA.
Compared with diatoms, dinoflagellates are more sensitive to phosphorus stress and express AP in the same environment [50, 62, 63], as was observed in our field study. Even when phosphate was lower than 0.10 μmol l−1, the diatom S. costatum ELF was less intense compared with the dinoflagellates represented by three Prorocentrum species. No obvious difference in the AP expression of three Prorocentrum species could be observed in the field with the single-cell ELF assay. However, with the combination of both bulk and single-cell ELF assays in the laboratory, the similarities and differences in AP characteristics of three Prorocentrum species were observed. All three Prorocentrum species were inducible, and increased with the decrease of phosphate. Considerable amounts of AP were released into the water as the soluble state. The repletion of phosphate could not completely inhibit APA in several days. Obvious differences existed in the AP of three Prorocentrum species. First of all, the Prorocentrum species differed in AP location sites. Most AP of P. donghaiense and P. minimum is localized on or close to the cell surface, which might be more efficient in the coupling effect of hydrolysis and uptake of DOP, as suggested by some studies [64, 65]. In contrast, most AP expression was intracellular in P. micans. It requires more energy and time to translocate the AP on the cell surface to directly interact with exteral orgainc phosphorus substrates [23]. Alternatively, the intracelular AP is an isoform that plays a distinct role, such as hydrolyzing the internal organic phosphorus [27, 30, 31]. Second, a higher AP affinity for DOP substrate was observed in P. donghaiense and P. minimum whereas a higher maximum hydrolysis rate for DOP in P. micans was observed. P. donghaiense and P. minimum seemed to be more efficient in hydrolyzing the relatively low amount of organic phosphorus compounds in natural seawaters. Finally, and most importantly, the APA expression levels and response time profiles are quite different among the three Prorocentrum species. APA of P. donghaiense rapidly increased to the peak value of 3.40 fmol cell−1 h−1 when phosphate was lower than 0.2 μmol l−1 in the early stage, and then decreased; APA reached the second peak value of 1.63 fmol cell−1 h−1 when PP was exhausted. As for P. minimum or P. micans, a sharp increase in APA was observed until the celluar phosphorus pool was exhausted and the maximum APA was two to three orders of magnitude higher than that of P. donghaiense. The maximum growth rates of Prorocentrum were 0.35~0.51 day−1 in this study. Suppose Prorocentrum cells could growth at a very high growth rate of 1 day−1, the phosphorus requirement of Prorocentrum species in the unit of fmol cell−1 h−1 could be roughly calculated as the minimum PP (PPmin) plus a growth rate of 1 day−1 and then divided by 24 h. The AP of P. donghaiense is sufficient in hydrolyzing organic phosphorus compounds once they are available in seawater (Table 4). In contrast, the over-expression of AP in the other two Prorocentrum species, especially P. micans, might be a waste of energy. Young et al. [66] also observed an over-expression of APA in phytoplankton and they suggested this characteristic might not benefit the algae itself. In summary, the AP characteristic of P. donghaiense suggests that it benfits from its efficiency in utilizing DOP when compared with the other two Prorocentrum species.
Results from the bulk AP and single cell AP assays in the laboratory were compared. Considering that phytoplankton AP would be released into seawater as the soluble state that could not be measured by the single-cell ELF assay, as shown in our results, high bulk APA did not necessarily accompany with high ELF results at some time nodes. Cells with low ELF or without ELF might still suffer phosphorus stress or limitation. Furthermore, although three Prorocentrum species show significant differences in AP quantity, that could not be differentiated with the single-cell ELF assay no matter in the field or the laboratory study. The bulk AP assay failed to show the detailed information of phosphatase producers whereas the single-cell ELF assay was merely a qualitative estimate [26, 50]. Thus, the combination of the bulk and single-cell AP assays are strongly recommended to give an accurate analysis in future studies.
Conclusion
The species of P. donghaiense, P. minimum, and P. micans all suffered from P stress and expressed abundant AP, in particular when DIP was lower than 0.10 μmol l−1 during the P. donghaiense bloom. The APA expression was regulated by both external DIP and DOP, and internal phosphorus pool. Compared with P. minimum and P. micans, P. donghaiense showed some unique AP characteristics that might help it efficiently utilize organic phosphorus substrates and outcompete other concurrent species to outburst in the P-deficient ECS.
References
Lu DD, Qi YZ, Gu HF, Dai XF, Wang HX, Gao YH, Shen PP, Zhang QC, Yu RC, Lu SH (2014) Causative species of harmful algal blooms in Chinese coastal waters. Algol Stud 145:145–168
Zhou ZX, Yu RC, Zhou MJ (2017) Seasonal succession of microbial blooms from diatoms to dinoflagellates in the East China Sea: A numerical simulation study. Ecol Model 360:150–162
Lu DD, Goebel J (2001) Five red tide species in genus Prorocentrum including the description of Prorocentrum donghaiense Lu sp. nov. from the East China Sea. Chin J Oceanol Limnol 19:337–344
Zhou MJ, Zhu MY (2006) Progress of the project “Ecology and Oceanography of harmful algal blooms in China”. Adv Earth Science 21:673–679
Chen GF, Ma CS, Zhang CY, Zhou J, Wang YY, Wang GC, Zhang BY, Xu Z, Lu DD (2013) A rapid and sensitive method for field detection of Prorocentrum donghaiense using reverse transcription-coupled loop-mediated isothermal amplification. Harmful Algae 29:31–39
Liu LS, Zhou J, Zheng BH, Cai WQ, Lin KX, Tang JL (2013) Temporal and spatial distribution of red tide outbreaks in the Yangtze River Estuary and adjacent waters, China. Mar Pollut Bull 72:213–221
Tang DL, Di BP, Wei GF, Ni IH, Oh IS, Wang SF (2006) Spatial, seasonal and species variations of harmful algal blooms in the South Yellow Sea and East China Sea. Hydrobiologia 568:245–253
Wang JT, Zhang YW, Li H, Cao J (2013) Competitive interaction between diatom Skeletonema costatum and dinoflagellate Prorocentrum donghaiense in laboratory culture. J Plankton Res 35:367–378
Shi XG, Lin X, Li L, Li MZ, Palenik B, Lin SJ (2017) Transcriptomic and microRNAomic profiling reveals multi-faceted mechanisms to cope with phosphate stress in a dinoflagellate. ISME J 11:2209–2218
Zhou YP, Zhang YM, Li FF, Tan LJ, Wang JT (2017) Nutrients structure changes impact the competition and succession between diatom and dinoflagellate in the East China Sea. Sci Total Environ 574:499–508
Glibert PM, Burkholder JM, Kana T (2012) Recent insights about relationships between nutrient availability, forms, and stoichiometry, and the distribution, ecophysiology, and food web effects of pelagic and benthic Prorocentrum species. Harmful Algae 14:231–259
Li Y, Lu SH, Jiang TJ, Xiao YP, You SP (2011) Environmental factors and seasonal dynamics of Prorocentrum populations in Nanji Islands National Nature Reserve, East China Sea. Harmful Algae 10:426–432
Huang BQ, Ou LJ, Wang XL, Huo WY, Li RX, Hong HS, Zhu MY, Qi YZ (2007) Alkaline phosphatase activity of phytoplankton in East China Sea coastal waters with frequent HAB occurrences. Aquat Microb Ecol 49:195–206
Van Mooy BAS, Fredricks HF, Pedler BE, Dyhrman ST, Karl DM, Koblížek M, Lomas MW, Mincer TJ, Moore LR, Moutin T, Rappé MS, Webb EA (2009) Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 458:69–72
Dyhrman ST, Jenkins BD, Rynearson TA, Saito MA, Mercier ML, Alexander H, Whitney LP, Drzewianowski A, Bulygin VV, Bertrand EM, Wu ZJ, Benitez-Nelson C, Heithoff A (2012) The transcriptome and proteome of the diatom Thalassiosira pseudonana reveal a diverse phosphorus stress response. PLoS One 7:e33768
Lin SJ, Litaker RW, Sunda WG (2016) Phosphorus physiological ecology and molecular mechanisms in marine phytoplankton. J Phycol 52:10–36
Cembella AD, Antia NJ, Harrison PJ (1984) The utilization of inorganic and organic phosphorous compounds as nutrients by eukaryotic microalgae: a multidisciplinary perspective: part 1. CRC Crit Rev Microbiol 10:317–391
Hoppe HG (2003) Phosphatase activity in the sea. Hydrobiologia 493:187–200
Duhamel S, Dyhrman ST, Karl DM (2010) Alkaline phosphatase activity and regulation in the North Pacific Subtropical Gyre. Limnol Oceanogr 55:1414–1425
Labry C, Delmas D, Youenou A, Quere J, Leynaert A, Fraisse S, Raimonet M, Ragueneau O (2016) High alkaline phosphatase activity in phosphate replete waters: The case of two macrotidal estuaries. Limnol Oceanogr 61:1513–1529
Davis CE, Mahaffey C (2017) Elevated alkaline phosphatase activity in a phosphate-replete environment: Influence of sinking particles. Limnol Oceanogr 62:2389–2403
Jones JG (1972) Studies on freshwater micro-organisms: phosphatase activity in lakes of differing degrees of eutrophication. J Ecol 60:777–791
González-Gil S, Keafer BA, Jovine RVM, Aguilera A, Lu SH, Anderson DM (1998) Detection and quantification of alkaline phosphatase in single cells of phosphorus-starved marine phytoplankton. Mar Ecol Prog Ser 164:21–35
Nicholson D, Dyhrman S, Chavez F, Paytan A (2006) Alkaline phosphatase activity in the phytoplankton communities of Monterey Bay and San Francisco Bay. Limnol Oceanogr 51:874–883
Ou LJ, Huang BQ, Hong HS, Qi YZ, Lu SH (2010) Comparative alkaline phosphatase characteristics of the algal bloom species Prorocentrum donghaiense, Alexandrium catenella and Skeletonema costatum. J Phycol 46:260–265
Vrba J, Macholdová M, Nedbalová L, Nedoma J, Šorf M (2018) An experimental insight into extracellular phosphatases-differential induction of cell-specific activity in Green algae cultured under various phosphorus conditions. Front Microbiol 9:271
Lin X, Wang L, Shi X, Lin S (2015) Rapidly diverging evolution of an atypical alkaline phosphatase (PhoAaty) in marine phytoplankton: insights from dinoflagellate alkaline phosphatases. Front Microbiol 6:868
Pereira N, Shilova IN, Zehr JP (2016) Molecular markers define progressing stages of phosphorus limitation in the nitrogen-fixing cayanobacterium, Crocosphaera. J Phycol 52:274–282
Zhang C, Luo H, Huang LM, Lin SJ (2017) Molecular mechanism of glucose-6-phosphate utilization in the dinoflagellate Karenia mikimotoi. Harmful Algae 67:74–84
Lin X, Zhang H, Cui YD, Lin SJ (2012) High sequence variability, diverse subcellular localizations, and ecological implications of alkaline phosphatase in dinoflagellates and other eukaryotic phytoplankton. Front Microbiol 3:235
Li TC, Guo CT, Zhang YQ, Wang C, Lin X, Lin SJ (2018) Identification and expression analysis of an atypical alkaline phosphatase phosphatase in Emiliania huxleyi. Front Microbiol 9:2156
Meseck SL, Alix JH, Wikfors GH, Ward JE (2009) Differences in the soluble, residual phosphate concentrations at which coastal phytoplankton species up-regulate alkaline-phosphatase expression, as measured by flow-cytometric detection of ELF-97® fluorescence. Estuar Coasts 32:1195–1204
Hung JJ, Chen CH, Gong GC, Sheu DD, Shiah FK (2003) Distributions, stoichiometric patterns and cross-shelf exports of dissolved organic matter in the East China Sea. Deep-Sea Res II 50:1127–1145
Li J, Glibert PM, Zhou MJ, Lu SH, Lu DD (2009) Relationships between nitrogen and phosphorus forms and ratios and the development of dinoflagellate blooms in the East China Sea. Mar Ecol Prog Ser 383:11–26
Fang TH (2004) Phosphorus speciation and budget of the East China Sea. Cont Shelf Res 24:1285–1299
Li MZ, Li L, Shi XG, Lin LX, Lin SJ (2015) Effects of phosphorus deficiency and adenosine 5’-triphosphate (ATP) on growth and cell cycle of the dinoflagellate Prorocentrum donghaiense. Harmful Algae 47:35–41
Ou LJ, Huang XY, Huang BQ, Qi YZ, Lu SH (2015) Growth and competition for different forms of organic phosphorus by the dinoflagellate Prorocentrum donghaiense with the dinoflagellate Alexandrium catenella and with the diatom Skeletonema costatum s.l. Hydrobiologia 754:29–41
Ou LJ, Wang D, Huang BQ, Hong HS, Qi YZ, Lu SH (2008) Comparative study on phosphorus strategies of three typical harmful algae in Chinese coastal waters. J Plankton Res 30:1007–1017
Heil CA, Glibert PM, Fan C (2005) Prorocentrum minimum (Pavillard) Schiller A review of a harmful algal bloom species of growing worldwide importance. Harmful Algae 4:449–470
Ou LJ, Lundgren V, Lu SH, Granéli E (2014) The effect of riverine dissolved organic matter and other nitrogen forms on the growth and physiology of the dinoflagellate Prorocentrum minimum (Pavillard) Schiller. J Sea Res 85:499–507
Grasshoff K, Kremling K, Ehrhardt M (1999) Methods of Seawater Analysis. Wiley-VCH, Weinheim
Sunda WG, Price NM, Morel FMM (2005) Trace metal ion buffers and their use in culture studies. In: Andersen RA (ed) Algal Culturing Techniques. Academic, Burlington, pp 35–64
Guillard RRL (1973) Methods for microflagellates and nanoplankton. In: Stein JR (ed) Handbook of phycological methods: culture methods and growth measurements. Cambridge University Press, Cambridge, pp 69–85
Hillebrand H, Dürselen C, Kirschtel D, Pollingher U, Zohary T (1999) Biovolume calculation for pelagic and benthic microalgae. J Phycol 35:403–424
Parsons TR, Yoshiaki M, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford
Valderrama JC (1995) Methods of nutrient analysis. In: Hallegraeff GM, Anderson DM, Cembella AD (eds) Manual on harmful marine microalgae. IOC and Guides. UNESCO Publ, Paris, pp 251–568
Solórzano L, Sharp J (1980) Determination of total dissolved phosphorus and particulate phosphorus in natural waters. Limnol Oceanogr 25:754–758
Hoppe HG (1983) Significance of exoenzymatic activities in the ecology of brackish water: measurements by means of methylumbelliferyl-substrates. Mar Ecol Prog Ser 11:299–308
Kwon HK, Oh SJ, Yang H (2011) Ecological significance of alkaline phosphatase activity and phosphatase-hydrolyzed phosphorus in the northern part of Gamak Bay, Korea. Mar Pollut Bull 62:2476–2482
Dyhrman ST, Ruttenberg KC (2006) Presence and regulation of alkaline phosphatase activity in eukaryotic phytoplankton from the coastal ocean: implications for dissolved organic phosphorus remineralization. Limnol Oceanogr 51:1381–1390
Artigas J, Soley S, Pérez-Baliero MC, Romaní AM, Ruiz-González C, Sabater S (2012) Phosphorus use by planktonic communities in a large regulated Mediterranean river. Sci Total Environ 426:180–187
Boǵe G, Lespilette M, Jamet D, Jamet J (2017) Role of DOP on the alkaline phosphatase activity of size fractionated plankton in coastal waters in the NW Mediterranean Sea (Toulon Bay, France). Mar Pollut Bull 117:264–273
Bogé G, Lespilette M, Jamet D, Jamet J (2014) Analysis of the role of DOP on the particulate phosphatase activity in Toulon Bay (N.W. Mediterranean Sea, France). Mar Pollut Bull 86:342–348
Litchman E, Nguyen BLV (2008) Alkaline phosphatase activity as a function of internal phosphorus concentration in freshwater phytoplankton. J Phycol 44:1379–1383
Rychtecký P, Řeháková K, Kozlíková E, Vrba J (2015) Light availability may control extracellular phosphatase production in turbid environment. Microb Ecol 69:37–44
Jauzein C, Labry C, Youenou A, Quéré J, Delmas D, Collos Y (2010) Growth and phosphorus uptake by the toxic dinoflagellate Alexandrium catenella (Dinophyceae) in response to phosphate limitation. J Phycol 46:926–936
Bogé G, Lespilette M, Jamet D, Jamet JL (2012) Role of seawater DIP and DOP in controlling bulk alkaline phosphatase activity in N.W. Mediterranean Sea (Toulon, France). Mar Pollut Bull 64:1989–1996
Lim JH, Lee CW, Bong CW, Affendi YA, Hii YS, Kudo I (2018) Distributions of particulate and dissolved phosphorus in aquatic habitats of Peninsular Malaysia. Mar Pollut Bull 128:415–427
Nausch M (1998) Alkaline phosphatase activities and the relationship to inorganic phosphate in the Pomeranian Bight (southern Baltic Sea). Aquat Microb Ecol 16:87–94
Dignum M, Hoogveld H, Matthijs HCP, Laanbroek HJ, Pel R (2004) Detecting the phosphate status of phytoplankton by enzyme-labelled fluorescence and flow cytometry. FEMS Microbiol Ecol 48:29–38
Fuentes S, Wikfors GH, Meseck S (2014) Silicon deficiency induces alkaline phosphatase enzyme activity in cultures of four marine diatoms. Estuar Coasts 37:312–324
Ou LJ, Huang BQ, Lin LZ, Hong HS, Zhang F, Chen ZZ (2006) Phosphorus stress of phytoplankton in Taiwan Strait using bulk and single-cell alkaline phosphatase assay. Mar Ecol Prog Ser 327:95–106
Girault M, Arakawa H, Hashihama F (2013) Phosphorus stress of microphytoplankton community in the western subtropical North Pacfic. J Plankton Res 35:146–157
Xu Y, Wahlund TM, Feng L, Shaked Y, Morel FMM (2006) A novel alkaline phosphatase in the Coccolithophore Emiliania huxleyi (Prymnesiophyceae) and its regulation by phosphorus. J Phycol 42:835–844
Landry C, Tremblay L (2012) Compositional differences between size classes of dissolved rganic matter from freshwater and seawater revealed by an HPLC-FTIR system. Environ Sci Technol 46:1700–1707
Young EB, Tucker RC, Pansch LA (2010) Alkaline phosphatase in freshwater Cladophora-Epiphyte assemblages: regulation in response to phosphorus supply and localization. J Phycol 46:93–101
Acknowledgments
The authors thank the captain and crew of RV “Science 3,” who made concerted efforts during sampling. The authors thank Prof. Douding Lu and Dr. Xinfeng Dai for providing data of P. donghaiense density. The authors also thank the two anonymous reviewers for their helpful comments.
Funding
This work was financially supported by the National Key R&D Program of China (No. 2017YFC1404300) and the National Natural Science Foundation of China (grant numbers 41776121 and 41176087).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ou, L., Qin, X., Shi, X. et al. Alkaline phosphatase activities and regulation in three harmful Prorocentrum species from the coastal waters of the East China Sea. Microb Ecol 79, 459–471 (2020). https://doi.org/10.1007/s00248-019-01399-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-019-01399-3