Abstract
Streptomycetes are widely distributed in the marine environment, although only a few studies on their associations to algae and coral ecosystems have been reported. Using a culture-dependent approach, we have isolated antibiotic-active Streptomyces species associated to diverse intertidal marine macroalgae (Phyllum Heterokontophyta, Rhodophyta, and Chlorophyta), from the central Cantabrian Sea. Two strains, with diverse antibiotic and cytotoxic activities, were found to inhabit these coastal environments, being widespread and persistent over a 3-year observation time frame. Based on 16S rRNA sequence analysis, the strains were identified as Streptomyces cyaneofuscatus M-27 and Streptomyces carnosus M-40. Similar isolates to these two strains were also associated to corals and other invertebrates from deep-sea coral reef ecosystem (Phyllum Cnidaria, Echinodermata, Arthropoda, Sipuncula, and Anelida) living up to 4.700-m depth in the submarine Avilés Canyon, thus revealing their barotolerant feature. These two strains were also found to colonize terrestrial lichens and have been repeatedly isolated from precipitations from tropospheric clouds. Compounds with antibiotic and cytotoxic activities produced by these strains were identified by high-performance liquid chromatography (HPLC) and database comparison. Antitumor compounds with antibacterial activities and members of the anthracycline family (daunomycin, cosmomycin B, galtamycin B), antifungals (maltophilins), anti-inflamatory molecules also with antituberculosis properties (lobophorins) were identified in this work. Many other compounds produced by the studied strains still remain unidentified, suggesting that Streptomyces associated to algae and coral ecosystems might represent an underexplored promising source for pharmaceutical drug discovery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In nature, bacteria of the order Actinomycetales, particularly of Streptomyces genus, are the main producers of secondary metabolites of great medical and industrial relevance. Streptomyces are responsible for the production of most of the discovered secondary metabolites with activity as antibiotics, antitumor, or immunosuppressive agents, etc. Streptomyces species are widely distributed in nature, in both terrestrial and marine environments. Although most of the known species are of terrestrial origin and have been isolated from soils along the past few decades (since the 1950s), indigenous marine actinomycetes indeed exist in the oceans and are widely distributed in different marine organisms (animals and plants), besides seawater and sediments [12, 30, 45, 58]. An increasing number of novel potent bioactive metabolites have been isolated from marine actinomycetes during the last decade [7, 18, 30, 35, 42]. Since the greatest biodiversity and metabolic variety occur in the oceans, marine actinomycetes are emerging as a source of novel drugs [50]. The marine environment has become a prime resource in search and discovery for novel products, and marine actinomycetes turn out to be important contributors [53]. For future success on natural product discovery, it has been proposed to increase efforts on the isolation of microorganisms from the marine environment and also on those living in association with plants and animals [11].
Marine macroalgae (seaweeds) remains as a relatively underexplored source in the search of Streptomyces producing bioactive substances, expected to play an important role for its survival in this habitat. Only a few reports describe the isolation of actinomycetes on seaweeds on coastal ecosystems from temperate and cold waters of the North Atlantic Ocean, particularly from Iberian Peninsula coasts [19] and from the Kiel Fjord in the Baltic Sea [47, 55]. Phylogenetic analysis revealed a great diversity of antibiotic-producing actinobacteria, belonging most of the isolates to the Streptomyces genus [47, 55].
Coral reefs are among the most productive marine ecosystems. Considered as the “rain forests” of the oceans, coral reefs are the source of a large group of structurally unique biosynthetic products with biomedical potential [41]. The most prolific source of bioactive compounds consists in coral reef invertebrates, mainly sponges, ascidians, molluscs, and bryozoans, and it is becoming evident that some of the compounds are indeed produced by invertebrate-associated microorganisms [37]. Although little is known about the diversity of coral-associated actinobacteria, recent reports from the China Sea coral ecosystems show that diverse cultivable actinomycetes are associated with soft corals [51, 57] and also with stony corals [56]. These associated actinomycetes produce some antibacterial agents believed to protect their host against pathogens [57].
In this line of evidence, we initially isolated predominant Streptomyces strains associated to diverse intertidal seaweeds, collected in the temperate Cantabrian Sea (Southern Bay of Biscay in the North of Iberian Peninsula), and then explored their biosynthetic potential of medical relevance. We report here the isolation of two bioactive Streptomyces species, their identification by 16S RNA analysis and phylogenetic analysis, the corresponding determination of their metabolic profile, and finally the identification of some of the produced secondary metabolites with antibacterial, antifungal, antitumor, and anti-inflammatory activities. We have also investigated the distribution of these species in other marine environments, mainly focusing on their association to invertebrates from deep-sea coral ecosystems from the Avilés Canyon in the central Cantabrian Sea and also in other habitats. Knowledge of Streptomyces ecological distribution is important to obtain novel biologically active natural products, but also for the conservation of these unique marine ecosystems.
Methods
Sampling of Marine Macroalgae
Intertidal seaweed samples were collected at a coastal location nearby Gijón, in the central Cantabrian Sea (N Spain, Bay of Biscay; 43° 32′ 31″ N, 5° 39′ 6″ W), within an intertidal system with a tidal range of 5–6 m. A total of 35 samples were collected at different seasons during three consecutive years (2010–2012). Algae were transferred into sterile plastic bags and kept at 4 °C until immediate processing upon arrival in the laboratory, within 12–24 h after sampling.
Sampling of Deep-Sea Coral Reef Invertebrates
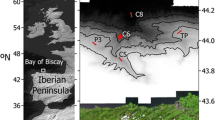
A total of 87 deep-sea invertebrates were collected at the submarine Avilés Canyon in April–May 2013, onboard RV Sarmiento de Gamboa during the BIOCANT3 expedition. Benthonic species were collected using a 5-m length Agassiz trawl with a beam width of 5 m and towed during 1 h at four stations located inside (C5, C8) and outside the Avilés Canyon (P3, TP, Fig. 1). One specimen of the coronate jellyfish Periphylla periphylla was captured within 1,200- and 2,000-m depth at station C5 (Fig. 1) using a pelagic MOCNESS with a square, 1-m2 frame and a 200 black-tinted nitex mesh sieve. After collection, invertebrate samples were aseptically and individually transferred to sterile plastic bags, washed with sterile marine water, and immediately processed in the onboard laboratory.
Sampling of Lichens
A total of about 120 lichen samples were collected in the North of the Iberian Peninsula (Northern Spain and Northern Portugal) and South of France since 2007. After collection, lichens were transferred into sterile plastic bags and kept at 4 °C until immediate processing upon arrival in the laboratory.
Sampling of Rain Water and Hailstone Precipitations
Different precipitations (rain water, hailstone, and snow) were taken within a year time at Gijón and Oviedo stations. Samples of 2–3 ml were collected in sterile recipients and immediately plated on selective media upon arrival in the laboratory, usually within 1–24 h.
Streptomyces Strain Isolation
All different samples were fragmented in empty Petri dishes, with the aid of a sterile scalpel or hammer in the case of stony corals, and transferred to tubes containing 1–2 ml of sterile marine water from the Cantabrian Sea. After vortex, 0.2 ml of each sample was plated on selective media containing the antifungal cycloheximide (80 μg/ml) and anti-Gram negative bacteria nalidixic acid (20 μg/ml), reported to be used previously for actinomycete isolation [21]. Different media were used, either prepared with distilled water or seawater from the same habitat: TSA (Merk) for seaweeds, TSA1/3 and Bleb 1/6 (Oxoid) for deep sea invertebrates and rain water samples, and TSA for lichens. Incubation was carried out for 2 weeks at 28 °C. Colonies growing on agar plates were selected based on different colony morphologies and pigment production. Isolates obtained in pure culture were frozen in 20 % glycerol at −20 and at −70 °C for long-term storage.
Biological Activities
Antimicrobial Bioassays
For antibiotic production, streptomycetes cultures were routinely grown on R5A medium [16]. Antibiotic production was determined by means of bioassays against the following microorganisms as indicators: the Gram-positive bacteria Staphylococcus aureus ATCC 6538P, the Gram-negative Escherichia coli ESS, and the yeast Sacharomyces cerevisiae. Bioassays against bacteria were carried out in TSA1/2 and for yeast in Sabouraud 1/2 (Pronadisa). These analyses were performed with agar plugs and also with ethyl acetate extracts from solid cultures.
Cytotoxic Assays
Determination of viable cells in cytotoxicity assays was carried out against two tumor lines: HeLa, from cervical carcinoma, and HCT116, from colorectal carcinoma, by using the Cell counting kit-8-(96992) from Sigma-Aldrich. Cytotoxic activities were determined with ethyl acetate extracts, obtained in both neutral and acidic conditions, for undiluted extracts and also for extracts diluted 1/10 and 1/100 times. Finally, 2 μl of each extract was added to each well containing 200 μl of cell suspension, and triplicate assays were carried out for every sample.
16S RNA Analysis Identification
The isolated strains were subjected to phylogenetic analysis based on 16S rRNA sequence analysis. DNA was extracted using a microbial DNA isolation kit (Ultra Clean, MoBio Laboratories, Inc.). The DNA was checked for purity, using standard methods [44]. The almost-complete 16S rRNA gene sequence of the bacterial strains was obtained by PCR amplification on a Genius thermocycler (Techne), followed by direct sequencing using primers 616V (forward) and 699R (reverse), as described [3], to target about 1,000 nt close to the 5′ end and primers P609D and P1525R to target positions 785–802 and 1525–1541, respectively (Escherichia coli numbering), as described [34]. The amplification mixture (50 μl) comprised 1 μl (50 pmol/μl) each of 616V and 699R primers, 0.5 μl (5U/μl) of DFS-Taq DNA Polymerase (Bioron), 5 μl of 10× incompleted reaction buffer (Bioron), 0.75 μl of MgCl2 100 mM, 1 μl dNTP mixture (containing 100 μM each of dATP, dGTP, dCTP, and dTTP, Roche), 35.75 μl of sterile filtered water (Milli-Q purification system, Millipore), and 5 μl of DNA template. The DNA templates were amplified by initial denaturation at 94 °C for 10 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 56 °C for 45 s, extension at 72 °C for 45 s, and a final extension at 72 °C for 10 min. Controls, devoid of DNA, were simultaneously included in the amplification process. The integrity of PCR products was assayed by development of single bands following electrophoresis for 25 min at 135 V in 1 % (w/v) agarose gels in Tris-borate EDTA buffer.
Sequences here obtained were compared to public sequences in databases using basically the BLAST program (Basic Local Alignment Search Tool) against the National Center for Biotechnology Information (NCBI) and EzTaxon.org server version 2 [10], submitted and deposited in the EMBL sequence database with Accession numbers HG965212, HG965214, HG965215, and HG965216.
Chromatographic Analysis
Routinely, compounds produced by Streptomyces strains were assessed in cultures on R5A solid medium. Agar plugs of about 7 ml that were taken from the plates were extracted with ethyl acetate in neutral and acidic (after addition of 1 % formic acid) conditions. The organic fraction was evaporated and the residue redissolved in 100 μl of a mixture of DMSO and methanol (50:50). These samples were analyzed by reversed phase chromatography in an Acquity UPLC equipment employing a BEH C18 column (1.7 μm, 2.1 × 100 mm, Waters), with acetonitrile and 0.1 % trifluoroacetic acid as solvents. Samples were eluted with 10 % acetonitrile during 1 min, followed by a linear gradient from 10 to 100 % during 7 min and an additional isocratic hold with 100 % acetonitrile during 2 min, at a flow rate of 0.5 ml/min and at a column temperature of 35 °C. Detection and spectral characterization of peaks were performed in both cases by photodiode array detection and Empower software (Waters).
Identification of Compounds by HPLC Analysis
The chromatographic system consisted of an HP 1090M liquid chromatograph equipped with a diode-array detector and Kayak XM 600 Workstation (Agilent Technologies, Waldbronn, Germany). Multiple wavelength monitoring was performed at 210, 230, 260, 280, 310, 360, 435, and 500 nm, and UV-Vis spectra measured from 200 to 600 nm. Samples (10 ml) of the culture broths were centrifuged; the supernatants were adjusted to pH 5.0 and extracted with an equivalent volume of ethyl acetate. The mycelial pellet was extracted with 10-ml MeOH-acetone (1:1). The organic layers were concentrated, dried in vacuo, and resuspended in 1 ml MeOH. A total of 5-μl aliquots of the samples were injected onto an HPLC column (125 × 3 mm) fitted with a guard-column (20 × 3 mm) filled with 5-μm Nucleosil-100 C-18 (Maisch, Ammerbuch, Germany). The samples were analyzed by linear gradient elution using 0.1 % ortho-phosphoric acid as solvent A and acetonitrile as solvent B at a flow rate of 0.85 ml/min. The gradient was from 4.5 to 100 % for solvent A in 15 min with a 3-min hold at 100 % for solvent B. Evaluation was carried out by means of an in-house HPLC-UV-Vis database which contained nearly of 1,000 reference compounds, mostly antibiotics [17].
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
Qualitative analysis was performed by GC-MS (Chromatograph Agilent 6890N coupled with a 5975B mass spectrometer) mainly as described for geosmin detection [24]. Volatiles released from Streptomyces strains during 2-week growth on R5A plates at 28 °C were absorbed onto 100 mg of activated charcoal (Norit GAC 1240) placed in the lid of each Petri dish. The charcoal was extracted with 0.5 ml of chloroform (Merck) for an hour, and then filtered through cotton wool. One microliter of each extract was analyzed by GC-MS as follows: capillary column, fused silica (30 m; 0.25-mm inside diameter; 0.25-mm film thickness); carrier gas, He (0.82 kPa on column injection); temperature program, isothermal for 1 min at 40 °C, change from 40 to 210 °C at a rate of 10 °C per min, and isothermal for 25 min at 210 °C; energy of ionization, 70 eV. The identity of these volatile compounds was determined by comparing their mass spectra with the Whiley and NIST (National Institute of Standards and Technology) libraries.
Results
Isolation of Bioactive Streptomyces Species Associated to Macroalgae from the Central Cantabrian Sea
Preliminary analysis of different intertidal marine macroalgae (brown, red, and green) collected in the central Cantabrian coast revealed the striking generalized presence of diverse cultivable Streptomyces populations. Morphologically different colonies of bacteria of this genus were isolated in a great number from many of the seaweed samples screened by using TSA selective media. All the isolates were able to grow in different media independently if they contain distilled water (TSA and R5A) or seawater from the same habitat and tolerate up to 7.5 % NaCl.
Among the predominant isolates from diverse seaweed species, two phenotypically different strains, M-27 and M-40 (Supplemental material 1), displaying strong antibiotic activities against Gram-positive, Gram-negative bacteria, and fungi (Supplemental material 2), were selected for further studies. When growing in R5A production media, the different isolates showed diverse phenotypic features and, according to mycelium, spore pigmentation and the color of pigments released to the medium, they were initially named as “Orange” and “Beige.” The first strain showed an orange mycelium and pink pale spores, releasing an intense orange pigment to the medium, while displaying very strong antibacterial activities against both Gram-positive and Gram-negative bacteria. The strain initially named Beige showed both mycelium and spores of this peculiar color, not showing pigment production, and presented anti-Gram-positive activity.
In addition to antimicrobial activities, ethyl acetate extracts of the strains displayed diverse ranges of cytotoxic activities against two different tumor cell lines: HeLa and HCT116, being most active to the Orange strain against both cell lines even after 1/100 dilution (Supplemental material 3). Extracts of this strain were also active against DLD-1 and MC-F7 cell lines, from colorectal and breast adenocarcinomas, respectively (Santiago Cal, personal communication).
We focused on these widely distributed strains, which were further characterized by 16S RNA phylogenetic analysis. We studied their biogeographically distribution among different ecosystems and also, their metabolic profiling, with identification of some of the bioactive compounds produced.
Identification of Predominant Marine Streptomyces Strains by 16S RNA Phylogenetic Analysis
For taxonomic identification, an isolate of each type of strain was further analyzed for 16S RNA sequencing. The strain M-27 with Orange phenotype displayed 99.9 % similarity with the S. cyaneofuscatus (AY999770/Type strain JCM4364) belonging to the S. griseus clade [43], and we will refer to it as S. cyaneofuscatus M-27 strain (accession number HG965212). However, to the best of our knowledge, there is no precedent on the presence of this species in marine environments.
The strain M-40 with Beige phenotype showed 100 % similarity to Streptomyces carnosus (accession number KC522300), and we will refer to it as S. carnosus M-40 strain (accession number HG965214). Members of this species have been previously isolated from an intertidal sponge from the China Sea [54], and as far as we know, they have not been reported in terrestrial habitats.
Distribution of Streptomyces Species in Intertidal Seaweeds
The distribution of Streptomyces strains among the different seaweed species sampled is shown in Table 1. The presence of these bacteria in seawater obtained from the same habitat was of 2–4 orders of magnitude less than that in a similar volume of seaweed, depending on the season.
The strain S. cyaneofuscatus M-27 was first isolated in September 2010 from the perennial brown alga Fucus spiralis. This strain was isolated mainly from the inner part of the receptacles in the fucals F. spiralis and Pelvetia canaliculata, as observed after ethanol immersion of the alga during 3 min. While most of streptomycetes colonizing algal surfaces are eliminated with this treatment, colonies corresponding to this species were still isolated in great number from the receptacles inner part. Also, seasonal differences were found in this case, since the number of isolates is highly reduced in winter, when receptacles are inactive, thus suggesting a possible role in algal development. Similar strains were isolated from brown, red, and green algae during 2011 and 2012. S. carnosus M-40 strain was first isolated in October 2011, from C. baccata, and similar strains were found to be present in brown and red algae collected in 2012.
Distribution of Streptomyces Species Among Deep-Sea Coral Reef Invertebrates from the Avilés Canyon
Surprisingly, phenotype features, biological activities, and metabolic profiling (see below) suggest that similar isolates to S. cyaneofuscatus M-27 and S. carnosus M-40 strains were also found to inhabit deep ecosystems from the Avilés Canyon. Samples were collected in Spring 2013 during a cruise expedition aboard the Sarmiento de Gamboa oceanographic ship. Table 2 displays the different invertebrate hosts from deep-sea coral ecosystems from which similar strains were isolated.
As shown in Table 2, similar S. cyaneofuscatus M-27 isolates were found to be mainly associated to invertebrates of the Phylum Cnidaria, which includes colonial and solitary stony corals (O. Scleractinia), soft gorgonian corals (O. Gorgonacea and O. Alcyonaea), but also in some actinia, ofiuroid, and polychaeta specimen. S. carnosus M-40 seems to be more widely distributed since in addition to the P. Cnidaria, it was isolated from different species of P. Echinodermata, P. Arthropoda, P. Sipuncula, and P. Anelida. In the Avilés Canyon, similar strains to these two species were also isolated from invertebrates collected at all depths tested up to 4,700 m (Table 2), thus indicating the barotolerant behavior of these streptomycetes, able to survive under 470 atm of hydrostatic pressure, but also at very low temperatures, up to 2–4 °C.
Presence of Similar Streptomyces Strains in Other Habitats
In the course of previous actinomycete isolation from terrestrial lichens in the North of Iberian Peninsula (Northern Spain and Northern Portugal), strains displaying similar phenotypic, antibiotic production, and metabolite profile features to S. cyaneofuscatus M-27 and S. carnosus M-40 were found. Identification by 16S RNA, bioactivity assays, and UPLC revealed that these isolates are similar to the respective marine strains. As shown in Table 3, T-178 terrestrial strain (HG965215) displays 100 % similarity to S. cyaneofuscatus M-27 (accession number HG965212), and T-145 terrestrial strain (HG965216) displays 100 % similarity to S. carnosus M-40 (accession number HG965214), and to our knowledge has not been reported in terrestrial habitats. Strikingly, these inland lichen isolates collected from forest and mountain habitats were able to grow in seawater prepared media, and under saline conditions up to 7.5 % NaCl, being phenotypically and metabolically indistinguishable from the marine-borne strains.
Recently, the species described here have been repeatedly isolated from different precipitations from tropospheric clouds (rain water and hailstone) collected at Gijón and Oviedo stations all along the last year (2013–2014).
Metabolite Profiling Analysis and Identification of Secondary Metabolites Produced
To uncover the biosynthetic abilities of the two identified species, ethyl acetate extracts of R5A solid cultures after 7 days of growth were analyzed by ultra-performance liquid chromatography (UPLC). This was followed by the screening for secondary metabolites by HPLC analysis and by means of UV-visible absorbance spectral libraries [17]. Although most of the compounds produced by these strains remain unidentified, some of them were identified as shown in Figs. 2 and 3. The chromatograms shown are maxplots, i.e., chromatogram at absorbance maximum for each analyte, obtained from spectrophotometric detection in the range from 210 to 500 nm.
Chromatogram of extract of S. cyaneofuscatus M-27. 2a Peak numbers indicate the identified compounds and correspond to the following: daunomycin (1), cosmomycin B (2), maltophilin (3), and galtamycin (4). The peaks corresponding to unidentified anthracyclines (*) and unidentified maltophilin (**) are also shown. The lower part of the figure represents U.V. absorption spectra of the identified molecules. 2b Chemical structures of bioactive secondary metabolites identified in extracts of S. cyaneofuscatus M-27
Chromatogram of extract of S. carnosus M-40. 3a Peak numbers indicate the identified compounds and correspond to the following: germicidin A (5), germicidin B (6), and lobophorin B (7). The peaks corresponding to unidentified lobophorines (*) are also shown. The lower part of the figure represents U.V. absorption spectra of the identified molecules. 3b Chemical structures of secondary metabolites with biological activity identified in extracts of S. carnosus M-40
Figure 2a shows the metabolite profile of ethyl acetate extracts of S. cyaneofuscatus M-27 as determined by UPLC chromatographic analysis, and Fig. 2b displays the chemical structures of the identified bioactive synthesized compounds. This strain has the ability to produce simultaneously several antitumor antibiotics of the anthracycline family (Table 4), three of them identified as daunomycin (1), cosmomycin B (2), and galtamycin B (4), respectively (Fig. 2a, b). Different members of the antracycline/angucycline family still remain unidentified (Fig. 2a). Galtamycin B, to our knowledge, has not been previously reported to be produced neither by Streptomyces nor in marine environments. In addition, S. cyaneofuscatus M-27 produces the antifungal macrolactam maltophilin (3) as well as an unidentified derivative.
Several compounds belonging to the lobophorines family have been detected in ethyl acetate extracts form S. carnosus M-40 (Fig. 3), among them only lobophorine B (7) has been identified. Lobophorines A and B are macrolides related to antibiotics of the Kijanimicin class with anti-inflammatory and antituberculosis properties (Table 4). It has been reported that an S. carnosus strain associated to a sponge from the China Sea produces lobophorines C and D with citotoxic activities [54].
Also produced by this strain are germicidin A (5) and B (6), both pyrone compounds (Fig. 3a, b) displaying a physiological role in spore germination and hypha elongation (Table 4).
Volatile Metabolite Profiling Analyses by GC-MS and Comparison among the Different Streptomyces Species
Most major volatile compounds produced by the studied strains were identified by comparison with Wiley database as sesquiterpene aromatics (Supplemental material 4). Only one major peak is detected in M-27, while two major peaks were detected in M-40. The two major products in M-40 were identified as geosmin (8), the compound responsible for the “earth smell” widespread among Streptomyces species [8, 26], and beta-patchoulene (9), traditionally obtained from the plant Pogostemon cablin (patchouli) and used as fragrance agent in perfume industry, whose production by the Streptomyces genus has been recently reported [6]. The main product in M-27 corresponds to geosmin. Many other volatile compounds produced by these two strains still remain unidentified.
Discussion
Macroalgae and corals are complex hosts harboring a rich diversity of associated microorganisms with functions related to host health and defense. It has been recently suggested that marine macroalgae and epiphytic bacteria communities interact in a tight relationship as a unified functional entity or holobiont [14], term due to [36]. The seaweed holobiont concept is analogous to the coral holobiont, which describes a complex symbiosis between the coral animal and the associated microorganisms [5]. We report here that Streptomyces species producing bioactive secondary metabolites are widespread among marine macroalgae in intertidal ecosystems and, interestingly, also among different invertebrates from deep-sea bottoms, including cold coral reefs. Clearly, this finding has both ecological and pharmacological interest.
We have isolated diverse cultivable Streptomyces populations colonizing marine macroalgae. Our focus was on two particularly abundant and widespread antibiotic producing strains, identified as S. cyaneofuscatus M-27 and S. carnosus M-40 after 16S rRNA sequencing and phylogenetic analysis. The Streptomyces-seaweed associations reported here were specific and persistent within the 3-year timespan of our study, which also illustrated some seasonal variability. Although most of them seem to be epiphytic forms associated to the surface of the alga, S. cyaneofuscatus M-27 was clearly endophytic and associated to the receptacles of Fucus spiralis and Pelvetia canaliculata. In these hosts, S. cyaneofuscatus M-27 populations vary dramatically from winter, when the receptacles are inactive, to spring and summer, when these organs are mature. It has been shown that bacteria are the most common colonizers on macroalgae surfaces and can influence their growth at various developmental stages (e.g., reproduction, [4]), while secreting bioactive compounds that regulate seaweed morphogenesis and increase survival under contrasting environmental conditions [46]. Specific associations of bacterial populations with different parts of seaweeds, showing seasonal differences, have been reported [47], and also that epibacterial community patterns are host-specific but temporally variable [29].
S. cyaneofuscatus M-27 and S. carnosus M-40 were found both in intertidal seaweeds and in several deep-sea invertebrates at the Avilés submarine Canyon, especially in association with the reef-forming corals Lophelia pertusa and Madrepora oculata, very abundant in this Canyon [33]. Overall, it was very surprising to uncover that these two strains are able to colonize plants and animals living in very different habitats such as temperate intertidal ecosystems and cold water coral ecosystems up to 4,700-m depth. Streptomyces living on intertidal macroalgae are subject to a variable range of physiochemical environmental conditions in which the physical conditions, such as temperature, oxygen concentration, desiccation degree, exposure to sun light, and salinity, are constantly changing. In contrast, the deep-sea coral ecosystem, although extreme, is relatively constant from 1,800- to 4,700-m depth in terms of temperature (2–4 °C), oxygen concentration (7,7 mg/l aprox.), salinity (3.5 %), and darkness, whereas the hydrostatic pressure rises from 180 up to 470 atm. It follows that these Streptomyces species are barotolerant since they are able to grow at high hydrostatic pressure, in addition to atmospheric pressure.
These findings are also of interest in the Streptomyces genus biogeography. Similar strains to the marine-derived strains reported here were also isolated from terrestrial lichens (although restricted to specific geographical areas), which raises interesting questions concerning their origin and evolution. Comparative analysis of marine strains with similar terrestrial isolates from the inland lichens here described revealed that all of them grow and produce bioactive compounds in culture media either prepared with marine or distilled water. It was really striking to find that all mentioned strains, independently of their marine or terrestrial origin, grow in media containing up to 7.5 % of NaCl. Although in general is assumed that marine streptomycetes are of terrestrial origin, other alternative scenarios could be also possible. As there was scientific literature on the fact that Streptomyces strains were found in tropospheric clouds [1], we also investigated their possible presence in rainwater and other precipitations taken over last year (2013–2014) in the same geographical region. With the recent finding of strains similar to S. cyaneofuscatus M-27 and S. carnosus M-40 isolated from different precipitations, rainwater, and hailstone, it is tempting to speculate that, in our planet, oceanic marine aerosols forming clouds might contribute to dissemination of streptomycetes spores from the sea to inland ecosystems.
Streptomyces-host interactions on marine ecosystems are also of pharmacological interest, since potential novel bioactive compounds might be produced in these interactions. Microbial communities associated with living organisms, such as corals, sponges, and algae, are influenced not only by physiochemical environmental factors but also by ecological interactions with their host organism [38]. New trends in marine research revealed that some of the natural products identified in marine organisms, initially thought to be invertebrate-derived, were later found to be produced by symbiotic or commensal microbes [20]. The “symbiont-product” hypothesis has emerged following this line of evidence [37, 40].
There is ample knowledge of retraction of the geographic range of several brown seaweed species in the Cantabrian Coast, some of them approaching regional extinction, a trend which has been explained at least partially by a global increase in sea surface temperature [13, 15, 31, 52]. The effect seems to be particularly evident in cold water algae of the order Fucales, to which Fucus and Pelvetia canaliculata belong. If there is some host specificity in the Streptomyces-alga association, such retraction may involve the loss of bacterial strains with potential medical interest. Likewise, climate change projections indicate that nearly 70 % of the deep corals in the world will experience corrosive effects by the end of this century due to acidification of the water, a direct consequence of the increasing levels of atmospheric CO2 levels [23]. Comprehensive analysis of the deep-ocean communities is now starting (e.g., see [33] for an overview of deep biodiversity at the Avilés Canyon); thus, we may already be losing a startling, undocumented diversity of species and their interactions, including host-specific animal-Streptomyces associations. Clearly, these two aspects require a concerted research effort to document and preserve a crucial but potentially declining resource.
As a matter of fact, the streptomycetes reported here produce different bioactive secondary metabolites, with antibacterial, antifungal, cytotoxic, and anti-inflammatory activities. Comparative analysis of Streptomyces metabolites with natural product databases lead to the identification of several chemically diverse compounds, whereas many others remain unidentified, and some of them might be new. Some of the compounds here identified have not been previously found in the marine environment or reported in the genus Streptomyces [25, 35, 42, 50]. This finding mainly reinforces the idea that Streptomyces associated to intertidal marine macroalgae from coastal ecosystems, and deep-sea coral reefs invertebrates, might represent a promising resource for the discovery of novel natural products biologically active as pharmacological compounds for medical use. But also, the results of this work make us aware that knowledge of Streptomyces distribution is essential for the conservation of these unique and delicate marine ecosystems.
References
Amato P, Parazols M, Sancelme M, Laj P, Mailhot G, Delort AM (2007) Microorganisms isolated from the water phase of tropospheric clouds at the Puy de Dôme: major groups and growth abilities at low temperatures. FEMS Microbiol Ecol 59:242–254
Aoki Y, Matsumoto D, Kawaide H, Natsume M (2011) Physiological role of germicidins in spore germination and hyphal elongation in Streptomyces coelicolor A3(2). J Antibiot 645:607–611
Arahal DR, Sánchez E, Macián MC, Garay E (2008) Value of recN sequences for species identification and as a phylogenetic marker within the family Leuconostocaceae. Int Microbiol 11:33–39
Armstrong E, Tyan L, Boyd KG, Wright PC, Burgess JG (2001) The symbiotic role of marine microbes on living surfaces. Hydrobiologia 461:37–40
Bourne DG, Garren M, Work TM, Rosenberg E, Smith GW, Harvell CD (2009) Microbial desease and the coral holobiont. Trends Microbiol 17:554–562
Braña AF, Rodríguez M, Pahari P, Rohr J, García LA, Blanco G (2014) Activation and silencing of secondary metabolites in Streptomyces albus and Streptomyces lividans after transformation with cosmids containing the thienamycin gene cluster from Streptomyces cattleya. Arch Microbiol 196:345–355
Bull AT, Stach JEM, Ward AC, Goodfellow (2005) Marine actinobacteria: perspectives, challenges, future directions. Antonie Van Leeuwenhoek 87:65–79
Cane DE, Ikeda H (2011) Exploration and mining of the bacterial terpenome. Acc Chem Res 45:463–472
Chen C, Wang J, Guo H, Hou W, Yang N, Ren B, Liu M, Dai H, Liu X, Song F, Zhang L (2013) Three antimycobacterial metabolites identified from a marine-derived Streptomyces sp. MS100061. Appl Microbiol Biotechnol 97:3885–3892
Chun J, Lee JH, Jung Y, Kim M, Kim S, Kim BK, Lim YW (2007) EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol 57:2259–2261
Demain AL (2009) Antibiotics: natural products essential to human health. Med Res Rev 29:821–842
Dharmaraj S (2010) Marine Streptomyces as a novel source of bioactive substances. World J Microbiol Biotechnol 26:2123–2139
Duarte L, Viejo RM, Martíñez B, de Castro M, Gómez-Gesteira M, Gallardo T (2013) Recent and historical range shifts of two canopy-forming seaweeds in North Spain and the link with trends in sea surface temperature. Acta Oecol 51:1–10
Egan S, Harder T, Burke C, Steinberg P, Kjelleberg S, Thomas T (2013) The seaweed holobiont: understanding seaweed–bacteria interactions. FEMS Microbiol Rev 37:462–476
Fernández C (2011) The retreat of large brown seaweeds on the north coast of Spain: the case of Saccorhiza polyschides. Eur J Phycol 46:352–360
Fernández E, Weissbach U, Sánchez Reillo C, Braña AF, Méndez C, Rohr J, Salas JA (1998) Identification of two genes from Streptomyces argillaceus encoding two glycosyltransferases involved in the transfer of a disaccharide during the biosynthesis of the antitrumor drug mithramycin. J Bacteriol 180:4929–4937
Fiedler HP (1993) Biosynthetic capacities of actinomycetes. 1. Screening for secondary metabolites by HPLC and UV-visible absorbance spectral libraries. Nat Prod Lett 2:119–128
Fiedler HP, Bruntner C, Bull AT, Ward AC, Goodfellow M, Potterat O, Puder C, Mihm G (2005) Marine actinomycetes as a source of novel secondary metabolites. Antonie Van Leeuwenhoek 87:37–42
Genilloud O, Peláez F, González I, Díez MT (1994) Diversity of actinomycetes and fungi on seaweeds from the Iberian coasts. Microbiologia 10:413–422
Giddings LA, Newman DJ (2013) Microbial natural products: molecular blueprints for antitumor drugs. J Ind Microbiol Biotechnol 40:1181–1210
González I, Ayuso-Sacido A, Anderson A, Genilloud O (2005) Actinomycetes isolated from lichens: evaluation of their diversity and detection of biosynthetic gene sequences. FEMS Microbiol Ecol 54:401–415
Grein A. (1987) in Advances in Applied Microbiology, ed. Laskin AI (Academic, Somerste, NJ) vol. 32, pp. 203–214
Guinotte J, Orr J, Cairns S, Freiwald A, Morgan L, George R (2006) Will human-induced changes in seawater chemistry alter the distribution of deep-sea scleractinian corals? Front Ecol Environ 4:141–146
Gust B, Challis GL, Fowler K, Kieser T, Chater K (2003) PCR targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci U S A 100:1541–1546
Hughes C, Fenical W (2010) Antibacterials from the sea. Chemistry 16:12512–12525
Jachymova J, Votruba J, Viden I, Rezanka T (2002) Identification of Streptomyces odor spectrum. Folia Microbiol 47:37–41
Jakobi M, Winkelmann G, Kaiser D, Kempler C, Jung G, Berg G, Bahl H (1996) Maltophilin: a new antifungal compound produced by Stenotrophomonas maltophilia R3089. J Antibiot 49:1101–1104
Jiang ZD, Jensen PR, Fenical W (1999) Lobophorins A and B, new anti-inflammatory macrolides produced by a tropical marine bacterium. Bioorg Med Chem Lett 9:2003–2006
Lachnit T, Meske D, Wahl M, Harder T, Schmitz R (2011) Epibacterial communitiy patterns on marine macroalgae are host-specific but temporally variable. Environ Microbiol 13:655–665
Lam KS (2006) Discovery of novel metabolites from marine actinomycetes. Curr Opin Microbiol 9:245–251
Lamela-Silvarrey C, Fernández C, Anadón R, Arrontes J (2012) Fucoid assemblages on the north coast of Spain: past and present (1977–2007). Bot Mar 55:199–207
Li M, Chen YL (1986) Structural studies on rhodilunancins A and B. J Antibiot 39:430–436
Louzao M, Anadón N, Arrontes J, Álvarez-Claudio C, Fuente DM, Ocharan F, Anadón A, Acuña JL (2010) Historical macrobenthic community assemblages in the Avilés Canyon, N Iberian Shelf: baseline biodiversity information for a marine protected area. J Mar Syst 80:47–56
Lucena T, Pascual J, Garay E, Arahal DR, Macián MC, Pujalte MJ (2010) Haliea mediterranea sp. nov., a new marine gammaproteobacterium. Int J Syst Evol Microbiol 60:1844–1848
Manivasagan P, Venkatesan J, Sivakumar K, Kim SK (2014) Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol Res 169:262–278
Margulis L, Fester R (1991) Symbiosis as a source of evolutionary innovation: speciation and morphogenesis. MIT Press. Boston, 454 pages.
Noro JC, Kalaitzis JA, Neilan BA (2012) Bioactive natural products from Papua New Guinea Marine Sponges. Chem Biodivers 9:2077–2095
Olson JB, Kellogg CA (2010) Microbial ecology of corals, sponges, and algae in mesophotic coral environments. FEMS Microbiol Ecol 73:17–30
Petersen F, Zähner H, Metzger JW, Freund S, Hummel RP (1993) Germicidin, an autoregulative germination inhibitor of Streptomyces viridochromogenes NRRL B-1551. J Antibiot 46:1126–1138
Piel J (2009) Metabolites from symbiotic bacteria. Nat Prod Rep 26:338–362
Radjasa OK, Vaste YM, Navarro G, Vervoort HC, Tenney K, Linington R, Crews P (2011) Highlights of marine invertebrate-derived biosynthetic products: their biomedical potential and possible production by microbial associants. Biorg Med Chem 19:6658–6674
Rahman H, Austin B, Mitchell W, Morris PC, Jamieson DJ, Adams DR, Spragg AM, Schweizer M (2010) Novel anti-infective compounds from marine bacteria. Mar Drugs 8:498–518
Rong X, Huang Y (2010) Taxonomic evaluation of the Streptomyces griseus clade using multilocus sequence analysis and DNA-DNA hybridization, with proposal to combine 29 species and three subspecies as 11 genomic species. Int J Syst Evol Microbiol 60:696–703
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Schneemann I, Nagel K, Kajahn I, Labes A, Wiese J, Imhoff JF (2010) Comprehensive investigation of marine Actinobacteria associated with the sponge Halichondia panicea. Appl Environ Microbiol 76:3702–3714
Singh RP, Mantri VA, Reddy CRK, Jha B (2011) Isolation of seaweed-associated bacteria and their morphogenesis-inducing capability in axenic cultures of the green alga Ulva fasciata. Aquat Biol 12:13–21
Staufenberger T, Thiel V, Wiese J, Imhoff JF (2008) Phylogenetic analysis of bacteria associated with Laminaria saccharina. FEMS Microbiol Ecol 64:65–77
Ströch K, Zeeck A, Antal N, Fiedler HP (2005) Retymicin, galtamycin B, Saquayamycin Z and ribofuranosyllumichome, novel secondary metabolites from Micromonospora sp. Tü6368. J Antibiotics 58:103–110
Stutzman-Engwall KJ, Hutchinson CR (1989) Multigene families for anthracycline antibiotic production in Streptomyces peucetius. Proc Natl Acad Sci U S A 86:3135–3139
Subramani R, Aalbersberg W (2012) Marine actinomycetes: an ongoing source of novel bioactive metabolites. Microbiol Res 167:571–580
Sun W, Peng C, Zhao Y, Li Z (2012) Functional gene-guided discovery of type II polyketides from culturable actinomycetes associated with soft coral Scleronephthya sp. PLoS ONE 7:e42847
Voerman SE, Llera E, Rico JM (2013) Climate driven changes in subtidal kelp forest communities in NW Spain. Mar Environ Res 90:119–127
Ward AC, Bora N (2006) Diversity and biogeography of marine actinobacteria. Curr Opin Microbiol 9:279–286
Wei RB, Xi T, Li J, Wang P, Li FC, Lin YC, Qin S (2011) Lobophorin C and D, new kijanimicin derivatives from a marine sponge-associated actinomycetal strain AZS17. Mar Drugs 9:359–368
Wiese J, Thiel V, Nagel KI, Staufenberger T, Imhobb JF (2009) Diversity of antibiotic-active bacteria associated with the brown alga Laminaria saccharina from the Baltic sea. Mar Biotechnol 11:287–300
Yang S, Sun W, Tang C, Jin L, Zhang F, Li Z (2013) Phylogenetic diversity of actinobacteria associated with soft coral Alcyonium gracllimum and stony coral Tubastraea coccinea in the East China Sea. Microb Ecol 66:189–199
Zhang XY, He F, Wang GH, Bao J, Xu XY, Qi SH (2013) Diversity and antibacterial activity of culturable actinobacteria isolated from five species of the South China Sea gorgonian corals. World J Microbiol Biotechnol 29:1107–1116
Zotchev SB (2012) Marine actinomycetes as an emerging resource for the drug development pipelines. J Biotechnol 158:168–175
Acknowledgments
This study was financially supported by the Universidad de Oviedo (UNOV-11-MA-02), Gobierno del Principado de Asturias (SV-PA-13-ECOEMP-62), and Ministerio de Economía y Competitividad, Proyecto DOSMARES/BIOCANT (MICINN-10-CTM2010-21810-C03-02). The authors are grateful to Ricardo Anadón and all other participants in the BIOCANT3 campaign. The authors want to thank all the people who contributed to sample collection, especially to Gloria Blanco Sotura, Manuela Blanco, Rubén Medina, and Noé Medina. We are also grateful to Santiago Cal for his valuable help and José L. Caso and José A. Guijarro for continuous support. We finally thank Miguel Campoamor and Marcos García for their excellent technical assistance and M.C. Macián (CECT) for her help in the identification of the strains. This is a contribution of the Asturian Marine Observatory.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary 1
Phenotypes of M-27 and M-40 strains grown in R5A agar plates. Pictures were taken after 5 days of growth and correspond to (A) sporulated cultures, and (B) the back side of the plates. (PDF 91 kb)
Supplementary 2
Antibiograms against M. luteus (A) and E. coli ESS (B) of ethyl acetate extracts from M-27 and M-40 strains obtained after 5 days of growth in solid R5A medium. The extracts were obtained from 7 ml of culture under neutral (n) and acidic (a) conditions and resuspended in 50 μl of DMSO-methanol from which 15 μl were loaded onto the discs. (PDF 74 kb)
Supplementary 3
Cell survival percentage in cytotoxicity assays with acidic ethyl acetate extracts from M-27 and M-40 strains carried out against two different tumour cell lines: HeLa, from cervical carcinoma, and HCT116, from colorectal carcinoma. The fact that the 1/10 diluted M-27 extracts appear more active than the undiluted ones against both cell lines could be explained by assay interferences due to the high complexity of the sample, which might contain other compounds with antagonist activity only observed at high concentrations. (PDF 31 kb)
Supplementary 4
Volatile profile of marine Streptomyces species obtained through GS-MS analysis. Peak numbers indicate the compounds identified by comparison with the Whiley dabase as: geosmin (8); beta-patchoulene (9). (PDF 221 kb)
Rights and permissions
About this article
Cite this article
Braña, A.F., Fiedler, HP., Nava, H. et al. Two Streptomyces Species Producing Antibiotic, Antitumor, and Anti-Inflammatory Compounds Are Widespread Among Intertidal Macroalgae and Deep-Sea Coral Reef Invertebrates from the Central Cantabrian Sea. Microb Ecol 69, 512–524 (2015). https://doi.org/10.1007/s00248-014-0508-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-014-0508-0