Abstract
Polycyclic aromatic hydrocarbons (PAHs) are common contaminants in terrestrial and aquatic environments and can represent a significant constituent of the carbon pool in coastal sediments. We report here the results of an 18-month seasonal study of PAH biodegradation and heterotrophic bacterial production and their controlling biogeochemical factors from 186 sediment samples taken in a tidally influenced freshwater estuary. For each sampling event, measurements were averaged from 25–45 stations covering ∼250 km2. There was a clear relationship between bacterial production and ambient temperature, but none between production and bottom water dissolved oxygen (DO) % saturation or PAH concentrations. In contrast with other studies, we found no effect of temperature on the biodegradation of naphthalene, phenanthrene, or fluoranthene. PAH mineralization correlated with bottom water DO saturation above 70% (r2 > 0.99). These results suggest that the proportional utilization of PAH carbon to natural organic carbon is as much as three orders of magnitude higher during cooler months, when water temperatures are lower and DO % saturation is higher. Infusion of cooler, well-oxygenated water to the water column overlying contaminated sediments during the summer months may stimulate PAH metabolism preferentially over non-PAH organic matter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural and anthropogenic processes are responsible for introducing PAHs into the environment [27, 28, 37, 3, 1]. Harbors, marinas, and wastewater treatment and industrial facilities in urban watersheds are entry points for PAHs and other contaminants which sorb to particles and eventually accumulate in sediments [34, 21, 16, 35], resulting in contamination of ∼10% of U.S. river, lake, and coastal sediments [58]. If extrapolated to global sediment, PAH may potentially represent a carbon pool of 0.01 × 1015 g (using lowest estimates of coastal sediment deposition [23]). Cleanup costs for affected sediments in the United States alone are estimated in the billions to trillions of dollars [56, 49]. Current and future urban development will likely increase the PAH loading in coastal and estuarine waterways [60, 44].
Following deposition, particle-bound PAHs may be removed by processes such as physical resuspension [33], bioturbation [48], porewater partitioning [40, 41, 38], ingestion or sorption by animals [59], or biodegradation [10, 9]. The environmental factors that regulate rates of in situ PAH biodegradation are poorly understood and difficult to model [43, 2]. Approaches to assess bioremediation potential have included both laboratory studies (to predict in situ rates) and field analysis (to measure processes in situ). These have produced disparate results, indicating that laboratory studies may not be sufficient to determine environmental rates of bioremediation [7].
For carbon-based contaminants, such as PAHs or petroleum, the primary goal of bioremediation efforts is to stimulate natural or introduced microbial populations to utilize more carbon, thereby degrading more contaminant. Efficacy testing usually involves nonspecific measures of bacterial metabolism such as total respiration (CO2 evolution), bacterial biomass, or O2 utilization [43]. Ideally, the optimal bioremediation approach would preferentially stimulate PAH carbon utilization relative to other forms of carbon. In this manner, costly remediation efforts, such as nutrient or cometabolyte additions, would be minimized. Knowledge of factors controlling biogeochemical cycles, particularly carbon flow through the microbial assemblage, is a critical first step in the ultimate design and implementation of any bioremediation strategy.
Two major factors affecting sediment microbial activities are ambient temperature and DO concentration. Studies in the Delaware Bay and other East Coast estuaries have shown water temperature correlates with both heterotrophic bacterial productivity (total carbon demand) [50, 17, 13, 25, 20] and contaminant biodegradation [26, 6, 9, 52]. DO has also been shown to affect the microbially mediated turnover of organic matter in estuarine sediments [62]. Low DO has been shown to limit microbial utilization of aromatic organic contaminants in uncontaminated sediments [9, 6, 14]; however, recent studies suggest PAH mineralization may occur at a rapid rate under anoxic conditions [12, 36, 47]. In previous studies in the Delaware and Schuylkill Rivers the sediment surface and nepheloid layer (flocculent material above the sediment surface) were found to be dynamic with significant transport and deposition of particles and particle-associated PAH [46]. Thus it was hypothesized that surface sediment should be roughly in equilibrium with the overlying water and that characteristics of the bottom water should affect microbial communities living in the uppermost surface sediments. In studying the surface sediment using short-term, field carbon-cycling techniques, a better understanding of the factors limiting PAH biodegradation could be obtained. A large temporal and spatial sampling program covering a 250-km2 portion of the Delaware and Schuylkill Rivers near Philadelphia, PA (USA), was undertaken primarily to determine the effect of temperature and DO, but also others parameters such as sediment organic carbon content, on sediment microbial activities and PAH biodegradation.
Methods
Study Site and Sample Collection
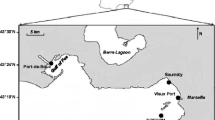
This study was conducted in a tidally influenced freshwater region of the Delaware Bay near Philadelphia, PA (USA) (Fig. 1). A series of stations was established in the industrialized region of the Delaware and Schuylkill Rivers, where environmental impacts have been well documented [54, 61, 39]. The large cluster of refineries located on the banks of the Schuylkill River is a suspected current source for hydrocarbon contaminants in the sample area. Samples were also taken within the Philadelphia Naval Complex Reserve Basin (PNCRB). Cruise dates were 17–19 December 1997, 18–20 June 1998, 16–18 September 1998, 14–16 December 1998, and 3–5 May 1999. Sediment samples were collected using a Petite Ponar within the PNCRB from an Avon inflatable and with a Smith-Mack grab from R/V Cape Henlopen in the Delaware and Schuylkill Rivers. Up to 46 stations were sampled during each event. Water samples (for use in bioassays) were collected with a 2.5-L Nansen-type bottle in the PNCRB and a 30-L Niskin bottle in the Delaware and Schuylkill Rivers.
Bacterial Production
Bacterial production was measured using a modification of the leucine incorporation method [31]. A 0.50-μL surface sediment subsample from each station was added to 2-mL centrifuge tubes (three experimental and one control) which were precharged with [3H-4,5]-L-leucine (1 mCi mmol−1). One mL of 0.22-μm filtered bottom water (collected <1 m above bottom) was then added to each tube to form a sediment slurry. Samples were incubated for 1–2 h at in situ temperatures and subsequently processed by the method of Smith and Azam [53]. A Beckman LS6500 scintillation counter was used for analysis. A constant isotope dilution factor of 1000 was used for all samples. This was estimated from actual measurements of sediment-dissolved free amino acids [8] and saturation experiment estimates [55]. The effects of atmospheric oxygen in the incubation tube headspace were assumed to be negligible by calculating the incubation tube diameter, assuming an oxygen diffusion coefficient of 2 × 10−5 cm2 s−1, and estimating the “depth” of oxygen penetration (<0.1 mm). Recent evidence suggests that even production data in anaerobic samples are not affected when using the 3H-leucine centrifugation method [5].
PAH Mineralization
PAH mineralization was measured using 14C-labeled substrates in a manner similar to previous studies [45, 15]. Briefly, [UL-14C] naphthalene (18.6 mCi mmol−1, >98% purity by HPLC), [3-14C] fluoranthene (45 mCi mmol−1, >98% purity by HPLC), or [9-14C] phenanthrene (47 mCi mmol−1, >98% purity by HPLC) was added to surface (1–5 mm) sediment samples (1 mL wet weight) in 100 × 16 mm test tubes to a final concentration of ∼500 ng g−1 (depending on specific activity). Three replicates were prepared with one control. Then, 0.5 mL of 0.22-μm filtered bottom water was added to each tube to make a sediment slurry. Sediment slurries were created in order to facilitate diffusion of labeled substrates throughout the sediment. Isotope dilution was calculated from the ambient test PAH concentration (determined by GC/MS—see below). Samples were incubated for <24 h at in situ temperature and evolved 14CO2 was captured on NaOH-soaked filter papers suspended in the headspace. One mL 2 N H2SO4 was added to end incubations and to force any remaining CO2 into headspace of the tube to the filter paper trap. This was done at the beginning of each incubation for the control tubes. Filter papers containing metabolic 14CO2 were removed and radioassayed on a Beckman LS6500 scintillation counter. The resultant disintegrations per minute (dpm) values were used to calculate substrate mineralization. Because it is not possible to predict the stochiometric relationships between 14CO2 molecules liberated and molecules of parent PAH degraded, mineralization rates were calculated directly from 14CO2 recovery and the specific activity of the initial 14C-labeled substrate on a per carbon basis. 14CO2 from control incubations (acidified at t = 0) were subtracted from the average of experimental triplicates. Controls generally yielded on the order of 100–300 dpm. Oxygen diffusion was calculated as with production measurements. In addition to facilitating the dispersion of added substrates in the incubation tubes, sediment slurries were used so that an overlying water could lens could provide additional protection against oxygen diffusion into the sediment during the course of incubation. Oxygen penetration was calculated to be <2 mm into the sediment slurry (∼25 mm).
Ambient PAH Concentrations
Ambient PAH concentrations were determined by drying of 10–15 g of wet sediment with diatamaceous earth, accelerated solvent extraction of dried samples, and GC/MS analysis of the extracts [19]. p-Terphenyl-d14 and 2-fluorobiphenyl were used as surrogate standards. Matrix spike duplicates were prepared, extracted, and analyzed from one sample per sampling event. A series of calibrations standards (Supelco) were run after every 12 unknown samples. A Hewlett-Packard 6890 GC coupled to a 5973 MS was used for all PAH analyses. A 60-m 5% phenyl 95% methyl (SPB-5) 0.250-μm ID capillary column was used. Samples were injected by means of an autosampler into a splitless mode inlet maintained at 250°C. Pressure pulse programming was used to increase inlet pressure from 16 to 25 psi prior to the septum purge at 2 min into the run. Overall column flow was 1.0 mL min−1. Helium was the carrier gas. The initial column temperature was 40°C. The temperature was ramped as follows: (1) at 1 min run-time, ramp 4.0°C min−1 to 180°C, hold 5 min; (2) ramp 4.0°C min−1 to 220, hold 5 min; (3) ramp 4.0°C min−1 to 280°C, hold 5 min; (4) ramp 4.0°C min−1 to 300°C, hold 10 min. The distal column end will empty directly in the source area of the 5973 MS. The solvent delay was 7.0 min and the MS was set to scan from 35 to 300 amu. The MS was tuned with perfluorotributylamine (PFTBA). The MS temperature was set to 106°C; the source was set to 230°C.
Total Organic Carbon
Total organic carbon (TOC) was measured in sediments by standard methods [57] using a Carlo Erba Model 1108 Elemental Analyzer equipped with Eager 300 software. Data are presented in percent carbon on a dry weight basis. Sediment samples from the May 1999 sampling were compromised during storage (cooler shipped to wrong address and allowed to warm) and were not analyzed for TOC.
Temperature and Dissolved Oxygen
Temperature, salinity, and DO were measured with a DataSonde IV in the Reserve Basin and with a SeaBird CTD in the Delaware and Schuylkill Rivers. For interseasonal comparisons, DO was converted from mg L−1 to % saturation (corrects for temperature-dependent oxygen solubility) using Seabird Seasave software. All sensors were calibrated prior to use.
Statistical Analysis
Statistical analyses were conducted using GraphPad® Instat and Microsoft® Excel software. Because samples were not taken in a completely random grid (for instance, samples within the PNCRB were taken very close to one another), a coefficient of skewness was calculated for each averaged variable. The concern was that samples very close to one another geographically would bias the data set toward a mean that was nonrepresentative. Data sets with coefficients greater than +1.0 are indicative of an abnormal distribution around the mean value. In order to determine if skewness in these data sets would change the conclusions of this work, entire distribution means were compared to data averaged from the second quartile of the distibutions with a coefficient of skewness >1.
Results
PAH Concentrations
Individual PAH concentrations were averaged for all stations from each sampling event (Table 1). Individual values ranged from nondetected to 8.2, 21.2, and 11.7 μg g−1 for naphthalene, phenanthrene, and fluoranthane, respectively (PAHs used to assay mineralization). Highest sediment concentrations for PAHs were found in Schuylkill River, Reserve Basin, and Station 9 (close to an outfall) sediments. QA/QC procedures for PAH analyses were conducted in accordance with standard EPA methods as referenced in the Methods section [19]. QA/QC values for only one sampling (June 1998) are presented (Table 2); however, each sampling event yielded similar results.
Ambient Conditions
Seasonal temperature and DO measurements were averaged for all stations from each sampling event (Table 3). Average temperatures ranged from 5.07 to 24.7°C. Average DO % saturations were highest in December 1997 and May 1999. TOC varied by approximately two orders of magnitude (∼0.1% to over 10%). Highest % carbon values were found in the Schuylkill River and Reserve Basin samples.
Microbial Activities
Average bacterial production was lowest in December 1997 and highest in June 1998 with individual measurements ranging from 1.5 μg C g−1 d−1 (Dec 1997) to 16 mg C g−1 d−1 (June 1998). Average mineralization rates for PAHs varied considerably, but were consistently highest in May 1999. Mineralization rates for individual stations ranged from nondetected (with each substrate) to 20, 25, and 94 ng g−1 h−1 (naphthalene, phenanthrene, and fluoranthane, respectively). The highest rates were observed in May 1999. Mineralization rates displayed the most skew, and although average values were lower in the second quartile, seasonal trends were consistent with watershed averages (Table 3).
PAH turnover times were calculated by dividing the total utilization (assuming a conservative metabolic efficiency of 50% [24, 52]) into the ambient concentration (Table 4). These values estimate the time required for all of a given PAH to be degraded given linear utilization (and no further input). Furthermore, a turnover time assumes as the concentration of ambient PAH decreases, PAH degraders maintain the same rate of PAH degradation. Neither assumption is ideal, as seasonal environmental changes are known to affect microbial activities and general enzyme kinetics clearly show a concentration–activity relationship. Findings for the May 1999 sampling were consistent with those in a recent survey of literature values [52], where similar tracer techniques were used. Turnover times from earlier months sampled during this study were longer than many turnover times surveyed from the literature [26, 6, 52, 29, 47]. Methods in this study differ from related studies in that they rely on shorter incubation times of <24 h (which reduces adaptation by the microbial community to “new” artificial conditions), tracer (below ambient) additions of 14C-labeled compounds (which should not stimulate natural microbial communities), and direct conversion of specific activity to amount of PAH degraded (on a per carbon basis rather than a percent 14C recovery). Additionally, this study site had almost no variation in salinity, which may affect PAH biodegradation [51].
Discussion
Factors Affecting Heterotrophic Production
Average heterotrophic production did not correlate with sediment PAH concentrations (r2 < 0.1) or with sediment total organic carbon (data not presented). However, there was a weak negative relationship between average bottom DO % saturation and average heterotrophic production (r2 = 0.32). Not surprisingly, there was a positive correlation between bottom water temperature and average heterotrophic production (r2 = 0.80) (Fig. 2). These results agree well with other investigations in the Delaware River and other watersheds in the U.S. mid-Atlantic region [18, 22, 25, 32, 50].
Factors Affecting PAH Mineralization
In contrast with bacterial production and most other studies of aromatic compound biodegradation [4, 6, 30, 42], there was no correlation between average temperature and average individual PAH utilization (r2 < 0.01). PAH mineralization also showed no correlation with PAH concentration (not shown) or sediment total organic carbon (Table 5). One other study using comparable tracer methods found little correlation between PAH mineralization and temperature at some sites [52], but not in all cases. When average PAH mineralization from this study was related to average bottom DO % saturation using nonlinear regression analysis, an exponential increase in PAH mineralization with increasing DO % saturation above ∼70% was observed (Fig. 3). A semilog plot of average bottom water temperature and average PAH mineralization gave r2 values >0.99 for all PAHs tested. Below 70% DO saturation, mineralization of PAHs was relatively low and unvaried. This relationship held true for all three PAHs tested. These results contrast with laboratory incubations (14–20 days) using natural sediments [6] in which DO % saturation below ∼40% inhibited PAH mineralization. Skew-adjusted (second-quartile average) PAH mineralization was also modeled using exponential regression; r2 values were >0.95 (Fig. 3, inset).
Average naphthalene (○), phenanthrene (□), and fluoranthene (▲) mineralization as a function of % DO saturation. Inset graph shows DO % saturation related to the log of mineralization rate equations. (1) naphthalene: y = 2 × 10−14e0.3103x; r2 = 0.99; (2) phenanthrene: y = 1 × 10−6e0.0901x; r2 = 0.99; (3) fluoranthene: y = 1 × 10−12e0.2486x; r2 = 1.0; (4) skew-adjusted (see text) naphthalene: y = 1.9 × 10−10e0.1733x; r2 = 0.99; (5) skew-adjusted phenanthrene: y = 9.5 × 10−10e0.1542x; r2 = 0.96; (6) skew-adjusted fluoranthene: y = 1.4 × 10−10e0.191x; r2 = 0.98.
Methodological differences most likely account for the disparity seen between turnover times calculated from the tracer methods used in this study and long-term hydrocarbon utilization studies conducted under anaerobic conditions in the laboratory [47, 12, 11]. The experiments in this study were conducted with only surface sediments where microbial populations would have fewer tendencies to adapt for anaerobic conditions. Tracer techniques measure the instantaneous rates of biodegradation which can then be related to the ambient pool of substrate [15], whereas long-term laboratory incubations (needed to assay appreciable mineralization of PAH) measure the potential for the microbial community to degrade contaminants. In instances where historical contamination is the prime source of hydrocarbons in sediments, degradation rates calculated from long-term laboratory incubations might approximate the in situ degradation rate. In complex, human-affected environments, continual deposition of PAH and other hydrocarbon contaminants makes the calculation of turnover times much more problematic [46].
Importance of PAH as a Carbon Source
PAHs compete with other (usually more labile) organic matter in natural biogeochemical cycles. Thus attempts to stimulate biodegradation of PAHs may also stimulate the utilization of non-PAH carbon or other natural organic matter (NOM), increasing the use of limiting factors such as nutrients and DO. Utilization of NOM preferentially to PAH would tend to enrich PAH content in sediment, particularly if PAH concentrations are normalized to sediment OM. Sediment OM concentrations (w/w) varied greatly from 0.1 to >10% (data not shown) and had only modest correlation with total PAH concentrations (r2 = 0.66). To determine the importance of PAH as a carbon source to natural sediment bacteria, the percentage of heterotrophic bacterial production accounted for by individual PAH biodegradation was calculated (Table 4). Production in this sense represents the total carbon demand of the microbial assemblage, and we make the assumption that this production is fueled primarily by NOM. Therefore the ratio of PAH utilization to production essentially is the ratio of PAH utilization to NOM utilization. This relative utilization of PAH:NOM was highest during December 1997, December 1998, and May 1999. Higher relative utilization of PAH carbon to NOM is the ultimate goal of hydrocarbon bioremediation efforts. Therefore, in seasons where the proportion of PAH utilization to heterotrophic production is higher, more intrinsic remediation may be expected to occur. However, in no case was more than 4% of the carbon demand of the microbial consortium supplied by any individual PAH.
Turnover times have been used to estimate the time of removal for contaminants (and natural organic matter). Overall, turnover times indicated that PAHs are likely to accumulate and persist during several seasons, especially in early summer. This is important as PAHs tend to persist much longer in impacted environments than can usually be predicted by calculated turnover times. Although fine-scale temporal resolution is lacking in this study (5 days sampled out of 500), if turnover times are averaged over the course of the year only phenanthrene appears to be degraded fast enough to prevent sediment accumulation (225 days). A previous analysis of PAH concentrations on suspended particles within the watershed indicated current deposition and/or resuspension likely occurs [46]. Mass balance calculations of sediment transport (using turbidity measurements) in and out of the PNCRB during tidal cycles also indicated net deposition of PAH-containing particles [46]. Turnover times can only approximate environmental persistence given no additional input. It appears that continuing deposition of PAH-containing particles allows net accumulation of PAHs in sediments in the watershed.
Results from this study indicate DO saturation in waters overlying sediments is a prime factor regulating surface sediment PAH biodegradation in this freshwater tidal estuary. In contrast with other studies, we found no apparent relationship between ambient temperature and PAH biodegradation. It may be that when DO is not limiting and water temperature is low (depressing total heterotrophic production), PAH-degrading assemblages have a selective advantage in hydrocarbon-contaminated sediments. This has implications for remediation strategies because effective contaminant utilization may occur in cooler months, where previously it has been assumed there is little biodegradation. We may predict that without other growth limitations, bioremediation should occur at a significant rate throughout the year. Results from this study indicate that bottom water DO saturation above ∼70% is critical for appreciable PAH biodegradation in surface sediments. A potential year-round remediation strategy for PAH-impacted sediments, on a limited-area approach, might be to introduce cool, well-oxygenated water to regions overlying surface sediments. This strategy would limit temperature-related total heterotrophic bacterial production, but preferentially stimulate PAH biodegradation.
References
JO Allen JL Durant NM Dookeran K Taghizadeh EF Plummer AL Lafleur AF Sarofim KA Smith (1998) ArticleTitleMeasurement of C24H14 polycyclic aromatic hydrocarbons associated with a size-segregated urban aerosol Environ Sci Technol 32 1928–1932 Occurrence Handle10.1021/es970919r
KH Baker DS Herson (1990) ArticleTitleIn situ bioremediation of contaminated aquifers and subsurface soils Geomicrobiol J 8 133–146
HA Bamford JH Offenberg RK Larsen F-C Ko JE Baker (1999) ArticleTitleDiffusive exchange of polycyclic aromatic hydrocarbons across the air–water interface of the Patapsco River, an urbanized subestuary of the Chesapeake Bay Environ Sci Technol 33 2138–2144 Occurrence Handle10.1021/es981324e
GW Bartholomew FK Pfaender (1983) ArticleTitleInfluence of spatial and temporal variations on organic pollutant biodegradation rates in an estuarine environment Appl Environ Microbiol 45 103–109 Occurrence Handle6824312
D Bastviken L Tranvik (2001) ArticleTitleThe leucine incorporation method estimates bacterial growth equally well in both oxic and anoxic lake waters Appl Environ Microbiol 67 2916–2921 Occurrence Handle10.1128/AEM.67.7.2916-2921.2001 Occurrence Handle11425702
JE Bauer DG Capone (1985) ArticleTitleDegradation and mineralization of the polycyclic aromatic hydrocarbons anthracene and naphthalene in intertidal marine sediments Appl Environ Microbiol 50 81–90
JW Blackburn (1998) ArticleTitleBioremediation scaleup effectiveness: a review Biorem J 1 265–282
DJ Burdige CS Martens (1990) ArticleTitleBiogeochemical cycling in an organic-rich coastal marine basin: 11. The sedimentary cycling of dissolved, free amino acids Geochim Cosmochim Acta 54 3033–3052 Occurrence Handle10.1016/0016-7037(90)90120-A
DG Capone JE Bauer (1992) Microbial processes in coastal pollution R Mitchell (Eds) Environmental Microbiology Wiley-Liss New York 191–237
CE Cerniglia MA Heitkamp (1989) Microbial degradation of polycyclic aromatic hydrocarbons (PAH) in the aquatic environment U Varanasi (Eds) Metabolism of Polycyclic Aromatic Hydrocarbons in the Aquatic Environment CRC Press Boca Raton, FL 41–68
JD Coates RT Anderson DR Lovley (1996) ArticleTitleOxidation of polycyclic aromatic hydrocarbons under sulfate-reducing conditions Appl Environ Microbiol 62 1099–1101
JD Coates J Woodward J Allen P Philp DR Lovley (1997) ArticleTitleAnaerobic degradation of polycyclic aromatic hydrocarbons and alkanes in petroleum-contaminated marine harbor sediments Environ Sci Technol 63 3589–3593
RB Coffin JH Sharp (1987) ArticleTitleMicrobial trophodynamics in the Delaware Estuary Mar Ecol Prog Ser 41 253–266
TJ Cutright SY Lee (1994) ArticleTitleMicroorganisms and metabolic pathways for remediation of PAH contaminated soil Fresenius Environ Bull 3 413–421
JW Deming (1993) 14C tracer method for measuring microbial activity in deep-sea sediments PF Kemp BF Sherr EB Sherr JJ Cole (Eds) Handbook of Methods in Aquatic Microbial Ecology Lewis Publishers Ann Arbor, MI 405–414
RM Dickhut KE Gustafson (1995) ArticleTitleAtmospheric washout of polycyclic aromatic hydrocarbons in the Southern Chesapeake Bay region Environ Sci Technol 29 1518–1525 Occurrence Handle10.1021/es00006a013
M Felip ML Pace JJ Cole (1996) ArticleTitleRegulation of planktonic bacterial growth rates: the effects of temperature and resources Microb Ecol 31 15–28 Occurrence Handle10.1007/BF00175072
S Findlay ML Pace D Lints JJ Cole NF Caraco B Peierls (1991) ArticleTitleWeak coupling of bacterial and algal production in a heterotrophic ecosystem: the Hudson River estuary Limnol Oceanogr 36 268–278
JA Fisher MJ Scarlett AD Stott (1997) ArticleTitleAccelerated solvent extraction: an evaluation for screening of soils for selected U.S. EPA semivolatile organic priority pollutants Environ Sci Technol 31 1120–1127 Occurrence Handle10.1021/es9606283
TR Fisher AB Gustafson K Sellner R Lacouture LW Haas RL Wetzel R Magnien D Everitt B Michaels R Karrh (1999) ArticleTitleSpatial and temporal variation of resource limitation in Chesapeake Bay Mar Biol 133 763–778 Occurrence Handle10.1007/s002270050518
TP Franz SJ Eisenreich TM Holsen (1998) ArticleTitleDry deposition of particulate polychlorinated biphenyls and polycyclic aromatic hydrocarbons to Lake Michigan Environ Sci Technol 32 3681–3688 Occurrence Handle10.1021/es980107a
X Gayte D Fontvielle KJ Wilkinson (1999) ArticleTitleBacterial stimulation in mixed cultures of bacteria and organic carbon from river and lake waters Microb Ecol 38 285–295 Occurrence Handle10541790
JI Hedges RG Keil R Benner (1997) ArticleTitleWhat happens to terrestrial organic matter in the ocean? Org Geochem 27 195–212 Occurrence Handle10.1016/S0146-6380(97)00066-1
SE Herbes LR Schwall GA Williams (1977) ArticleTitleRate of microbial transformation of polycyclic aromatic hydrocarbons: a chromatographic quantification procedure Appl Environ Microbiol 34 244–246 Occurrence Handle907346
MP Hoch DL Kirchman (1993) ArticleTitleSeasonal and interannual variability in bacterial production and biomass in a temperate estuary Mar Ecol Prog Ser 98 283–295
JP Hudak J McDaniel S Lee JA Fuhrman (1988) ArticleTitleMineralization potentials of aromatic hydrocarbons by estuarine microorganisms: variations with season, location, and bacterioplankton production Mar Ecol Prog Ser 47 97–102
M Kästner M Breuer-Jammali B Mahro (1998) ArticleTitleImpact of inoculation protocols, salinity, and pH on the degradation of polycyclic aromatic hydrocarbons (PAHs) and survival of PAH-degrading bacteria introduced into soil Appl Environ Microbiol 64 359–362 Occurrence Handle9435090
M Kästner M Breuer-Jammali B Mahro (1994) ArticleTitleEnumeration and characterization of the soil microflora from hydrocarbon-contaminated soil sites able to mineralize polycyclic aromatic hydrocarbons (PAH) Appl Microbiol Biotechnol 41 267–273 Occurrence Handle10.1007/s002530050142
RP Kerr DG Capone (1988) ArticleTitleThe effect of salinity on the microbial mineralization of 2 polycyclic aromatic-hydrocarbons in estuarine sediments Mar Environ Res 26 181–198 Occurrence Handle10.1016/0141-1136(88)90026-8
H Kim HF Hemon LR Krumholz BA Cohen (1995) ArticleTitleIn-situ biodegradation of toluene in a contaminated stream. 1. Field studies Environ Sci Technol 29 108–116 Occurrence Handle10.1021/es00001a014
DL Kirchman (1993) Leucine incorporation as a measure of biomass production by heterotrophic bacteria PF Kemp BF Sherr EB Sherr JJ Cole (Eds) Handbook of Methods in Aqautic Microbial Ecology Lewis Publishers Boca Raton, FL 509–512
AKT Kirschner B Velimirov (1999) ArticleTitleBenthic bacterial secondary production measured via simultaneous 3H-thymidine and 14C-leucine incorporation, and its implication for the carbon cycle of a shallow macrophyte-dominated backwater system Limnol Oceanogr 44 1871–1881
JS Latimer WR Davis DJ Keith (1999) ArticleTitleMobilization of PAHs and PCBs from in-place contaminated marine sediments during simulated resuspension events Estuar Coast Shelf Sci 49 577–595 Occurrence Handle10.1006/ecss.1999.0516
GG Leppard DT Flannigan D Mavrocordatos CH Marvin DW Bryant BE McCarry (1998) ArticleTitleBinding of polycyclic aromatic hydrocarbons by size classes of particulate in Hamilton Harbor water Environ Sci Technol 32 3633–3639 Occurrence Handle10.1021/es980055p
E Lipiatou I Tolosa R Simo I Bouloubassi J Dachs S Marti M-A Sicre JM Bayona JO Grimalt A Saliot J Albaigés (1997) ArticleTitleMass budget and dynamics of polycyclic aromatic hydrocarbons in the Mediterranean Sea Deep-Sea Res Part II 44 881–905 Occurrence Handle10.1016/S0967-0645(96)00093-8
DR Lovley (2001) ArticleTitleBioremediation—anaerobes to the rescue Science 293 1444–1446 Occurrence Handle10.1126/science.1063294 Occurrence Handle11520973
E Manoli C Samara (1999) ArticleTitlePolycyclic aromatic hydrocarbons in natural waters: sources, occurrence and analysis TRAC—Trends Anal Chem 18 417–428 Occurrence Handle10.1016/S0165-9936(99)00111-9
KA Maruya RW Risebrough AJ Horne (1996) ArticleTitlePartitioning of polynuclear aromatic hydrocarbons between sediments from San Francisco Bay and their porewaters Environ Sci Technol 30 2942–2947 Occurrence Handle10.1021/es950909v
JR Maxted RA Eskin SB Weisberg FW Kutz (1997) ArticleTitleThe ecological condition of dead-end canals of the Delaware and Maryland coastal bays Estuaries 20 319–327
AE McElroy JW Farrington JM Teal (1989) Bioavailability of polycyclic aromatic hydrocarbons in the aquatic environment U Varanasi (Eds) Metabolism of Polycyclic Aromatic Hydrocarbons in the Aquatic Environment CRC Press Boca Raton, FL 1–39
SE McGroddy JW Farrington PM Gschwend (1996) ArticleTitleComparison of the in situ and desorption sediment–water partitioning of polycyclic aromatic hydrocarbons and polychlorinated biphenyls Environ Sci Technol 30 172–177 Occurrence Handle10.1021/es950218z
D Muncnerova J Augustin (1993) ArticleTitlePhysical and chemical aspects affecting the process of biodegradation of xenobiotics of the type of aromates Chemicke Listy 87 484–492
National Research Council (1993) In Situ Bioremediation: When Does It Work? 1–207
B Ngabe TF Bidleman GI Scott (2000) ArticleTitlePolycyclic aromatic hydrocarbons in storm runoff from urban and coastal South Carolina Sci Total Environ 255 1–9 Occurrence Handle10.1016/S0048-9697(00)00422-8 Occurrence Handle10898390
FK Pfaender GW Bartholomew (1982) ArticleTitleMeasurement of aquatic biodegradation rates by determing heterotrophic uptake of radiolabeled pollutants Appl Environ Microbiol 44 159–164 Occurrence Handle7125647
JW Pohlman RB Coffin CS Mitchell MT Montgomery BJ Spargo JK Steele TJ Boyd (2002) ArticleTitleTransport, deposition and biodegradation of particle bound polycyclic aromatic hydrocarbons in a tidal basin of an industrial watershed Environ Monit Assess 75 155–167 Occurrence Handle10.1023/A:1014435830558 Occurrence Handle12002284
MM Rothermich LA Hayes DR Lovley (2002) ArticleTitleAnaerobic, sulfate-dependent degradation of polycyclic aromatic hydrocarbons in petroleum-contaminated harbor sediment Environ Sci Technol 36 4811–4817 Occurrence Handle10.1021/es0200241 Occurrence Handle12487304
LC Schaffner RM Dickhut S Mitra PW Lay C Brouwer-Riel (1997) ArticleTitleEffects of physical chemistry and bioturbation by estuarine macrofauna on the transport of hydrophobic organic contaminants in the benthos Environ Sci Technol 31 3120–3125 Occurrence Handle10.1021/es970054h
Sediment Management Work Group (1999) Contaminated Sediment Management Technical Papers, 1–37
F-K Shiah HW Ducklow (1994) ArticleTitleTemperature regulation of heterotrophic bacterioplankton abundance, production, and specific growth rate in Chesapeake Bay Limnol Oceanogr 39 1243–1258
MP Shiaris (1989) ArticleTitlePhenanthrene mineralization along a natural salinity gradient in an urban estuary, Boston Harbor, Massachusetts Microb Ecol 18 135–146
MP Shiaris (1989) ArticleTitleSeasonal biotransformation of naphthalene, phenanthrene, and benzo [a] pyrene in surficial estuarine sediments Appl Environ Microbiol 55 1391–1399
DC Smith F Azam (1992) ArticleTitleA simple, economical method for measuring bacterial protein synthesis rates in seawater using 3H-leucine Mar Microb Food Webs 6 107–114
AC Steyermark JR Spotila D Gillette H Isseroff (1999) ArticleTitleBiomarkers indicate health problems in brown bullheads from the industrialized Schuylkill River, Philadelphia Trans Am Fish Soc 128 328–338 Occurrence Handle10.1577/1548-8659(1999)128<0328:BIHPIB>2.0.CO;2
L Tuominen (1995) ArticleTitleComparison of leucine uptake methods and a thymidine incorporation method for measuring bacterial activity in sediment J Microbiol Methods 24 125–134 Occurrence Handle10.1016/0167-7012(95)00062-3
U.S. Environmental Protection Agency (1998) Contaminated Sediment Management Strategy. EPA 823-R-98-001
UNESCO (1994) Determination of POC and PON. Protocols for JGOFS Core Measurements 101–103
United States Environmental Protection Agency (1997) The Incidence and Severity of Sediment Contamination in Surface Waters of the United States. Vol 1. National Sediment Quality Survey. EPA 823-R-97-006
TIR Utvik S Johnsen (1999) ArticleTitleBioavailability of polycyclic aromatic hydrocarbons in the North Sea Environ Sci Technol 33 1963–1969 Occurrence Handle10.1021/es9804215
PC Metre Particlevan BJ Mahler ET Furlong (2000) ArticleTitleUrban sprawl leaves its PAH signature Environ Sci Technol 34 4064–4070 Occurrence Handle10.1021/es991007n
SB Weisberg WH Burton (1993) ArticleTitleSpring distribution and abundance of ichthyoplankton in the tidal Delaware River Fish Bull 91 788–797
AR Zimmerman EA Canuel (2000) ArticleTitleA geochemical record of eutrophication and anoxia in Chesapeake Bay sediments: anthropogenic influence on organic matter composition Mar Chem 69 117–137 Occurrence Handle10.1016/S0304-4203(99)00100-0
Acknowledgments
This work was funded by the NRL CORE Work Unit 61-7619/61153N MA013-01-44. The opinions and assertions contained herein are not to be construed as official or reflecting the views of the U.S. Navy or the naval service at large. The authors thank D.L. Kirchman for helpful comments on this manuscript, the captain, crew, and support personnel of the R/V Cape Henlopen, and Inactive Ships at the Philadelphia Naval Complex for logistical assistance during this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boyd, T., Montgomery, M., Steele, J. et al. Dissolved Oxygen Saturation Controls PAH Biodegradation in Freshwater Estuary Sediments. Microb Ecol 49, 226–235 (2005). https://doi.org/10.1007/s00248-004-0279-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-004-0279-0