Abstract
Background
Communicating bronchopulmonary foregut malformation is a rare anomaly characterized by a patent congenital communication between the esophagus or stomach and an isolated portion of the respiratory system. An esophagogram is taken as the gold standard for diagnosis. Compared with esophagography, computed tomography (CT) is more widely used and easily obtained, but CT findings have been described as nonspecific.
Purpose
To describe CT findings in 18 patients with communicating bronchopulmonary foregut malformation to assist with early diagnosis.
Material and methods
A retrospective review of 18 patients who had proven communicating bronchopulmonary foregut malformation between January 2006 and December 2021 was conducted. For each patient, the medical records, including demographics, clinical manifestations, upper gastrointestinal radiography, magnetic resonance imaging and CT findings, were reviewed.
Results
Among the 18 patients, there were 8 males. The right to left ratio was 3.5:1. An entire lung was involved in 10 patients, a lobe or a segment was involved in 7 patients and an ectopic lesion was located in the right neck in 1 patient. The isolated lung may arise from the upper esophagus, mid-esophagus, lower esophagus or stomach, which were detected in 1, 3, 13, and 1 patient, respectively. On chest CT, an extra bronchus which did not arise from the trachea was detected in 14 patients. Contrast-enhanced chest CT was performed in 17 patients, the isolated lung receiving its blood supply from the pulmonary artery in 13 patients, the systemic artery in 11 patients and both pulmonary and systemic arteries in 7 patients.
Conclusions
The presence of an extra bronchus, which does not arise from the trachea, highly suggests the diagnosis of communicating bronchopulmonary foregut malformation. Contrast-enhanced chest CT can provide accurate information regarding the airways, lung parenchyma and vascular structures that is useful to plan surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Communicating bronchopulmonary foregut malformation is a rare congenital anomaly defined by a patent congenital communication between the esophagus or stomach and an isolated portion of the respiratory tract. A lobar bronchus arising from the esophagus is described as an esophageal bronchus. If the main bronchus originates from the esophagus, it is termed the esophageal lung. In 1992, Srikanth et al. [1] reviewed 57 cases and proposed a system to classify communicating bronchopulmonary foregut malformation into four groups. Group I is associated with esophageal atresia and tracheoesophageal fistula and contains 2 subdivisions, group IA, defined as a whole lung involved, and group IB, defined as a portion of one lung involved. Group II is when the ipsilateral mainstem bronchus is absent from the trachea and the total sequestered lung (usually the right) originates from the esophagus. Group III is when an isolated part of the lung communicates with the esophagus. Group IV is when there is communication between a normal bronchial system and the esophagus, and the portion of the lung that is served by the communicating bronchus receives systemic blood supply. The early diagnosis of an esophageal bronchus can prevent complications such as aspiration and infection. A delayed diagnosis can result in recurrent pneumonia, bronchiectasis and pulmonary fibrosis, eliminating the option for lung salvage surgery as opposed to resection [2, 3]. The pediatric radiologist might be the first person to consider the diagnosis and plays an important role in advocating for appropriate imaging.
There are many imaging modalities for the diagnosis of communicating bronchopulmonary foregut malformation. With the more widespread use of magnetic resonance imaging (MRI), it is possible to make a diagnosis in the prenatal period. The presence of a T2 hyperintense structure arising from a lung lesion and directed toward the gastroesophageal junction strongly suggests the diagnosis of an esophageal bronchus [4, 5]. The volume of the affected lung is maintained in utero but decreases rapidly after birth, which leads to ipsilateral mediastinal shift and could potentially cause severe respiratory failure, so most patients experience recurrent aspiration pneumonia from birth [5]. An esophagogram is the gold standard in the diagnosis of communicating bronchopulmonary foregut malformation. However, communicating bronchopulmonary foregut malformation group IA is extremely difficult to diagnose due to the existence of esophageal atresia [6, 7]. For newborns and children who cannot cooperate with the test, there is also a high possibility of a false-negative result, and the potential risk of false aspiration. Compared with esophagography, computed tomography (CT) is more widely used and easily obtained. Consolidation, atelectasis, an abnormal carina and anomalous pulmonary anatomy can be adequately depicted on CT, but CT findings in the setting of an esophageal bronchus have been described as nonspecific in previous reports [2]. We describe 18 patients diagnosed with communicating bronchopulmonary foregut malformation and emphasize their CT imaging features to assist with making an early diagnosis.
Materials and methods
Our institutional review board approved this retrospective study with a waiver of informed consent. The charts of patients diagnosed with communicating bronchopulmonary foregut malformation between January 2006 and December 2021 were retrospectively reviewed. We classified all patients according to the system devised by Srikanth et al. [1]. The exclusion criteria were as follows: (1) patients were diagnosed with bronchopulmonary foregut malformation pathologically, but there was no communicating channel between the isolated lung and esophagus or stomach; (2) for patients meeting the criterion of communicating bronchopulmonary foregut malformation, there was no pre-surgery CT scan.
There were 18 patients enrolled in our study (8 males). The age at onset of symptoms varied from birth to 9 years old. Chest CT scans, contrast-enhanced CT, and upper gastrointestinal radiography were performed in 18, 17 and 13 patients, respectively. MRI examinations of the neck and chest were performed in 1 patient. For each patient, medical records, including demographics, clinical manifestations, upper gastrointestinal radiography, MRI and CT findings were reviewed. All imaging studies of the 18 patients were assessed by two pediatric radiologists (T.Y. and Z.L.H. with 13 and 11 years of experience, respectively).
Results
The clinical features are summarized in Table 1. The CT imaging features are summarized in Table 2.
Imaging findings
Communicating bronchopulmonary foregut malformation group IB
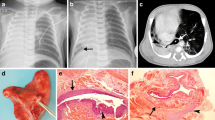
A 29-month-old boy was admitted to our hospital with suspected recurrence of a tracheoesophageal fistula and a history of primary repair of group C esophageal atresia in another hospital. There was a channel that originated from the mid-esophagus and ran into a mass in the left lower hemithorax on CT (Fig. 1). Pathologic examination of the specimen showed extralobar sequestration with a bronchogenic cyst.
a–e Images of a 29-month-old boy with a history of primary repair of type C esophageal atresia. a–c Axial CT images from the superior (a) mid (b) and inferior (c) chest on lung windows show a channel (arrow) originating from the mid-esophagus (arrowhead) and extending into a mass in the left lower hemithorax. There is a tube in the esophagus. d Anteroposterior chest radiograph from an upper gastroesophageal contrast study shows filling of the communicating channel, however the anomalous bronchus in the isolated lung is not visualized. e Axial contrast-enhanced CT image shows the isolated lung receiving its blood supply from the left pulmonary artery (arrow) and the splenic artery (not shown on the image)
Communicating bronchopulmonary foregut malformation group II
The lesion was located on the right side in all 10 patients. There was no associated esophageal atresia. A right main bronchus absent from the trachea was observed in 9 patients, and the shortened blind end of the right main stem bronchus was involved in 1 patient. The isolated lung arose from the lower esophagus in all 10 patients. The isolated lung was small and solid with no lobulation, therefore a hazy hemithorax, mediastinal shift with dextrocardia and compensatory hyperinflation of the left lung were present in all patients. The isolated lung may present as multiple heterogeneous cystic lesions (Fig. 2). An extra bronchus was demonstrated on CT in 8 patients, which did not arise from the trachea. Computed tomography showed the anomalous bronchus running toward the lower esophagus in all 8 patients. Among them, the opening of the distal esophagus was well visualized in 4 patients. In the 2 patients in whom an extra bronchus was not identified on CT, the isolated lung presented as macrocystic lesions in which the bronchus was difficult to identify. The branches of the pulmonary artery and vein feeding/draining to the malformation were hypoplastic, with systemic blood supply in 6 patients. The systemic feeding vessels arose from the thoracic aorta (n = 4), the infradiaphragmatic arteries (n = 3) and the infradiaphragmatic arteries including the celiac trunk (n = 1), abdominal aorta (n = 1) and one not detailed (n = 1). In one patient, there were multiple feeding vessels arising from the celiac trunk, thoracic aorta and right subclavian artery.
a–d Images of a 7-year-old boy suspected of having cystic lung disease. a Coronal CT minimum intensity projection reconstruction (MIP) image shows a small right lung, presenting with a “soap bubble” appearance, with the right main bronchus running toward the lower esophagus. b, c Coronal contrast-enhanced CT MIP reconstruction images show the sequestered lung receiving its blood supply from the infradiaphragmatic aorta (b, its origin not displayed) and from a hypoplastic right pulmonary artery (c, arrow) and draining into the right pulmonary vein (c, arrowhead). d 3D volume-rendered CT angiography (posterior view) shows the sequestered lung receiving both pulmonary (black arrow) and systemic (white arrow) blood supply, with the vein draining into the right pulmonary vein (arrowhead)
Tracheal stenosis at the level of the aortic arch was observed in all patients, and the anteroposterior diameter of the trachea decreased to 2 mm or even 1.5 mm. There was 1 patient in whom multiple anomalies occurred, including right lung agenesis, right-sided esophageal lung, congenital long segment tracheal stenosis with left pulmonary artery sling and an atrial septal defect (Fig. 3).
Computed tomography (CT) images of a 15-day-old female neonate with ongoing symptoms of tachypnoea after birth. a Axial CT image demonstrates hypoplasia of the right lung with ipsilateral mediastinal shift. b Axial contrast-enhanced CT image shows compression of the trachea by an aberrant left pulmonary artery (arrow), which arises from the right pulmonary artery and passes posterior to the left main bronchus and anterior to the esophagus. c Coronal contrast-enhanced CT maximum intensity projection reconstruction image shows hypoplasia of the right pulmonary artery (arrow) and vein (arrowhead). d, e Coronal (d) and sagittal (e) CT minimum intensity projection reconstruction images show a long segment congenital tracheal stenosis, from the level of the thoracic inlet to the carina, with the tracheal lumen narrowed to 2 mm. f 3-dimensional CT bronchography image shows a long segment congenital tracheal stenosis, an absent right main bronchus and an anomalous bronchus in the right thorax that runs toward the lower esophagus (arrow)
Communicating bronchopulmonary foregut malformation group III
All lesions were located in the thoracic cavity except one, which was located in the right neck (Fig. 4). Most patients present with segment involvement, but they may present with lobar involvement. The right upper lobe was involved in 1 patient in our study. The lesion was located in the lower left hemithorax in 3 patients and in the lower right hemithorax in 2 patients. The lesion may communicate with the upper esophagus, mid-esophagus (Fig. 5), lower esophagus or stomach (Fig. 6), which were detected in 1, 2, 3 and 1 patient, respectively, in our study. An extra bronchus which didn’t arise from the trachea was demonstrated in 5 patients on CT scan. The opening of the distal esophagus was well visualised in 2 patients. The communicating channel was not detected by either pre-surgery CT scan or a barium esophagogram study in 1 patient but was confirmed during the operation. Presentation as multiple cystic lesions was seen in 2 patients and mimicked esophageal diverticulum (Fig. 7) in 1 patient. The lesion was small and solid with atelectasis and consolidation in 5 patients. The systemic feeding vessels arose from the thoracic aorta (n =2) and infra-diaphragmatic arteries (n =1). Pulmonary artery supply was detected in 3 patients.
a–f, Images of a 1-year-and-7-month-old boy with symptoms of cough related to feeding, who also had a congenital scoliosis with vertebral deformity and anal atresia. a Axial computed tomography (CT) image shows an ectopic aerated bronchus (arrow) in the right cervical mass. b 3-dimensional (D) CT bronchography demonstrates an anomalous bronchus (arrow) running toward the cervical esophagus and poor visualization of the distal esophageal opening. c 3-D volume-rendered CT angiography shows that the isolated lung receives blood supply from the right subclavian artery (arrow). d Axial T2 fat suppressed magnetic resonance image shows the mass is composed of two parts with different signal intensities. e Oblique chest radiograph from an upper gastrointestinal (UGI) contrast study shows the isolated lung communicating with the upper esophagus (arrow). f Oblique chest radiograph from an UGI contrast study shows the isolated lung communicating with the upper esophagus via a second passage (arrow)
a–c Images of a 6-year-old girl with intermittent hematemesis for 4 days, fever for 6 days, cough for 1 month and black stool for 16 days. Axial computed tomography (CT) images from the superior (a) to inferior (c) chest on lung windows show an anomalous connection to the esophagus (black arrow), with atelectasis and consolidation in the associated lung. d Axial contrast-enhanced CT image shows the affected lung receiving blood supply from the contralateral pulmonary artery (arrow). e Coronal CT minimum intentsity projection reconstruction image clearly depicts the anomalous bronchus (arrow). f Coned anteroposterior chest radiograph obtained during an upper gastrointestinal study shows the anomalous bronchus (arrow) arising from the esophagus, confirming the diagnosis of esophageal bronchus
a–e Images of a 6-month-old girl with symptoms of recurrent vomiting after birth, with an associated duodenal septum resected during a second surgery. a Axial computed tomography (CT) image shows a mass in the right lower thorax with the air bronchogram sign. b Coronal CT minimum intensity projection reconstruction image showing a tubular air-filled fistula (arrows) running downward and toward the stomach. c Anteroposterior chest radiograph obtained during an upper gastrointestinal study shows the anomalous bronchus (arrow) originating from the gastric fundus and passing into a mass in the right lower lobe, which was confirmed at operation to be intralobar sequestration. d 3-dimensional (D) volume-rendered CT angiography (anterior view) shows the mass receiving abnormal systemic blood supply from the celiac trunk. e 3-D volume-rendered CT angiography (posterior view) shows the mass receiving abnormal systemic blood supply (arrowhead), with the vein draining into the right inferior pulmonary vein (arrow). A artery, R right
a–f Images of a 3-year-and-4-month-old girl diagnosed with leukemia, with fever and chronic cough. a Anteroposterior (AP) radiograph shows an opacity in the right lower hemithorax. b AP chest radiograph performed approximately 1.5 months after the image in (a) shows that the lesion in (a) has been replaced by an air-filled cyst (arrows). c Axial computed tomography (CT) image shows the cyst filled with iodinated contrast which was taken orally before the CT scan. d Axial CT obtained 6 days after the initial CT scan (c), shows an air bronchogram in the lesion. e An AP chest radiograph obtained during an upper gastrointestinal study shows the lesion communicating with the esophagus, mimicking an esophageal diverticulum. f An axial CT image obtained 5 months later, shows a marked decrease in diameter of the cyst and an anomalous bronchus (white arrow) running toward the lower esophagus (black arrow)
Discussion
The embryogenesis of communicating bronchopulmonary foregut malformation is not clear, it is treated as a sub-type of bronchopulmonary foregut malformation including congenital anomalies associated with the development of the foregut, pulmonary airways and vascular components [8]. The clinical presentation of communicating bronchopulmonary foregut malformation is not unique. Symptoms can present anytime from birth to adulthood and can include respiratory distress, cough during feeding and recurrent pneumonia. Cough during feeding and recurrent pneumonia were detected in 12 (66.7%) patients. However, certain groups of patients, especially groups I and II, have esophageal atresia or have an entire lung affected, and these groups are more likely to present very early in life. Most group IA patients develop respiratory distress shortly after birth due to esophageal atresia and a dysfunctional unilateral lung [3, 6, 7, 9]. The right lung was more commonly involved, with the right to left ratio being 14 to 4. Patients with bilateral disease [3, 10, 11] are rare, and there were no patients with bilateral disease in our study. The lower esophagus or the gastroesophageal junction were involved in 13 patients and are the most common sites of communication, as reported previously [1,2,3,4].
Communicating bronchopulmonary foregut malformation is a rare congenital anomaly in which the isolated lung communicates with the esophagus or stomach. It may be an intralobar or an extralobar isolated lung. The extralobar sequestration is wrapped with independent pleura, so it usually presents as a solid lesion and is asymptomatic. However, it becomes inflamed when it communicates with the esophagus or stomach. Gastric or esophageal reflux through the communication could contribute to inflammation of the isolated lung tissue, which can be greatly relieved by feeding through a nasoduodenal tube in infants. N-butyl 2-cyanoacrylate has been used as palliative treatment to occlude the bronchoesophageal fistula [6].
The chest radiograph in communicating bronchopulmonary foregut malformation may show a number of features, including persistent lower lobe consolidation, pulmonary hypoplasia, varying degrees of atelectasis or a “soap-bubble” appearance with ipsilateral mediastinal shift [10]. We show similar findings. For such patients, the diagnosis of communicating bronchopulmonary foregut malformation should be suspected and further evaluated.
Esophagography remains the gold standard for diagnosing communicating bronchopulmonary foregut malformation. The fistula is delineated by a positive contrast agent (barium or water-soluble iodine contrast agent) in esophagography and by a negative contrast agent (air) on CT. The existence of air in the esophagus or stomach enables the anomalous bronchus to be detected on CT. Identification of all the bronchi arising from the trachea is the first step, and the presence of “an extra bronchus” highly suggests the nature of the esophageal lung or bronchus. If the opening at the esophageal end is filled with air, the diagnosis of communicating bronchopulmonary foregut malformation can be made based on CT findings. This sign was detected in 7 (38.9%) patients in our study. For patients with an obscure opening at the esophageal end, we noticed that the anomalous bronchus ran toward the lower esophagus or stomach, rather than the hilum, and the cross point with the trachea was lower than the actual or presumed tracheal carina, which in turn indicated that it could not arise from the trachea. Therefore, a diagnosis of communicating bronchopulmonary foregut malformation was also highly likely. This sign was detected in 7 (38.9%) patients in our study. For such patients, an esophagogram should be undertaken to confirm the diagnosis. Postprocessing technology (such as minimum intensity projection, volume render reconstruction, curved planar reformation) contributes to the recognition of anomalous bronchi [6, 7, 12, 13].
Patients with an entire lung involved present with an opaque hemithorax with ipsilateral mediastinal shift. In neonates, the most common causes of these radiographic findings are lung collapse, severe pulmonary hypoplasia and pulmonary agenesis, but communicating bronchopulmonary foregut malformation should also be suspected [2]. Chest radiography does not provide sufficient information, and a chest CT is necessary. The right main bronchus is absent, so the presence of a consolidated or cystic lung confirms extralobar pulmonary sequestration. The existence of air (even just a little) in the isolated lung means that it must communicate with the upper digestive tract, so a diagnosis of communicating bronchopulmonary foregut malformation should be suspected. For patients with a lobe or segment involved, it may be easier to trace along the esophagus in an attempt to find a channel extending into the lung parenchyma. For patients with esophageal atresia and tracheoesophageal fistula, preoperative esophagogram cannot be performed, but chest CT can be useful for achieving an early preoperative diagnosis [9].
For our group II patients, an entire lung was involved, and the isolated lung drew arterial supply from the pulmonary artery in all 9 patients undergoing contrast-enhanced CT; this is seldom seen in extralobar pulmonary sequestration [14]. The term “esophageal lung” defines its nature well. All these patients had lesions that were located on the right side, and only 4 patients have been reported to have lesions located on the left [3]. There was 1 patient presenting with right lung aplasia rather than lung agenesis, which is rare. The supply and drainage of the affected lungs were similar to those of the normal lung in most patients, although they were hypoplastic, which is in accordance with previous reports. A systemic blood supply was found in 6 (60%) patients. Blood may drain into the azygos vein [1, 3], but there was no patient with this in our study. Drainage was via the contralateral pulmonary vein in 1 patient. The right main bronchus was absent from the trachea. The esophageal lung was small, presenting as a solid or cystic mass with ipsilateral mediastinal shift. The anomalous bronchus can be easily recognized in the solid mass. However, there were two patients presenting with multiple cystic lesions with a “soap-bubble” appearance, in which the anomalous bronchus was poorly visualized. Under such circumstances, the normal distribution of gas in the gastrointestinal tract will help to differentiate it from diaphragmatic hernia. The presence of air in the extralobar isolated lung suggests communication with the gastrointestinal tract, so a communicating bronchopulmonary foregut malformation should be suspected and can be confirmed by an esophagogram.
In our study, CT manifestations of the patients in group III were more variable than those in group II. In most patients, the lesion was located in the thoracic cavity, but there was 1 patient with an ectopic lesion located in the right neck in our study. This is the first patient with two adjacent passages communicating with the cervical esophagus, which was revealed by esophagogram and later confirmed by surgery. It may be difficult to detect the anomalous bronchus arising from the mid-esophagus. For such cases, trace along the esophagus and the communicating channel may be depicted by the negative contrast agent (air), just as in the 2 cases in our study. A similar case has been reported [2]. There was one patient in whom the right upper lobe was involved and the anomalous bronchus did not arise from the trachea but ran downward and toward the lower esophagus (rather than the upper or middle esophagus). In another patient who had a diaphragmatic hernia, the communication channel was not shown by CT or esophagogram; perhaps it was tiny and compressed by adjacent herniated tissues; and the inflammation around the channel also contributed to its occlusion. There has been a case reported in whom the esophageal bronchus was visualized by prenatal MRI, but after birth, it was not visualized by either CT or barium esophagography [4]. The involved lung may present as multiple cystic lesions and may be filled with air or fluid. If the lesion is filled with fluid from the outset, it may be easily missed in the chest radiograph, and present as new pathology on a subsequent radiograph. The abnormal blood supply from the systemic artery is helpful in identifying the nature of pulmonary sequestration.
Group IB is rare and difficult to diagnose early. To the best of our knowledge, there are only 6 patients, including one of ours [3, 15], who was the first patient with a lesion located on the left. Most patients were diagnosed after their operation for esophageal atresia/tracheoesophageal fistula. Unfortunately, our cohort does not include any patients in group IA. We believe the advantage of CT in the detection of the anomalous bronchus in group IA patients only holds true so long as there is air in the gastrointestinal tract.
Group IV is rare; diagnosis can only be made after comprehensive evaluation and to date, only 3 patients have been reported. There were no Group IV patients in our study. Further imaging studies such as esophagogram, bronchography and arteriography can help in making a diagnostic evaluation [3].
Computed tomography and MRI are both reliable imaging modalities for demonstrating the anomalous artery supplying the isolated lung. The advantages of MRI include high soft tissue resolution and no radiation, but MR is limited in assessing lung parenchymal abnormalities. The advantages of multidetector CT include high spatial resolution and fast acquisition times. Once an axial CT dataset is acquired, 3-D reformations can be constructed of the lung, airway and vascular structures. Multidetector CT is more sensitive in detecting small systemic vessels than MRI, especially in small children, and allows evaluation of the lung parenchyma [16]. Multidetector CT is the technique of choice, especially in children with airway obstruction related to cardiovascular compression [17]. Given that children are more sensitive to radiation, a low-dose scanning scheme is adopted in our hospital to keep the radiation dose as low as possible. The systemic artery may arise from the celiac trunk, abdominal aorta or splenic artery, so contrast-enhanced CT axial scanning should extend to just below the level of the renal arteries, otherwise the origin of the supplying artery may not be clearly displayed. We have been confronted with such a problem in one patient.
Bronchopulmonary foregut malformation without tracheobronchial stenosis may be adequately managed by resecting the involved lung or by preservation of the lung by tracheobronchial reconstruction [2, 3, 9]. However, surgical management becomes extremely difficult when tracheobronchial stenosis is present. Tracheobronchial stenosis may be congenital or acquired, focal or diffuse. It may lead to life-threatening airway obstruction dependent on the length, location and severity of tracheal narrowing. Congenital tracheal stenosis is characterized by the presence of complete tracheal rings along the trachea of different lengths, presenting as a circular constriction with a luminal diameter as small as 1–2 mm. Slide tracheoplasty can be safely performed for the correction of congenital tracheal stenosis. The combination of communicating bronchopulmonary foregut malformation and congenital tracheal stenosis is extremely rare. There have only been a few reports to date [12, 18]. Computed tomography, especially contrast-enhanced CT, is a good imaging modality for evaluating the extent of stenosis as well as assessing surrounding structures and vasculature.
Communicating bronchopulmonary foregut malformation may be associated with congenital malformations such as cardiovascular anomalies, congenital diaphragmatic hernia, vertebral deformities, anorectal malformation and VACTERL associations (vertebral anomalies, anal atresia, cardiac anomalies, tracheoesophageal fistula, esophageal atresia, renal anomalies and limb anomalies). Congenital pulmonary airway malformation, bronchogenic cyst, congenital tracheal stenosis, duodenal septum and intestinal malrotation were also found in our study. Some congenital lung malformations are hybrid lesions, pathologically composed of two coexisting lesions. An overlap between pulmonary sequestration and congenital pulmonary airway malformation was noted in 2 patients. Congenital lung malformations may share a common embryological origin, in particular, in utero airway obstruction; the lesion type is dependent on the timing, level and severity of obstruction [19,20,21].
The current study has some limitations. First, there were no patients in groups IA and IV. Therefore, the value of CT in the diagnosis of communicating bronchopulmonary foregut malformation needs further evaluation in these groups. Second, some patients did not undergo surgery, with 6 (33.3%) patients receiving only medical treatment.
Conclusion
In patients presenting with cough during feeding and recurrent pneumonia, a chest CT should be undertaken. The presence of an extra bronchus, which does not arise from the trachea, is highly suspicious of a communicating bronchopulmonary foregut malformation. In most cases, the anomalous bronchus is well demonstrated by the negative contrast medium (air), even when the opening at the distal esophagus is obscure. The diagnosis can be confirmed by an esophagogram. A pre-operative contrast-enhanced chest CT provides accurate information about the airway, lung parenchyma and vascular structures, that is useful to plan surgery. Computed tomography also offers some clues towards the differential diagnosis. Tracheobronchial stenosis, which may lead to life-threatening airway obstruction, should be excluded.
References
Srikanth MS, Ford EG, Stanley P, Mahour GH (1992) Communicating bronchopulmonary foregut malformations: classification and embryogenesis. J Pediatr Surg 27:732–736
Colleran GC, Ryan CE, Lee EY et al (2017) Computed tomography and upper gastrointestinal series findings of esophageal bronchi in infants. Pediatr Radiol 47:154–160
Yang G, Chen L, Xu C et al (2019) Congenital bronchopulmonary foregut malformation: systematic review of the literature. BMC Pediatr 19:305–311
Partridge EA, Victoria T, Coleman BG et al (2015) Prenatal diagnosis of esophageal bronchus -first report of a rare foregut malformation in utero. J Pediatr Surg 50:306–310
Kobayashi H, Miyakoshi K, Koinuma G (2019) Communicating bronchopulmonary foregut malformation: Volume change of the affected lung after birth. Pediatr Pulmonol 54:669–671
He QM, Xiao SJ, Zhu XC et al (2015) Communicating bronchopulmonary foregut malformation type IA: radiologic anatomy and clinical dilemmas. Surg Radiol Anat 37:1251–1256
Boersma D, Koot BG, van der Griendt EJ et al (2012) Congenital bronchopulmonary foregut malformation initially diagnosed as esophageal atresia type C: challenging diagnosis and treatment. J Pediatr Surg 47:e59-62
Choo JY, Hwang J, Lee JH, Lee KY (2017) Bronchopulmonary foregut malformation presenting as extralobar pulmonary sequestration associated with a bronchogenic cyst: an unusual clinical and radiological feature in an adolescent patient. J Thorac Dis 9:E632-635
Chung JH, Lim GY, Kim SY (2014) Esophageal lung diagnosed following the primary repair of esophageal atresia with tracheo-esophageal fistula in a neonate. Surg Radiol Anat 36:397–400
Murray ME, Given-Wilson RM, Christopher JA, Jeffrey IJ (1994) Bilateral communicating bronchopulmonary foregut malformations in an infant with multiple congenital anomalies. Pediatr Radiol 24:128–130
Singal AK, Kumar VR, Rao M et al (2006) Bilateral communicating bronchopulmonary foregut malformations in a child. Ann Thorac Surg 82:330–332
Takamizawa S, Yoshizawa K, Machida M et al (2012) Successful tracheobronchial reconstruction of communicating bronchopulmonary foregut malformation and long segment congenital tracheal stenosis: a case report. J Pediatr Surg 47:E41-46
Alsaadi A, Alsufiani HA, Allugmani MD et al (2018) Esophageal lung with rare associated vascular and anorectal malformations. Radiol Case Rep 13:444–448
Verma A, Mohan S, Kathuria M, Baijal SS (2008) Esophageal bronchus: case report and review of the literature. Acta Radiol 49:138–141
Harumatsu T, Kaji T, Nagano A et al (2021) Successful thoracoscopic treatment for tracheoesophageal fistula and esophageal atresia of communicating bronchopulmonary foregut malformation group IB with dextrocardia: a case report of VACTERL association. Surg Case Rep 7:11–15
Lee EY, Siegel MJ, Sierra LM, Foglia RP (2004) Evaluation of angioarchitecture of pulmonary sequestration in pediatric patients using MDCT angiography. Am J Roentgenol 183:183–188
Lambert V, Signal-Cinqualbre A, Belli E et al (2005) Preoperative and postoperative evaluation of airway compression in pediatric patients with 3-dimensional multislice computed tomographic scanning: effect on surgical management. J Thorac Cardiovasc Surg 129:1111–1118
Tsugawa J, Tsugawa C, Satoh S et al (2005) Communicating bronchopulmonary foregut malformation: particular emphasis on concomitant congenital tracheobronchial stenosis. Pediatr Surg Int 21:932–935
Langston C (2003) New concepts in the pathology of congenital lung malformations. Semin Pediatr Surg 12:17–37
Riedlinger WFJ, Vargas SO, Jennings RW et al (2006) Bronchial atresia is common to extralobar sequestration, intralobar sequestration, congenital cystic adenomatoid malformation, and lobar emphysema. Pediatr Dev Pathol 9:361–373
Lee EY, Dorkin H, Vargas SO (2011) Congenital pulmonary malformations in pediatric patients: review and update on etiology, classification, and imaging findings. Radiol Clin North Am 49:921–948
Funding
The Special Fund of The Pediatric Medical Coordinated Development Center of Beijing Municipal Administration, No.: XTCX201814. Beijing Hospitals Authority’s Ascent Plan, Code: DFL20221002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, Z., Yu, T., Huang, J. et al. Computed tomography findings of communicating bronchopulmonary foregut malformation: a retrospective study of 18 patients. Pediatr Radiol 53, 1063–1075 (2023). https://doi.org/10.1007/s00247-023-05610-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-023-05610-z