Abstract
Functional imaging is playing an increasingly important role in pediatric radiology. Hybrid imaging techniques utilizing PET/CT (positron emission tomography/computed tomography), PET/MRI (positron emission tomography/magnetic resonance imaging), or SPECT/CT (single photon emission computed tomography/computed tomography) are now available in nearly every clinical practice. There are an increasing number of indications for the use of functional imaging, including oncologic and infectious indications, and it is essential to select and design the hybrid imaging protocol in order to optimize both the functional and anatomic components of the examination. Optimizing the protocol includes strategies for dose reduction, judicious use of contrast media and diagnostic quality imaging as appropriate, and for the greatest reduction in exposure to ionizing radiation, utilizing PET/MRI, whenever available. This review will provide an overview of hybrid imaging protocol considerations with a focus on oncologic and infectious indications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of functional imaging techniques in pediatric radiology is playing an increasingly important role in many clinical scenarios. PET/CT, PET/MRI, and SPECT/CT imaging is now performed in nearly every clinical practice. There are a variety of indications for the use of functional imaging, including neurologic, endocrinologic, orthopedic, oncologic, cardiac and infectious indications. The purpose of this review is to provide an overview of protocol considerations when designing a functional imaging study using one of the above hybrid imaging techniques, with a focus on oncologic and infectious indications.
Attenuation correction
Prior to reviewing the indications for PET and SPECT imaging, it is important to understand the role of attenuation correction for processing the imaging data. Attenuation correction accounts for a variety of factors influencing the PET image reconstruction. Viewed simply, different locations and different tissue attenuation values will influence the path traveled by the 511 keV annihilation photons following the positron emission event, thereby influencing the accuracy with which the location of the positron emission can be determined [1, 2]. Historically tissue attenuation correction factors were determined using a 68Ge rod source that rotated around the patient, creating a transmission scan that was used for attenuation correction. With the development of PET/CT, the attenuation values of the different tissues could be directly calculated from the Hounsfield unit (HU) density measurements obtained on CT. The CT data are acquired over the same field of view (FOV) as the PET data. A multi-linear transformation is then applied to estimate the linear attenuation coefficient at 511 keV (μ511) from the CT HU density numbers to yield a μ map, which is the spatial representation of tissue attenuation values. After the transformation is applied, the data are down-sampled and adapted to the resolution of the PET data and then used to generate a CT-based attenuation correction dataset, which is applied to the PET emission data [1, 2].
Attenuation correction CT examinations are typically performed using a non-diagnostic quality CT technique without intravenous contrast, although several approaches are possible. The attenuation correction CT can be done using an extremely low dose technique, which provides little anatomic detail but is adequate to accomplish attenuation correction, a low-dose, non-diagnostic quality CT technique, with or without contrast, that provides sufficient anatomic detail for co-localization, or using a standard diagnostic quality CT technique with intravenous and oral contrast. Depending on a particular patient’s imaging requirements, the imaging protocol should be designed to balance the need for diagnostic quality information with a desire to minimize the delivery of excess radiation to the extent possible [3,4,5], although it should be noted that reducing the tube voltage (kVP) of the attenuation correction CT below 100 kVp in larger patients may lead to under-correction of the PET data [1].
The development of PET/MR presented new challenges for attenuation correction of the PET data. Whereas CT-based attenuation correction allows tissue attenuation values to be derived from CT Hounsfield unit density measurements, MR-based attenuation correction relies on a 2-point Dixon technique [2]. The 511 keV μ map derived from this technique creates a 4 compartmental model with air, lung, fat, and soft tissue represented. There are some well recognized challenges inherent in this technique, including misclassification of high-density structures such as bone and truncation artifacts that occur from using a smaller MR image acquisition field-of-view as compared to the PET imaging field-of-view.
Despite these technical differences, when directly comparing standardized uptake value (SUV) measurements between PET/CT and PET/MR, these two PET imaging techniques are considered essentially interchangeable [6, 7]. Whereas the use of PET/MR was initially considered exploratory, most clinical protocols now allow either PET/CT or PET/MR imaging to be performed, depending on institutional preference and scanner availability, with the many advantages of PET/MRI being widely recognized [6, 8]. Indeed, in some parts of Europe PET/MR is ubiquitous and its use is encouraged, while PET/CT imaging is less frequently performed, particularly in children [9].
SPECT/CT techniques also offer the possibility of performing CT-based attenuation correction of the SPECT imaging data. This is most useful for quantitative SPECT/CT imaging [10]. For most pediatric indications CT-based attenuation correction is not essential for interpreting the SPECT imaging data and the CT data is largely utilized for anatomical co-localization. As such, the decision to perform a diagnostic quality CT as part of the SPECT/CT acquisition or to include intravenous contrast in the low-dose attenuation correct exam will depend on the clinical indication and intended diagnostic purpose of the examination [11].
Radiopharmaceuticals
18F-FDG is the most commonly used radiopharmaceutical for clinical PET imaging in pediatrics, although increasingly other isotopes are becoming clinically available or being validated for specific indications (Table 1). Notable among these is 68Ga-DOTATATE, a radioconjugate consisting of the somatostatin analogue tyrosine-3-octreotate (Tyr3-octreotate or TATE) conjugated to the bifunctional chelator DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid). 68Ga-DOTATATE is seeing increased use for imaging pediatric neuroendocrine tumors, including neuroblastoma [12, 13], as well as other somatostatin receptor expressing tumors such as medullary thyroid cancer. Other clinically available, but less frequently used PET radiopharmaceuticals include 18F-sodium fluoride (18F-NaF), 18F-FLT (3'-deoxy-3'-18F-fluorothymidine), and 18F-DOPA (18F-dihydroxyphenylalanine). 64Cu-SARTATE™ [14], a 64Cu-labeled radioconjugate of octreotate (Tyr3-octreotate) and the multi-functional sarcophagine cage-based chelator MeCOSar, and 18F-MFBG (18F-meta-fluorobenzylguanidine) [15] are undergoing active clinical evaluation for imaging of somatostatin receptor bearing tumors and neuroblastoma, respectively.
For SPECT/CT imaging in pediatric oncology,123I-MIBG (123I-meta-iodobenzylguanidine) is the gamma-emitting radiopharmaceutical most commonly used clinically, mainly for evaluation of patients with neuroblastoma [16]. 99mTc-sestamibi (99mTc-methoxyisobutylisonitrile) is used for localizing parathyroid adenomas, while 123I radioiodine is used in the evaluation of patients with thyroid cancer. 99mTc-sulfur colloid is used for sentinel lymph node detection, although in practice there are only selected indications (e.g., melanoma and certain sarcoma subtypes, such as rhabdomyosarcoma) where sentinel lymph node detection plays a role in disease management [17]. 67 Ga-citrate has been completely replaced by 18F-FDG for lymphoma imaging, while 111In-Octreotide has been largely replaced by 68Ga-DOTATATE.
Protocol strategies for optimizing the hybrid imaging technique
Functional imaging is primarily used for disease staging and response assessment based on data showing increased sensitivity compared to conventional imaging techniques, which in turn influences disease stage, risk stratification and treatment strategy [18, 19]. Interim response assessments provide an important biomarker allowing response-based treatment decisions to be made, distinguishing early treatment responders from non-responders. There is little role for the routine use of functional hybrid imaging techniques in off-therapy surveillance, although MIBG scintigraphy is still included in surveillance imaging recommendations for advanced stage neuroblastoma.
Optimizing the hybrid imaging technique to best accomplish the aims of staging and treatment response assessment focuses on reducing unnecessary exposure to ionizing radiation, eliminating the length of sedation and in some instances the requirement for multiple sedations, and reducing or eliminating repeat administrations of intravenous contrast [4, 5]. Depending on the indications for the functional imaging exam it may not be necessary to perform a dedicated diagnostic quality cross-sectional imaging evaluation, relying on the attenuation correction CT or MR images for anatomical reference, reserving diagnostic quality imaging for the characterization of specific functional imaging abnormalities, when needed. Improving the quality of the low dose attenuation correction CT images, for example by including intravenous contrast during the attenuation correction CT acquisition, in many instances may be adequate for colocalizing abnormalities detected by PET and obviate the need for a diagnostic quality CT [5]. Furthermore, it may not be necessary to obtain a separate diagnostic CT or MRI if the corresponding FDG PET imaging is normal.
Precise determination of the effective dose deriving from the PET and CT components of the exam will vary depending on CT technique, injected dose and type of radiopharmaceutical. For FDG PET/CT the dose delivered by the radiopharmaceutical is typically 5.2–7.4 mSv, based on North American and EANM consensus guidelines, while the effective dose from the CT component of exam can range from 1–2 mSv for a small child using a non-diagnostic low dose attenuation correction CT to as much as 10–12 mSv for a larger child when diagnostic quality CT imaging is incorporated into the PET/CT exam [1, 3]. The importance of having a strategy for dose reduction is underscored by comparison with estimates of adult effective dose determined for various radiopharmaceuticals using standard adult PET/CT parameters, in which the major contributor to total ED for body PET/CT protocols is CT [20].
We have focused on incorporating diagnostic imaging techniques, when required, into the PET/CT imaging exam, using a multi series approach in which both non-diagnostic and diagnostic quality CT imaging data are merged into a single study, which can be used for both diagnostic interpretation and for attenuation correction purposes [3, 5]. This provides both high quality CT imaging for anatomical localization of PET abnormalities, while still allowing areas outside of the region of interest to be imaged with a lower dose non-diagnostic quality CT.
All of the major vendors offer variations of this technique, which allows CT-based attenuation correction protocols to be optimized based on selected, user-defined regions of interest [21]. At Boston Children's Hospital PET/CT exams are performed using a Siemens Biograph mCT Flow PET/CT scanner equipped with 64 slice CT. Low dose attenuation correction CT images utilize CARE kV dose reduction and CARE Dose 4D tube current modulation. For patients having diagnostic CT imaging as part of the PET/CT examination, attenuation correction CT images (reference mAs 20–35) are acquired and combined with diagnostic CT images (reference mAs 150) to generate a single merged dataset, which is used for both attenuation correction and diagnostic interpretation [5] (Table 2, Fig. 1). At Boston Children's Hospital, more than 50% of the PET/CT imaging studies performed since 2016 have used the multi-series technique, incorporating a diagnostic CT into some portion of the PET/CT exam.
Whole-body PET/CT with diagnostic CT of chest, abdomen and pelvis in an 8-year-old with posterior mediastinal Ewing sarcoma. The diagnostic CT (Dx) portion of the exam (chest, abdomen and pelvis) is combined with the low attenuation correction CT (Ac) imaging of the head/neck and extremities (a) to produce a merged whole body composite image (b). The merged CT data is used to process the PET data, allowing creation of fused PET/CT images (c) and whole-body maximum intensity projection (MIP) images (d). There is no appreciable FDG uptake above background in the residual mediastinal mass (e, f, arrows), indicating a response to therapy. The diagnostic CT (e) is interpreted separately and confirms the mass has decreased substantially in size compared to the baseline exam (g, h)
An alternative strategy for dose reduction with PET imaging is PET/MR. The MRI acquired as part of the PET imaging examination, in addition to eliminating the additional exposure to ionizing radiation from PET/CT, also provides the added benefit of superior soft tissue characterization by MR as compared to CT [8]. The greatest challenge with PET/MR is protocol length. The basic PET/MR protocol (Fig. 2) is frequently supplemented with additional MR sequences [22] which can add as much as 60 min to the length of the exam. For young children in particular, this may result in an undesirable increase in sedation, and close attention to overall exam time is needed when developing the combined PET and MR imaging protocols.
Basic PET/MRI protocol. Schematic diagram with example images showing both standard core PET/MRI sequences, with options for additional focused MRI imaging. Adapted from Gatidis and Schäfer [22]. Abbreviations: AC attenuation correction, PET positron emission tomography, T2 Cor T2-weighted coronal, T2 tra T2-weighted axial, CE T1 Contrast-enhanced T1 weighted, T2-FLAIR T2-FLuid Attenuated Inversion Recovery, ADC apparent diffusion coefficient
Recently, total body PET/CT was introduced by United Health Care, in which ultra-high-resolution digital PET/CT is combined with a 194 cm axial PET field of view (FOV), as compared to the standard ~ 26 cm axial PET FOV [23]. This enables the entire body to be scanned in one bed position, as compared to multiple sequential acquisitions from the smaller FOV scanners. This allows the simultaneous acquisition of the PET imaging data from the entire body without the need for either continuous table feed or multiple overlapping bed positions, resulting in a dramatic reduction in imaging time. For pediatric imaging, this could potentially reduce or eliminate the need for sedation, or allow for a reduction in administered dose, given the increased sensitivity of the digital detectors. In addition to total body PET/CT, which has specific space and computer processing requirements that may limit the feasibility of this system in certain locations, a smaller, long axial field-of-view system has recently been approved for use. This system offers a 106 cm axial PET FOV, which for many young pediatric patients may be adequate for allowing the entire PET imaging examination to be performed with a single acquisition [24]. This smaller long axial field-of-view system system also has fewer space constraints, and in most instances can replace existing equipment without the need for enlarging the existing PET imaging suite. In both cases, these improvements in scanner technology offer the advantages of decreased acquisition time and reduced need for sedation, potential reductions in administered dose, and improved PET image quality resulting from the use of digital image detectors as compared to conventional lutetium oxyorthosilicate (LSO) detector systems, and are likely to dramatically transform the approach to PET imaging in pediatrics.
As noted above, in practice the use of attenuation corrected SPECT images provides little additional benefit in terms of improvements in image quality, but can be helpful if quantitative SPECT imaging is desired. However, as with PET/CT, the inclusion of intravenous contrast for the attenuation correction CT used during the SPECT/CT acquisition can be helpful for co-localizing uptake in very small structures or demonstrating the association of MIBG uptake with a particular anatomic region such as the adrenal gland, allowing the uptake to be cross-referenced against other imaging modalities such as MRI. In other instances, a contrast enhanced attenuation correction CT may provide sufficient anatomic detail to allow the SPECT imaging data to be interpreted with confidence, eliminating the need for any additional cross-sectional imaging (Fig. 3). As with PET/CT, optimization of the SPECT/CT examination should include a review of exam length, necessity of acquiring images from multiple bed positions, choice of CT imaging coverage, use of IV contrast, and need for diagnostic CT image quality [11].
14 day-old with prenatally diagnosed right adrenal mass and elevated catecholamines. MIBG SPECT/CT was obtained without sedation to assess for metastatic disease in the setting of presumed neuroblastoma. The right adrenal mass seen on the contrast enhanced low dose attenuation correction CT (a) shows intense MIBG uptake on the axial fused SPECT/CT (b) and coronal SPECT MIP (c) images. No other sites of MIBG avid disease were seen and the patient was managed with expectant observation. By 2 years of age catecholamine levels were near normal and the adrenal mass had nearly resolved
Lung nodules: Lung nodules require particular attention for PET/CT, PET/MR and SPECT-CT imaging. Several studies in both children and adults have shown that large pulmonary nodules are readily detected by both PET/CT and PET/MR. However, for small nodules, due to the quiet breathing technique required for the PET and SPECT acquisition, small nodules can go undetected, as compared to their ready detectability on a suspended respiration, high quality diagnostic CT [25, 26]. In a review of 103 patients undergoing PET/CT who also had a separate diagnostic chest CT, pulmonary nodules were identified in 61 (59%) of the patients (M. Robertson; S.D. Voss, unpublished). Of those patients with pulmonary nodules, concordant findings (all nodules were present on both the attenuation correction CT and the diagnostic chest CT) in only 40 of the 61 patients (66%). Approximately 16% of the nodules seen on the diagnostic CT were not detected based on the attenuation correction CT. Similar results have been published for PET MRI, using a free breathing technique. Nodules less than 4 mm in size had a low rate of detectability, whereas larger nodules greater than 4 mm in size were readily detected [27, 28].
Based on this, the recommendation is to obtain a diagnostic quality chest CT with suspended respiration, when possible, for patients at risk of pulmonary metastatic disease, such as those patients with pediatric sarcomas.
Patient preparation and sedation
In addition to the technical factors described above, attention must also be directed at individualizing the functional imaging examination for patients of varying ages, anxiety levels and maturity. Along with the concerns about ionizing radiation noted earlier, over the past 5 years there has been an increasing awareness of the potential risks of sedation and anesthesia in the pediatric population. This is particularly important in children undergoing functional imaging studies, where exam times may last from 30 min to 2–3 h and sedation is often required to complete the examination. As a general rule, patients under the age of 6 years may have difficulty lying still for a lengthy examination without some form of monitored sedation or anesthesia, although studies in MRI have demonstrated success when consultation with a child life specialist takes place prior to the study [29]. The challenge in extrapolating the success of try-without programs used in MRI to PET/CT and SPECT/CT lies in the fact that injecting a child with a radiopharmaceutical obliges us to complete the examination, without the luxury of re-scheduling, should a try-without sedation attempt fail. In practice we have found that encouraging a proactive discussion between the nuclear medicine physician, referring clinician, parents, nuclear medicine technologists and nursing staff prior to scheduling the study is most successful in identifying which children will require sedation and anesthesia and which can go without.
Indications for PET/CT, PET/MRI, and SPECT/CT imaging
Oncologic applications
The pediatric malignancies for which FDG PET imaging is now in routine use include Hodgkin and non-Hodgkin lymphoma, as well as post-transplant lymphoproliferative disease (PTLD), Ewing sarcoma, osteosarcoma, and rhabdomyosarcoma, and increasingly non-rhabdomyomatous soft tissue sarcomas and osteosarcoma [18, 19, 30, 31]. For each of these tumor types PET imaging plays an important role in both staging and response assessment, with increased sensitivity and specificity relative to conventional imaging techniques such as CT and MRI.
FDG PET/CT is used for imaging patients with neurofibromatosis type-1 (NF-1), where there is concern for malignancy, and complements routine whole-body MRI screening in the evaluation for potential degeneration of plexiform neurofibromas into malignant peripheral nerve sheath tumors (MPNST) [32, 33].
FDG PET/CT has also been increasingly used for staging patients with Langerhans' cell histiocytosis, and has been shown to complement skeletal survey, particularly for assessing visceral sites of disease [34] (Fig. 4). Indeed, in one recent study, it was shown that portions of the routine skeletal survey could be eliminated, particularly the axial skeleton imaging, based on improved sensitivity and specificity for detecting disease by FDG PET/CT [35]. Whole body MRI has also been shown to have higher sensitivity for detecting skeletal disease than plain radiography and skeletal scintigraphy, and in some centers, particularly in Europe, whole MRI is preferred over skeletal survey for staging LCH [36]. The opportunity to perform simultaneous FDG PET/CT and whole-body MRI on integrated PET/MRI scanners is appealing, however there are no large prospective studies directly comparing the respective sensitivity and specificity of these techniques.
18F-FDG PET/CT in an 11-year-old with Langerhans Cell Histiocytosis (LCH). Whole-body coronal maximum intensity projection (MIP) image (a) shows intense FDG uptake (solid arrow) in the left proximal femur lytic lesion seen radiographically (solid arrow) (b). Axial fused 18F-FDG PET/CT (c) and contrast enhanced axial attenuation correction CT (d) show focal intense FDG uptake in the thymus (open arrow, a, c, d), indicating an additional site of visceral LCH disease that could not be detected by skeletal survey, emphasizing the importance of 18F-FDG PET imaging in staging LCH [35]
Neuroendocrine tumor imaging, which had historically been accomplished with 111In-octreotide, is now almost exclusively imaged with 68Ga-DOTATATE, although in some neuroendocrine tumor subtypes FDG PET still plays a role in disease management. Although neuroendocrine tumors are relatively rare in children, with the recognition of an increasing number of cancer predisposition syndromes (CPS) that include neuroendocrine neoplasms, the use of 68Ga DOTATATE PET/CT and PET/MRI is increasing [12, 13]. Importantly 68Ga-DOTATATE and 18F-FDG PET imaging may be complementary to one another, particularly among patients with CPS in whom histologically and functionally distinct tumors are present (Fig. 5). Where distinct tumor phenotypes, based on differences in tumor glucose metabolism and expression of somatostatin receptors, are identified by a combination of functional imaging techniques [12].
68Ga-DOTATATE and 18F-FDG PET/CT in 18-year-old girl with succinate dehydrogenase B (SDHB) germline mutation, metastatic gastrointestinal stromal tumor (GIST) and paraspinal paraganglioma. She had previously undergone resection of a gastric GIST. Coronal MIP 68Ga-DOTATATE PET/CT (a) shows focal update in a paraspinal paraganglioma (solid arrow), but no uptake in known hepatic lesions. Coronal MIP with 18F-FDG PET/CT (b) shows multifocal FDG-avid hepatic GIST metastases (open arrows) as well as uptake in the paraspinal paraganglioma, (solid arrow) emphasizing the complementary nature of these functional imaging techniques in patients with hereditary pheochromocytoma paraganglioma cancer predisposition syndromes [12]
FDG PET imaging currently plays relatively little role in the routine evaluation of neuroblastoma patients. 123I-MIBG planar scintigraphy and SPECT/CT are considered standard of care for both staging and response assessment [37, 38], and current guidelines do not recommend the routine use of 18F-FDG for staging neuroblastoma. Although there have been no prospective studies comparing FDG with 123I-MIBG scintigraphy, 18F-FDG PET/CT remains an important alternative to 123I-MIBG, particularly in the case of non-MIBG-avid neuroblastoma (~ 10% of the cases). Despite the limited use of FDG for imaging neuroblastoma, it has been shown that the majority of neuroblastoma cells, in addition to expressing the norepinephrine transport receptors targeted by MIBG, also express type-2 somatostatin receptors (SSTR-2) and there is an increasing evidence to suggest that 68Ga-DOTATATE may be more sensitive than 123I-MIBG for detecting metastatic disease in neuroblastoma [37, 39,40,41].
The challenge clinically relates to the fact that MIBG scintigraphy has been an integral part of neuroblastoma imaging for more than 2 decades and has been shown to be essential for staging, up front risk classification, and response assessment. Furthermore, based on evidence showing that treatment with131I-MIBG improves outcome for patients with advanced stage neuroblastoma [42,43,44], 131I-MIBG is now being incorporated into induction therapy regimens for patients with high risk MIBG-positive neuroblastoma, making it essential to establish whether patients have MIBG-avid disease prior to beginning treatment. With the development of 18F-MFBG [15] — the PET imaging counterpart to 123I-MIBG — it is likely that PET/CT and PET/MR will eventually replace 123I-MIBG SPECT/CT imaging for the majority of neuroblastoma patients. Whether SSTR-2 targeted imaging with either 68Ga-DOTATATE or 64Cu-SARTATE will see increasing use in these patients, particularly with availability of 177Lu-DOTATATE (LUTATHERA)® and 67Cu-SARTATE for receptor targeted therapy, remains to be seen.
For most other pediatric solid tumors there are no established indications or recommendations for the routine use of FDG PET/CT in either for staging or response assessment, although in certain clinical scenarios FDG PET is still used to complement existing imaging techniques. For example, in patients with malignant germ cell neoplasms and rising tumor markers FDG PET can localize occult sites of disease recurrence. In other situations, FDG PET can help distinguish between localized and metastatic disease and can aid in distinguishing residual disease from post-operative changes and scarring.
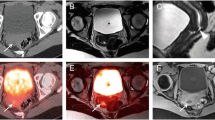
In addition to its use in neuroblastoma imaging, SPECT/CT is also used selectively for certain other indications such as parathyroid imaging, to improve the accuracy of localizing functional parathyroid adenomas (which often reside in ectopic locations) (Fig. 6), in the evaluation of thyroid cancer patients undergoing whole body imaging with 123I radioiodine, and to aid in sentinel lymph node localization for patients undergoing sentinel lymph node dissection [17]. Although the use of SPECT/CT for these indications is helpful, and in the case of parathyroid imaging is standard of care, in practice SPECT/CT is used far less frequently than PET/CT, owing both to a limited number of clinically useful radiopharmaceuticals and the inherently better spatial resolution of PET/CT compared to SPECT/CT.
SPECT/CT and ectopic parathyroid adenoma. Axial SPECT/CT images of the lower neck and upper chest (a, b) in a 12-year-old with hypercalcemia were obtained ~ 90 min after injection of 300 µCi 99mTc-Sestamibi. There is intense tracer uptake in the anterior/superior mediastinum consistent with ectopic parathyroid adenoma (a and b, arrow), corresponding to the enhancing mass in the anterior mediastinum on the subsequent axial contrast-enhanced chest CT (c, arrow). Pathology confirmed parathyroid adenoma
PET imaging and infection
Historically, infection imaging was performed with a 111In-labeled tagged white cell scan. The so-called WBC scan was very specific at identifying potential sources of infection, but had several limitations, including poor imaging characteristics inherent to the 111In radiotracer, poor sensitivity and relatively high radiation dose. With the development of FDG PET/CT, WBC scanning is used very infrequently. Based on high sensitivity and moderate specificity at identifying sites of metabolic activity, FDG PET/CT is increasingly being used to evaluate patients with fever of unknown origin. In one study, in 62% of the patients a cause of fever was identified [45]. In more than half of these patients a treatment modification was undertaken based on the PET imaging results. Because of its relatively high sensitivity, the negative predictive value of PET/CT is also high. That is, in the setting of fever of unknown origin, a negative FDG PET/CT scan offers assurance that an occult site of infection has not been overlooked.
In addition to evaluating patients with fever of unknown origin, FDG PET/CT has shown to be of value in the assessing patients with implanted hardware, such as spinal fusion rods, pacemakers, and similar devices, with the potential for infection around the hardware (Fig. 7) [46, 47]. Similarly, FDG PET/CT is playing an increasingly important role in the evaluation of pediatric cardiology patients with suspected endocarditis related to implanted cardiac devices, including prosthetic valves, vascular conduits, and pacemakers (J. Gillum, S.D Voss, unpublished). In some instances, an additional whole-body examination can be performed to complement the dedicated cardiac PET/CT when there is suspected systemic infection and evidence of sepsis elsewhere.
18F-FDG PET/CT for evaluation of hardware infection. 15-year-old with neurofibromatosis type 1(NF-1) and posterior spinal fusion presented with weight loss, elevated C-reactive protein and concern for infected hardware. 18F-FDG PET/CT coronal maximum intensity projection (MIP) image (a) and fused coronal PET and CT images (b) show intense uptake around the entire length of the hardware implant. At surgery, abscess pockets were found around the fusion rods, which were removed. Cultures revealed Staphylococcus aureus
Future directions
This article has largely focused on the technical aspects of functional imaging, protocol considerations and clinical applications. Artificial intelligence and machine learning approaches offer the potential to dramatically improve our current approaches to functional imaging [48]. AI techniques have already been applied to computer-aided diagnosis, and deep learning techniques with convolutional neural network-based algorithms can be leveraged at the image acquisition and data processing stage, allowing clinically interpretable images to be generated from noisy raw data stemming from, for example, patient motion or low counts, and inherently poor image contrast [49]. In addition to applications in image processing, deep learning techniques can also be used to generate attenuation corrected PET (and SPECT) images without the need for either a separately acquired CT or MRI [50], and there is increasing recognition of the value of AI in extracting valuable quantitative information from the PET imaging data itself, rather than relying solely on reconstructed images for semi-quantitative analysis and clinical interpretation [51]. The timeline for these techniques, which are still largely investigational, to reach routine clinical practice is likely to accelerate as the interest in AI and machine learning increases amongst both practicing radiologists and our industry partners.
Summary
Most of the common pediatric solid tumors now include PET/CT, PET/MRI, or SPECT-CT in the staging and post treatment response assessment algorithm. It is important to identify the most appropriate test for given tumor type, as well as to design the hybrid imaging protocol in order to optimize both the functional and anatomic components of the examination. Optimizing the protocol includes strategies for dose reduction in PET/CT and SPECT/CT, judicious use of diagnostic quality imaging as appropriate and when possible combining the diagnostic examination with the PET/CT and/or SPECT/CT study. For the greatest reduction in exposure to ionizing radiation, utilizing PET/MRI is desirable, whenever available. The elimination of duplicate studies, when appropriate, can dramatically reduce patient's exposure to ionizing radiation, prolonged and repeated sedation, and use of intravenous contrast. The use of functional imaging for the evaluation and management of suspected infection also requires careful study design in order to optimize the hybrid imaging examination for the greatest diagnostic yield.
References
Fahey FH (2009) Dosimetry of pediatric PET/CT. J Nucl Med 50:1483–1491
Wagenknecht G, Kaiser HJ, Mottaghy FM, Herzog H (2013) MRI for attenuation correction in PET: methods and challenges. MAGMA 26:99–113
Fahey FH, Goodkind A, MacDougall RD et al (2017) Operational and dosimetric aspects of pediatric PET/CT. J Nucl Med 58:1360–1366
Parisi MT, Bermo MS, Alessio AM et al (2017) Optimization of pediatric PET/CT. Semin Nucl Med 47:258–274
Colleran GC, Kwatra N, Oberg L et al (2017) How we read pediatric PET/CT: indications and strategies for image acquisition, interpretation and reporting. Cancer Imaging 17:28
Gatidis S, Schmidt H, Gucke B et al (2016) Comprehensive oncologic imaging in infants and preschool children with substantially reduced radiation exposure using combined simultaneous (1)(8)F-Fluorodeoxyglucose positron emission tomography/magnetic resonance imaging: a direct comparison to (1)(8)F-Fluorodeoxyglucose positron emission tomography/computed tomography. Invest Radiol 51:7–14
Schafer JF, Gatidis S, Schmidt H et al (2014) Simultaneous whole-body PET/MR imaging in comparison to PET/CT in pediatric oncology: initial results. Radiology 273:220–231
States LJ, Reid JR (2020) Whole-body PET/MRI applications in pediatric oncology. AJR Am J Roentgenol 215:713–725
Umutlu L, Beyer T, Grueneisen JS et al (2019) Whole-body [18F]-FDG-PET/MRI for oncology: a consensus recommendation. Nuklearmedizin 58:68–76
Bailey DL, Willowson KP (2014) Quantitative SPECT/CT: SPECT joins PET as a quantitative imaging modality. Eur J Nucl Med Mol Imaging 41:S17–S25
Nadel HR (2014) SPECT/CT in pediatric patient management. Eur J Nucl Med Mol Imaging 41:S104–S114
Krokhmal AA, Kwatra N, Drubach L et al (2022) (68) Ga-DOTATATE PET and functional imaging in pediatric pheochromocytoma and paraganglioma. Pediatr Blood Cancer 69:e29740
McElroy KM, Binkovitz LA, Trout AT et al (2020) Pediatric applications of Dotatate: early diagnostic and therapeutic experience. Pediatr Radiol 50:882–897
Hicks RJ, Jackson P, Kong G et al (2019) (64)Cu-SARTATE PET imaging of patients with neuroendocrine tumors demonstrates high tumor uptake and retention, potentially allowing prospective dosimetry for peptide receptor radionuclide therapy. J Nucl Med 60:777–785
Pandit-Taskar N, Zanzonico P, Staton KD et al (2018) Biodistribution and dosimetry of (18)F-Meta-Fluorobenzylguanidine: a first-in-human PET/CT imaging study of patients with neuroendocrine malignancies. J Nucl Med 59:147–153
Sharp SE, Trout AT, Weiss BD, Gelfand MJ (2016) MIBG in neuroblastoma diagnostic imaging and therapy. Radiographics 36:258–278
Wagner LM, Kremer N, Gelfand MJ et al (2017) Detection of lymph node metastases in pediatric and adolescent/young adult sarcoma: Sentinel lymph node biopsy versus fludeoxyglucose positron emission tomography imaging-A prospective trial. Cancer 123:155–160
Uslu L, Donig J, Link M et al (2015) Value of 18F-FDG PET and PET/CT for evaluation of pediatric malignancies. J Nucl Med 56:274–286
States LJ, Voss SD (2019) PET/CT in pediatric oncology. In: Voss SD, McHugh K (eds) Imaging in pediatric oncology. Springer Nature, Switzerland, pp 29–62
Marti-Climent JM, Prieto E, Moran V et al (2017) Effective dose estimation for oncological and neurological PET/CT procedures. EJNMMI Res 7:37
Qi Z, Gates EL, O’Brien MM, Trout AT (2018) Radiation dose reduction through combining positron emission tomography/computed tomography (PET/CT) and diagnostic CT in children and young adults with lymphoma. Pediatr Radiol 48:196–203
Gatidis S, Schäfer JF (2019) PET/MRI. In: Voss SD, McHugh K (eds) Imaging in pediatric oncology. Springer Nature, Switzerland, pp 63–74
Badawi RD, Shi H, Hu P et al (2019) First human imaging studies with the EXPLORER total-body PET scanner. J Nucl Med 60:299–303
Alberts I, Hunermund JN, Prenosil G et al (2021) Clinical performance of long axial field of view PET/CT: a head-to-head intra-individual comparison of the Biograph Vision Quadra with the Biograph Vision PET/CT. Eur J Nucl Med Mol Imaging 48:2395–2404
Jh O, YooIe R, Kim SH et al (2007) Clinical significance of small pulmonary nodules with little or no 18F-FDG uptake on PET/CT images of patients with nonthoracic malignancies. J Nucl Med 48:15–21
McCarville MB, Billups C, Wu J et al (2013) The role of PET/CT in assessing pulmonary nodules in children with solid malignancies. AJR Am J Roentgenol 201:W900-905
Burris NS, Johnson KM, Larson PE et al (2016) Detection of small pulmonary nodules with ultrashort echo time sequences in oncology patients by using a PET/MR system. Radiology 278:239–246
Chandarana H, Heacock L, Rakheja R et al (2013) Pulmonary nodules in patients with primary malignancy: comparison of hybrid PET/MR and PET/CT imaging. Radiology 268:874–881
Jaimes C, Robson CD, Machado-Rivas F et al (2021) Success of nonsedated neuroradiologic MRI in children 1–7 years old. AJR Am J Roentgenol 216:1370–1377
Masselli G, De Angelis C, Sollaku S et al (2020) PET/CT in pediatric oncology. Am J Nucl Med Mol Imaging 10:83–94
Cederberg KB, Iyer RS, Chaturvedi A et al (2022) Imaging of pediatric bone tumors: A COG Diagnostic Imaging Committee/SPR Oncology Committee White Paper. Pediatr Blood Cancer e30000
Tsai LL, Drubach L, Fahey F et al (2012) [18F]-Fluorodeoxyglucose positron emission tomography in children with neurofibromatosis type 1 and plexiform neurofibromas: correlation with malignant transformation. J Neurooncol 108:469–475
Evans DGR, Salvador H, Chang VY et al (2017) Cancer and central nervous system tumor surveillance in pediatric neurofibromatosis 1. Clin Cancer Res 23:e46–e53
Jessop S, Crudgington D, London K et al (2020) FDG PET-CT in pediatric Langerhans cell histiocytosis. Pediatr Blood Cancer 67:e28034
Rameh V, Voss S, Bedoya MA et al (2022) The added value of skeletal surveys in the initial evaluation of children diagnosed with Langerhans cell histiocytosis in the era of staging (18) F-FDG PET/CT: A retrospective study. Pediatr Blood Cancer e30057
Schafer JF, Granata C, von Kalle T et al (2020) Whole-body magnetic resonance imaging in pediatric oncology - recommendations by the Oncology Task Force of the ESPR. Pediatr Radiol 50:1162–1174
Pfluger T, Piccardo A (2017) Neuroblastoma: MIBG imaging and new tracers. Semin Nucl Med 47:143–157
Sharp SE, Shulkin BL, Gelfand MJ et al (2009) 123I-MIBG scintigraphy and 18F-FDG PET in neuroblastoma. J Nucl Med 50:1237–1243
Maaz AUR, O’Doherty J, Djekidel M (2021) (68)Ga-DOTATATE PET/CT for neuroblastoma staging: utility for clinical use. J Nucl Med Technol 49:265–268
Gains JE, Aldridge MD, Mattoli MV et al (2020) 68Ga-DOTATATE and 123I-mIBG as imaging biomarkers of disease localisation in metastatic neuroblastoma: implications for molecular radiotherapy. Nucl Med Commun 41:1169–1177
Kong G, Hofman MS, Murray WK et al (2016) Initial experience With Gallium-68 DOTA-Octreotate PET/CT and peptide receptor radionuclide therapy for pediatric patients with refractory metastatic neuroblastoma. J Pediatr Hematol Oncol 38:87–96
DuBois SG, Chesler L, Groshen S et al (2012) Phase I study of vincristine, irinotecan, and (1)(3)(1)I-metaiodobenzylguanidine for patients with relapsed or refractory neuroblastoma: a new approaches to neuroblastoma therapy trial. Clin Cancer Res 18:2679–2686
George SL, Falzone N, Chittenden S et al (2016) Individualized 131I-mIBG therapy in the management of refractory and relapsed neuroblastoma. Nucl Med Commun 37:466–472
Polishchuk AL, Dubois SG, Haas-Kogan D et al (2011) Response, survival, and toxicity after iodine-131-metaiodobenzylguanidine therapy for neuroblastoma in preadolescents, adolescents, and adults. Cancer 117:4286–4293
Pijl JP, Kwee TC, Legger GE et al (2020) Role of FDG-PET/CT in children with fever of unknown origin. Eur J Nucl Med Mol Imaging 47:1596–1604
Bagrosky BM, Hayes KL, Koo PJ, Fenton LZ (2013) 18F-FDG PET/CT evaluation of children and young adults with suspected spinal fusion hardware infection. Pediatr Radiol 43:991–1000
Kwee RM, Kwee TC (2020) (18)F-FDG PET for diagnosing infections in prosthetic joints. PET Clin 15:197–205
Matsubara K, Ibaraki M, Nemoto M et al (2022) A review on AI in PET imaging. Ann Nucl Med 36:133–143
Liu J, Malekzadeh M, Mirian N et al (2021) Artificial intelligence-based image enhancement in PET imaging: noise reduction and resolution enhancement. PET Clin 16:553–576
McMillan AB, Bradshaw TJ (2021) Artificial intelligence-based data corrections for attenuation and scatter in position emission tomography and single-photon emission computed tomography. PET Clin 16:543–552
Orlhac F, Nioche C, Klyuzhin I et al (2021) Radiomics in PET imaging: a practical guide for newcomers. PET Clin 16:597–612
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Voss, S.D. SPECT/CT, PET/CT and PET/MRI: oncologic and infectious applications and protocol considerations. Pediatr Radiol 53, 1443–1453 (2023). https://doi.org/10.1007/s00247-023-05597-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-023-05597-7