Abstract
Background
Quantification of organ size has utility in clinical care and research for diagnostics, prognostics and surgical planning. Volumetry is regarded as the best measure of organ size and change in size over time. Scarce reference values exist for liver and spleen volumes in healthy children.
Objective
To report liver and spleen volumes for a sample of children defined by manual segmentation of contrast-enhanced CT images with the goal of defining normal values and thresholds that might indicate disease.
Materials and methods
This retrospective study included clinically acquired contrast-enhanced CTs of the abdomen/pelvis for children and adolescents imaged between January 2018 and July 2021. Liver and spleen volumes were derived through manual segmentation of CTs reconstructed at 2.5-, 3- or 5-mm slice thickness. A subset of images (5%, n=16) was also segmented using 0.5-mm slice thickness reconstructions to define agreement based on image slice thickness. We used Pearson correlation and multivariable regression to assess associations between organ volumes and patient characteristics. We generated reference intervals for the 5th, 25th, 50th (median), 75th and 95th percentiles for organ volumes as a function of age and weight using quantile regression models. Finally, we calculated Bland–Altman plots and intraclass correlation coefficients (ICC) to quantify agreement.
Results
We included a total of 320 children (mean age ± standard deviation [SD] = 9±4.6 years; mean weight 38.1±18.8 kg; 160 female). Liver volume ranged from 340–2,002 mL, and spleen volume ranged from 28–480 mL. Patient weight (kg) (β=12.5), age (months) (β=1.7) and sex (female) (β = −35.3) were independent predictors of liver volume, whereas patient weight (kg) (β=2.4) and age (months) (β=0.3) were independent predictors of spleen volume. There was excellent absolute agreement (ICC=0.99) and minimal absolute difference (4 mL) in organ volumes based on reconstructed slice thickness.
Conclusion
We report reference liver and spleen volumes for children without liver or spleen disease. These results provide reference ranges and potential thresholds to identify liver and spleen size abnormalities that might reflect disease in children.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Quantification of organ size has utility in clinical care and research for diagnostics, prognostics and surgical planning for operations such as liver transplant or laparoscopic splenectomy [1,2,3,4,5,6,7,8]. The size of the liver and the spleen in part represents their functional capacity, and it can be influenced by various organ-specific or systemic diseases [9, 10]. Traditionally, noninvasive assessment of liver and spleen sizes by imaging has relied upon subjective visual inspection, linear measures or formulaic indices using multiplanar linear measurements from cross-sectional imaging [3, 11]. Given the variation in the shape of the liver and spleen, traditional approaches of assessment can either over- or underestimate organ size [2, 12]. As such, volumetry is regarded as the best measure of organ size and change in size over time [2, 3, 13].
Organ volumetry by CT was first described in the 1970s and allows for precise determination of organ size [14,15,16]. In adults, CT-based volumetric segmentation of the liver and spleen has been shown to provide an objective and highly accurate assessment of sizes as compared to linear measures [2, 3]. Manual segmentation is a common method for volumetry and consists of drawing a binary label map on each image. A total volume is determined by summing all labeled voxels, and multiplying slice thickness and voxel area [3, 17, 18]. With increasing computational power and increasingly available approaches to assist in manual segmentation, hepatic and splenic CT-based volume determination and reporting are likely to become more common in clinical practice for diagnostic and opportunistic screening purposes [5, 13].
The liver and spleen grow during childhood. Scarce reference data exist for liver and spleen volumes in healthy children. The studies that do exist include either small samples or lack representation across all pediatric ages [4, 9, 18,19,20]. Therefore, we report values for liver and spleen volumes in a sample of children, defined by manual segmentation of contrast-enhanced CT images, with the goal of defining normal values and potential thresholds that could indicate disease.
Materials and methods
This retrospective study was approved by the institutional review board at Cincinnati Children’s Hospital Medical Center. All research activities complied with the Health Insurance Portability and Accountability Act.
Study participants
Children ages 2–17 years who underwent a clinically indicated CT of the abdomen/pelvis with intravenous contrast material in the Emergency Department at Cincinnati Children’s Hospital Medical Center between January 2018 and July 2021 and children who had CT of the abdomen/pelvis with intravenous contrast agent performed at an outside institution and interpreted at our institution during this same period were considered for inclusion in this study. Exclusion criteria included (a) the presence of any reported chronic disease; (b) height/weight outside the 5th to 95th percentiles for age according to the Centers for Disease Control and Prevention criteria [21, 22]; (c) laboratory abnormalities (liver enzymes more than three times the upper normal limit or abnormal complete blood count); (d) physical exam findings of hepatosplenomegaly, jaundice or lymphadenopathy; (e) imaging reports of liver or spleen abnormalities; and (f) CT examinations with motion artifacts or incompletely included organs that precluded full segmentation of the liver or spleen.
Sample selection
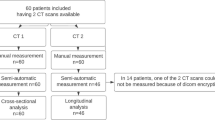
We extracted CT datasets from the institutional picture archiving and communication system (PACS) for research post-processing and analysis. We arbitrarily included 10 patients per year of age (2–17 years) and sex (male and female), totaling 32 age- and sex-stratified groups. We chose 2 years of age as the lower threshold given the paucity of CT examinations in children younger than 2 years. A research assistant (V.P.V.A.) used an imaging report search engine (Illuminate Insight; Softek, Overland Park, KS) to identify children for potential inclusion, yielding 3,136 unique patients. Children were then sorted according to age, biological sex and date of CT examination. For each age–sex group, we consecutively screened children until 10 children of each sex were included. In total, 641 patients were screened and 321 excluded, yielding a final sample of 320 children as detailed in Fig. 1. Race and ethnicity were not used as sample selection variables. The same research assistant (V.P.V.A.) then reviewed the electronic medical record (Epic Hyperspace; Epic Systems, Verona, WI) and CT reports for exclusion criteria. We reviewed laboratory data, and physical exam notes when available, for the period 90 days before and after the date of the CT examination. We also reviewed medical history summaries from Emergency Department admission notes. Height measurements within 90 days before or after the CT examination were reviewed for inclusion purposes, but because height is not routinely obtained at the time of CT examination, we did not analyze height as a predictor of liver and spleen volumes. Patient sex and weight, which are routinely obtained, were extracted from clinical notes on the day of the CT examination.
Computed tomography examination
All CT examinations performed at Cincinnati Children’s Hospital Medical Center (93.8%, 300/320) had been obtained using 16-cm z-axis coverage, 320-row detector scanners with size-specific techniques. Portal venous phase images were acquired 50 s after triggering at 120 Hounsfield units (HU) in the descending thoracic aorta. All images were reconstructed axially at 0.5-mm and either 3-mm (children <15 kg) or 5-mm (children ≥15 kg) slice thicknesses, with no gap between slices. Examinations performed at outside institutions had been reconstructed at 2.5-, 3- or 5-mm axial slice thicknesses with no gap.
Liver and spleen segmentation
Digital Imaging and Communications in Medicine (DICOM) images at 2.5-, 3- or 5-mm axial slice thickness were uploaded to post-processing software (3D Slicer, The Slicer Community) for manual segmentation. All segmentations were performed by one research assistant (V.P.V.A.) (Figs. 2 and 3). The intrahepatic vena cava, the portal vein in the liver hilum and the splenic hilar vessels were excluded from segmentation. Following segmentation, liver and spleen volumes (in mL) were extracted and recorded.
Organ volume agreement
All CT data sets with overlaid segmentation masks were reviewed and corrected, if needed, by one of two board-certified pediatric radiologists (A.T.T. and J.R.D.), each with more than 10 years of experience and certificates of advanced qualification in pediatric radiology. Corrected organ volumes were used for the final data analysis.
To define organ volume agreement based on reconstructed image slice thickness, the same research assistant (V.P.V.A.) performed segmentations in the same manner using the images reconstructed at 0.5-mm slice thickness for 16 children randomly selected from our included sample (one from each group from age 2 years to 17 years; 5% of included children). These segmentations were performed blinded to the segmentations performed using the 2.5-, 3- or 5-mm images and were similarly reviewed and corrected as needed. Agreement with organ volumes derived from the 5-mm slice thickness images was quantified based on the reference standard of 0.5-mm images.
Statistical analysis
We performed a descriptive analysis of demographic and clinical data to summarize sample characteristics. We summarized liver and spleen volume measures using means, medians, standard deviations (SDs) and interquartile ranges (IQRs), as appropriate. For the primary analysis, we used Pearson correlation to assess univariable associations between organ volumes and patient characteristics (age and weight). We used multiple linear regression modeling with forward variable selection to generate models for predicting organ volumes based on patient characteristics (age, weight and sex). Variables were allowed to enter the model if P<0.05 and were dropped from the model if P>0.1. We calculated Bland–Altman plots, intraclass correlation coefficients (ICC) and percentage differences (absolute difference/total volume at 0.5-mm slice thickness) to quantify agreement between organ volumes acquired with CTs reconstructed at different slice thicknesses [23].
We used quantile regression analysis to plot liver and spleen volumes as a function of patient age and weight. This method most completely characterizes the distribution of the data across the study sample. We generated reference intervals with regression models for the 5th, 25th, 50th (median), 75th and 95th percentiles of the organ volumes as a function of age and weight, and we provide regression formulas for prediction of volumes. We reviewed study sample organ volume scatter plots with overlaid quantile regression curves for outliers, with outliers defined as volumes outside the 95th and 5th quantile regression plots; then we reviewed images and medical records for each outlier to confirm the absence of exclusion criteria. All statistical analyses were performed with MedCalc Statistical Software version 20.009 (MedCalc Software Ltd., Ostend, Belgium) and GraphPad Prism version 8.0.0 for Windows (GraphPad Software, San Diego, CA). A P-value <0.05 was considered statistically significant.
Results
Study sample characteristics
A total of 320 children and adolescents (160 boys, 160 girls) were included in this study as detailed in Fig. 1. Mean age ± SD was 9±4.6 years, with a range of 2–17 years, and mean weight was 38.1±18.8 kg, with a range of 10.0–86.2 kg. The most common specific indications for CT examination were right lower quadrant pain/suspected appendicitis (48%, 155/320), abdominal trauma (28%, 90/320), and diffuse abdominal pain (16%, 51/320) (Table 1).
Liver and spleen volume
Liver volume in the study sample ranged from 340–2,002 mL, with a median of 424 mL at 2 years of age and 1,343 mL at 17 years of age. Spleen volume in the study sample ranged from 28–480 mL, with a median of 58 mL at 2 years of age and 225 mL at 17 years of age.
Effects of slice thickness on segmented volumes
The 16 sets of images segmented from both 0.5-mm and 5-mm thickness reconstructions showed excellent absolute agreement in liver volumes, ICC=0.999 (95% confidence interval [CI]: 0.998, 0.999) and spleen volumes, ICC=0.997 (95% CI: 0.991, 0.999). On Bland–Altman analysis, the mean difference in liver volumes was −4.6 mL (95% limits of agreement: −34.8 to +25.6 mL) with proportional bias manifest as increasing bias with increasing liver volume (P=0.03). The percentage difference for liver volumes ranged from −1.5% to +2.6%. For spleen volumes, the mean difference was +4.0 mL (95% limits of agreement: −8.9 to +16.8 mL) without proportional bias. The percentage difference for spleen volumes ranged from −3.9% to 8.1% (Fig. 4).
Uni- and multivariable predictors of liver volumes
We found a strong positive correlation between liver volumes and patient weight (r=0.91 [0.89 to 0.93]; P<0.001) and age (r=0.89 [0.86 to 0.91]; P<0.001). Using multivariable modeling, independent predictors of liver volumes were patient weight with a β coefficient of 12.5 (P=0.02), age (months) with a β coefficient of 1.7 (P<0.001) and sex (female) with a β coefficient of −35.3 (P<0.001) (Table 2).
Uni- and multivariable predictors of spleen volumes
There was a strong positive correlation between spleen volumes and patient weight (r=0.74 [0.68 to 0.78]; P<0.001) and age (r=0.72 [0.66 to 0.76]; P<0.001). At multivariable modeling, independent predictors of spleen volumes were patient weight with a β coefficient of 2.4 (P<0.001) and age (months) with a β coefficient of 0.3 (P=0.04) (Table 2). Sex was not independently associated with spleen volumes (P>0.15).
Liver volume quantiles
Quantile regression analysis of liver volume according to patient characteristics is detailed in Fig. 5 and Table 3. Formulas to estimate liver volume reference intervals based on weight and age are provided in Table 4. Formulas are also provided using only age as the predictor. Lookup tables of liver volumes as a function of age and sex, based on the quantile regression curves, are available as Online Supplementary Materials 1 and 2.
Spleen volume quantiles
Quantile regression analysis of spleen volume according to patient characteristics is detailed in Fig. 5 and Table 3. Formulas to estimate spleen volume reference intervals are provided in Table 4. Lookup tables of spleen volumes as a function of age and sex, based on the quantile regression curves, are available as Online Supplementary Material.
Outlier analysis
Based on quantile regression of liver and spleen volumes as a function of age and of weight, 32 values were outliers for liver volume (10%, 32/320) and 30 for spleen volume (9.3%, 30/320). No technical or documented clinical or pathological reason was identified to explain these outliers.
Discussion
Volumetric segmentation of the liver and spleen from cross-sectional imaging, with either manual or automated tools, provides a highly accurate assessment of organ size and is considered the reference standard for organ size assessment [2, 3]. Adult studies have provided reference values for liver and spleen volumes [1, 3, 24] but sparse data have been reported for children. Data for children are particularly important because the liver and spleen grow during childhood, necessitating age- and size-specific reference ranges. In this retrospective study, we provide reference intervals for liver and spleen volumes throughout childhood derived from a carefully selected sample of children and adolescents with no known condition that might impact liver or spleen volume. In our sample, liver volumes ranged from 340–2,002 mL and spleen volumes ranged from 28–480 mL, with increases in both organs throughout childhood. Patient age, weight and sex were independent predictors for liver volumes, whereas patient weight and age were independent predictors for spleen volumes. These results provide reference ranges and potential thresholds to identify liver and spleen size abnormalities that might reflect disease in children.
The patient-specific predictors of liver volume identified in our study are concordant with the literature. In a study of 54 adolescents and young adults from Japan with no known liver disease, Noda et al. [9] showed liver volume derived from CT to be positively correlated (r=0.93) with patient age through adolescence. Additionally, liver volume had a strong positive correlation with body weight (r=0.96). In an autopsy study of 388 Caucasian children and adolescents ages 0 to 16 years without known liver disease, Herden et al. [25] found that body weight and body length were independent predictors of liver volumes, and the authors proposed a formula for standard liver volume using patient weight and length. Furthermore, in a prospective study of 744 pediatric patients from a Chinese cohort with no known liver disease who underwent upper abdominal CT, Yang et al. [4] showed liver volume to have a strong correlation with age (r=0.90) and weight (r=0.95) and reported formulas for median liver volume based on body surface area. In that study, median liver volumes at 2 years and 17 years of age were 392 mL and 1,181 mL, respectively, compared to 424 mL and 1,343 mL in our study. This volumetric disparity could be attributed to differences in sample size, geography and methodology for liver volumetry estimation (manual segmentation in our study versus semi-automated software in the prior study). An important limitation of the prior study is that it did not exclude children with weight or height outside the normal percentiles for age and did not provide quantile thresholds to allow identification of outliers potentially indicative of disease. Finally, similar to prior pediatric [4] and adult [3] studies, our work found that sex had an association with liver volume.
In our analysis, spleen volumes were highly correlated with patient age (r=0.72) and weight (r=0.74), while the latter was the strongest independent predictor in multivariate analysis. These results are concordant with prior literature. In a study of 48 children and adolescents from a U.S. cohort with no known disease, Schlesinger et al. [19] showed spleen volumes to correlate better with body weight than with age. Similarly, in a retrospective study of 49 Japanese children, Watanabe et al. [18] demonstrated that the best predictor of spleen volumes was body weight, with better correlation than patient age.
Compared with prior studies, our study could be considered limited because we did not collect height, race or ethnicity as predictor variables, and we did not collect data into adulthood to characterize the known plateau in liver and spleen volumes that occurs with age. Weight is routinely collected as part of performing CT examinations at our institution to appropriately dose intravenous contrast material and age is readily known, making these useful variables with which to stratify patients. However, height is not routinely collected in our practice and might not be collected in other practices, making it less useful as a stratification variable in clinical practice. Regarding race and ethnicity, some studies have linked these variables to liver and spleen volumes [3, 9, 10, 12, 18, 26, 27]. However, to our knowledge, no defined biological reason exists to believe that self-reported race or ethnicity would impact organ size independent of age or patient size [28]. Any potential interplay among race, ethnicity, social determinants of health, and organ volume were not explored in this work.
Use of relatively thick (2.5–5 mm) axial images, which less perfectly represent the contours of the liver and spleen and might less accurately characterize volume because of partial volume effects, could be considered an additional limitation of our study [29]. However, in agreement with a prior study by Hori et al. [30], our analysis of a subset of segmentations performed using both 0.5-mm- and 5-mm-thick image slices showed excellent agreement and minimal absolute difference in extracted volumes, on the order of 4 mL larger for 0.5-mm segmentations for both liver and spleen. These differences are unlikely to be of clinical significance, and use of thick-slice images is more applicable to clinical practice given the laboriousness associated with segmenting 0.5-mm images [13]. Future work utilizing automated or deep-learning-based algorithms might reduce the laboriousness of segmentation, allowing use of thinner-slice images as part of routine clinical work [2, 3, 5, 13].
Further limitations of our study include a sample derived from emergency visits, which might mean less complete medical records, potentially missing the presence of chronic diseases that could affect liver and spleen volumes. Additionally, a fraction (28%) of our sample was imaged following abdominal trauma, which has been related to both increases and decreases in splenic volume and thus might have skewed our results [31, 32]. Finally, all included examinations were enhanced with intravenous contrast material, preventing exclusion of “silent” hepatic steatosis, which could have biased liver volumes.
Conclusion
Based on manual segmentation of a carefully selected sample of children and adolescents without known liver or splenic disease, we report reference volumes for liver and spleen in the pediatric population. Further work is needed to validate these reference values in larger populations and to test 5th and 95th percentile thresholds for identifying liver and spleen pathology.
References
Kromrey ML, Ittermann T, vWahsen C et al (2018) Reference values of liver volume in Caucasian population and factors influencing liver size. Eur J Radiol 106:32–37
Perez AA, Noe-Kim V, Lubner MG et al (2021) Deep learning CT-based quantitative visualization tool for liver volume estimation: defining normal and hepatomegaly. Radiology 302:336–342
Kim DW, Ha J, Lee SS et al (2021) Population-based and personalized reference intervals for liver and spleen volumes in healthy individuals and those with viral hepatitis. Radiology 301:339–347
Yang X, Wang H, Dong B et al (2021) Standard liver volume-predicting formulae derived from normal liver volume in children under 18 years of age. Front Pediatr 9:1–7
Pickhardt PJ (2022) Value-added opportunistic CT screening: state of the art. Radiology 303:241–254
Pattanayak P, Turkbey EB, Summers RM (2017) Comparative evaluation of three software packages for liver and spleen segmentation and volumetry. Acad Radiol 24:831–839
Chow KU, Luxembourg B, Seifried E, Bonig H (2016) Spleen size is significantly influenced by body height and sex: establishment of normal values for spleen size at US with a cohort of 1,200 healthy individuals. Radiology 279:306–313
Linguraru MG, Sandberg JK, Jones EC, Summers RM (2013) Assessing splenomegaly. Automated volumetric analysis of the spleen. Acad Radiol 20:675–684
Noda T, Todani T, Watanabe Y, Yamamoto S (1997) Liver volume in children measured by computed tomography. Pediatr Radiol 27:250–252
Yang X, Yang JD, Lee S et al (2018) Estimation of standard liver volume using CT volume, body composition, and abdominal geometry measurements. Yonsei Med J 59:546–553
Bezerra AS, D’Ippolito G, Faintuch S et al (2005) Determination of splenomegaly by CT: is there a place for a single measurement? AJR Am J Roentgenol 184:1510–1513
Olthof PB, van Dam R, Jovine E et al (2019) Accuracy of estimated total liver volume formulas before liver resection. Surgery 166:247–253
Ringl H (2021) Personalized reference intervals will soon become standard in radiology reports. Radiology 301:348–349
Heymsfield SB, Fulenwider T, Nordlinger B et al (1979) Accurate measurement of liver, kidney, and spleen volume and mass by computerized axial tomography. Ann Intern Med 90:185–187
Saunders SL, Clark JM, Rudser K et al (2022) Comparison of automatic liver volumetry performance using different types of magnetic resonance images. Magn Reson Imaging 91:16–23
Urata K, Kawasaki S, Matsunami H et al (1995) Calculation of child and adult standard liver volume for liver transplantation. Hepatology 21:1317–1321
Kaneko J, Sugarawa Y, Matsui Y et al (2002) Normal splenic volume in adults by computed tomography. Hepatogastroenterology 49:1726–1727
Watanabe Y, Todani T, Noda T, Yamamoto S (1997) Standard splenic volume in children and young adults measured from CT images. Surg Today 27:726–728
Schlesinger A, Hildebolt CF, Siegel MJ, Pilgrim T (1994) Splenic volume in children: simplified estimation at CT. Radiology 193:578–580
Schlesinger AE, Edgar KA, Boxer LA (1993) Volume of the spleen in children as measured on CT scans: normal standards as a function of body weight. AJR Am J Roentgenol 160:1107–1109
Centers for Disease Control and Prevention (2022) BMI percentile calculator for child and teen. CDC. https://www.cdc.gov/healthyweight/bmi/calculator.html. Accessed 9 Sep 2021
Centers for Disease Control and Prevention (2001) Data table of stature-for-age charts. CDC. https://www.cdc.gov/growthcharts/html_charts/statage.htm. Accessed 5 Jul 2022
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Roloff AM, Heiss P, Schneider TP et al (2016) Accuracy of simple approaches to assessing liver volume in radiological imaging. Abdom Radiol 41:1293–1299
Herden U, Wischhusen F, Heinemann A et al (2013) A formula to calculate the standard liver volume in children and its application in pediatric liver transplantation. Transpl Int 26:1217–1224
Tsehay B, Shitie D, Afenigus A, Essa M (2021) Sonographic evaluation of spleen size in apparently healthy children in north-west Ethiopia, 2020: time to define splenomegaly. BMC Pediatr 21:318
Feng LM, Wang PQ, Yu H et al (2017) New formula for predicting standard liver volume in Chinese adults. World J Gastroenterol 23:4968–4977
Flanagin A, Frey T, Christiansen SL, AMA Manual of Style Committee (2021) Updated guidance on the reporting of race and ethnicity in medical and science journals. JAMA 326:621–627
Triche BL, Nelson JT, McGill NS et al (2019) Recognizing and minimizing artifacts at CT, MRI, US, and molecular imaging. Radiographics 39:1017–1018
Hori M, Suzuki K, Epstein ML, Baron RL (2011) Computed tomography liver volumetry using 3-dimensional image data in living donor liver transplantation: effects of the slice thickness on the volume calculation. Liver Transpl 17:1427–1436
Cruz-Romero C, Agarwal S, Abujudeh HH et al (2016) Spleen volume on CT and the effect of abdominal trauma. Emerg Radiol 23:315–323
Goodman LR, Aprahamian C (1990) Changes in splenic size after abdominal trauma. Radiology 176:629–632
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 52 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Padua V. Alves, V., Dillman, J.R., Somasundaram, E. et al. Computed tomography-based measurements of normative liver and spleen volumes in children. Pediatr Radiol 53, 378–386 (2023). https://doi.org/10.1007/s00247-022-05551-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-022-05551-z