Abstract
Background
Left ventricular strain may be a more sensitive marker of left ventricular dysfunction than ejection fraction in pediatric cancer survivors after anthracycline therapy, but there is limited validation of strain measurement by feature tracking on cardiovascular magnetic resonance (MR) images.
Objective
To compare left ventricular circumferential and radial strain by feature tracking vs. harmonic phase imaging analysis (HARP) in pediatric cancer survivors.
Materials and methods
Twenty-six patients (20.2 ± 5.6 years old) underwent cardiovascular MR at least 5 years after completing anthracycline therapy. Circumferential and radial strain were measured at the base, midventricle and apex from short-axis myocardial tagged images by HARP, and from steady-state free precession images by feature tracking.
Results
Left ventricular ejection fraction more closely correlated with global circumferential strain by feature tracking (r = −0.63, P = 0.0005) than by HARP (r = −0.39, P = 0.05). Midventricular circumferential strain did not significantly differ by feature tracking or HARP (−20.8 ± 3.4 vs. −19.5 ± 2.5, P = 0.07), with acceptable limits of agreement. Midventricular circumferential strain by feature tracking strongly correlated with global circumferential strain by feature tracking (r = 0.87, P < 0.0001). Radial strain by feature tracking had poor agreement with HARP, particularly at higher values of radial strain. Intraobserver and interobserver reproducibility was excellent for feature tracking circumferential strain, but reproducibility was poor for feature tracking radial strain.
Conclusion
Midventricular circumferential strain by feature tracking is a reliable and reproducible measure of myocardial deformation in patients status post anthracycline therapy, while radial strain measurements are unreliable. Further studies are necessary to evaluate potential relation to long-term outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pediatric cancer survivors who have undergone anthracycline therapy are at risk for subsequent left ventricular systolic dysfunction, with a significant impact on long-term morbidity and mortality [1, 2]. Current guidelines for survivors include regular monitoring of left ventricular function with echocardiography [3], but limitations in detecting early changes have prompted evaluation of other methodologies to detect earlier signs of dysfunction, which could lead to earlier referral and treatment [4]. Left ventricular strain is a measure of myocardial deformation and may be a more sensitive marker than global left ventricular ejection fraction (LVEF), because decreased strain has been demonstrated in this population in patients with normal LVEF [5, 6].
Myocardial tagged imaging on cardiovascular magnetic resonance is considered the gold standard for strain imaging [7]. Strain can be quantified from these images using harmonic phase (HARP) imaging analysis software, but these tagged cardiovascular MR sequences require additional scanner time, have limited temporal resolution, and need to be acquired prospectively for analysis. Feature tracking is a promising technique that can measure strain from standard cardiovascular MR steady-state free precession cine images, but it has limited validation vs. myocardial tagged images [8, 9].
This study compared strain by feature tracking and HARP on cardiovascular MR images in pediatric cancer survivors.
Materials and methods
Patients were prospectively recruited from the Childhood Cancer Survivorship Program at the University of Michigan from May 2011 through August 2012 as part of a prospective pilot study evaluating echocardiography and cardiovascular MR for detection of ventricular dysfunction; the current report is a validation study of feature tracking on cardiovascular MR images in this population. Patients were eligible if they were 7–45 years of age, received a high cumulative dose of anthracyclines (≥250 mg/m2) before the age of 18, and were at least 5 years from the last dose of chemotherapy. Patients were excluded if they received radiation in the cardiac field, were placed on cardioprotectants during chemotherapy administration, had contraindication for cardiovascular MR, were currently on cardiac medications, or had a known history of congenital heart defect or recent evidence of cardiac dysfunction on echocardiogram. This study was compliant with the Health Information Portability and Accountability Act and was approved by the institutional review board. All subjects and parents of minors provided informed consent.
Cardiovascular MR was performed using a commercially available 1.5-T scanner (Philips Achieva or Ingenia; Best, The Netherlands). Cine images were obtained with a breath-hold, electrocardiographic-gated, segmented k-space, steady-state free precession sequence, with 20 non-interpolated phases per cardiac cycle. Scan parameters were repetition time (TR) 3.1–3.4 ms, echo time (TE) 1.5–1.7 ms, flip angle 60°, field of view 26–38 cm, slice thickness 8 mm, gap 1–2 mm, with in-plane spatial resolution 1.7–1.9 mm and temporal resolution 39 ± 7.5 ms. Ventricular volumes and ejection fraction were measured by Simpson’s method from short-axis images [10].
Myocardial tagged images were obtained with a breath-hold, electrocardiographic-gated, segmented k-space echo planar imaging sequence with spatial modulation of magnetization in orthogonal planes. Tagged images were acquired at three slices of the short-axis of the left ventricle: at the base, midventricle and apex, with uniform gap between slices. Scan parameters were TR 25–40 ms and TE 4.3–4.9 ms (Achieva) or TR 11–12 ms and TE 5.5–6.1 ms (Ingenia), echo planar imaging (EPI) factor 9 (Achieva) or 5 (Ingenia), 21 ± 4 phases per cardiac cycle, field of view 28–36 cm, slice thickness 8–10 mm, with in-plane spatial resolution 2–3 mm, and grid tag spacing 7–9 mm.
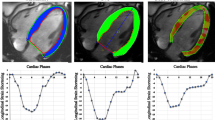
Myocardial tagged images were analyzed with HARP software (Diagnosoft, Morrisville, NC). Points were manually selected along the endocardium and epicardium (Fig. 1), with subsequent automated tracking. Contours were visually evaluated for adequate tracking of the endocardium and epicardium and were adjusted as necessary by a single experienced observer with 3 years of experience in pediatric cardiovascular MR, until the automated tracking was visually adequate. Peak midwall circumferential and radial Lagrangian strain were measured at each level and averaged for global strain.
Cardiovascular MR short-axis images were analyzed by feature tracking software (TomTec, Unterschleissheim, Germany). Slices were chosen for analysis at the base (the most basal slice did not include the left ventricular outflow tract), midventricle (level of the papillary muscles), and apex (below insertion of the papillary muscles). The most representative slices were chosen and were not constrained to the slices for which tagged images were available. Points were manually selected along the endocardium and epicardium (Fig. 1), with subsequent automated tracking. Contours were visually evaluated for adequate tracking and adjusted as necessary, and automated tracking was re-performed until tracking was visually adequate. Circumferential (measured at the endocardium) and radial Lagrangian strain were measured as the peak of the average curve at each level. Peak strain at each level was averaged for global strain, weighted for number of segments per level.
Images for feature tracking strain for all patients were re-analyzed >3 months later for analysis of intraobserver variability. A subset of 10 patients was analyzed by a second experienced reader with 9 years of experience in pediatric cardiovascular MR, blinded to initial results, for analysis of interobserver variability. Myocardial tagged images were not evaluated for reproducibility because it is the accepted standard with established good reproducibility [11, 12].
Data are presented as mean ± standard deviation. Means of HARP and feature tracking strain measurements were compared using paired t-test. Correlation analysis was performed using the Pearson correlation coefficient. Agreement of the two methods was evaluated by Bland–Altman analysis. Coefficients of variation (COV) and Bland–Altman analysis are presented for intraobserver and interobserver variability. P-values <0.05 were considered statistically significant.
Results
A total of 26 patients were included, with demographic data presented in Table 1. All patients successfully underwent cardiovascular MR and had adequate images for analysis, with visually adequate tracking. No patients had significant dyssynchrony or ventricular conduction delay.
Left ventricular ejection fraction (LVEF) more closely correlated with global circumferential strain by feature tracking (r = −0.63, P = 0.0005) than with global circumferential strain by HARP (r = −0.39, P = 0.05). Circumferential strain at the midventricular level correlated with LVEF both by feature tracking (r = −0.55, P = 0.003) and HARP (r = −0.62, P = 0.0008). Global radial strain did not reach statistical significance with LVEF by either feature tracking (r = 0.38, P = 0.06) or HARP (r = 0.30, P = 0.13). Radial strain at the midventricular level also did not correlate with LVEF by either method.
Global circumferential strain was mildly overestimated by feature tracking relative to HARP (−22.6 ± 3.0 vs. −18.9 ± 2.0, P < 0.0001), and correlation did not reach significance (r = 0.35, P = 0.08). However, circumferential strain at the midventricular level did not significantly differ by feature tracking and HARP (−20.8 ± 3.4 vs. −19.5 ± 2.5, P = 0.07), with modest correlation (r = 0.39, P = 0.047). Bland–Altman analysis is presented in Fig. 2.
Bland–Altman analysis of circumferential strain by feature tracking and harmonic phase imaging analysis (HARP), when measured globally (a) and at the midventricular level (b). Differences are reported as feature tracking (FT) - HARP. Dotted lines represent mean difference and limits of agreement (±1.96 standard deviations)
Radial strain was markedly underestimated by feature tracking relative to HARP, whether measured globally (43.9 ± 9.1 vs. 74.4 ± 23.9, P < 0.0001) or at the midventricular level (41.5 ± 9.9 vs. 68.7 ± 28.6, P = 0.0003). This systematic bias was much more pronounced at higher values of radial strain (Fig. 3). Radial strain at the midventricular level by feature tracking and HARP did not correlate (r = −0.32, P = 0.11).
Bland–Altman analysis of radial strain by feature tracking and harmonic phase imaging analysis (HARP), when measured globally (a) and at the midventricular level (b). Differences are reported as feature tracking (FT) - HARP. Dotted lines represent mean difference and limits of agreement (±1.96 standard deviations)
Circumferential strain measurement by feature tracking at the midventricular level strongly correlated (r = 0.87, P < 0.0001) with global circumferential strain measurement by feature tracking (Fig. 4). Radial strain measurement by feature tracking at the midventricular level correlated moderately (r = 0.49, P = 0.01) with global radial strain measurement by feature tracking (Fig. 4).
Intraobserver and interobserver reproducibility for global circumferential strain was excellent, with fairly similar reproducibility at each level (Table 2). Intraobserver and interobserver reproducibility for radial strain was poor, whether evaluated globally or at any level (Table 2). Bland–Altman analysis of intraobserver and interobserver reproducibility of global circumferential strain is presented in Fig. 5. Midventricular circumferential strain had similar mean difference and limits of agreement (intraobserver 0, −2.7 to 2.7; interobserver 0.3, −2.1 to 2.7).
Discussion
We have demonstrated that midventricular circumferential strain measurement by feature tracking best agrees with HARP, with excellent reproducibility. However, radial strain by feature tracking varies widely from HARP, particularly at higher values, and has poor reproducibility. Feature tracking strain measurement at the midventricular level provides similar values to global strain measurement. To our knowledge, this is the first study to compare and validate strain by feature tracking vs. HARP in this population.
Myocardial tagged imaging on cardiovascular MR has become the standard for analyzing myocardial deformation [8, 13, 14], with established good reproducibility for HARP analysis [11, 12]. However, this does require time to obtain additional sequences prospectively, and can be limited by tag fading later in the cardiac cycle. Feature tracking is attractive because it does not require additional imaging and typically requires less post-processing time [9]. It is unclear which method best represents myocardial deformation in this population; differences between methods do not necessarily imply inferiority of feature tracking. In our cohort, LVEF more closely correlated with global circumferential strain by feature tracking than by HARP. LVEF would not be expected to correlate perfectly with strain measurements because strain may be a more sensitive marker of dysfunction. Without a true independent gold standard, it is unclear which method better detects dysfunction not detected by LVEF. The difference in global strain may reflect variable reliability of measurements at different levels, with decreased reproducibility demonstrated particularly in the apex for both HARP and feature tracking [8, 9, 11]. In our cohort, circumferential strain reproducibility for feature tracking was relatively uniform by level, including at the apex. This may explain the similar relation of LVEF to midventricular circumferential strain by either feature tracking or HARP.
Previous studies compared feature tracking and HARP in normal patients and in Duchenne muscular dystrophy [8, 9]. Our results are consistent with prior findings of similar means with reasonable limits of agreement for circumferential strain, and confirm that circumferential strain by feature tracking is more reliable than radial strain. Hor et al. [11] demonstrated higher correlation of circumferential strain measurement by feature tracking and HARP at the midventricular level in patients with Duchenne muscular dystrophy than in our study, which could be related to differences in populations. HARP calculates strain based on myocardial tag deformation in the midwall, while feature tracking tracks voxels and reports strain at the endocardium. The subendocardium is more sensitive to damage from anthracyclines [15], and transmural strain differences have been reported in this population [16]. Differences between feature tracking and HARP from midmyocardial vs. endocardial strain could be amplified in this population, particularly in our cohort with high prior anthracycline doses. This underscores the need for validation across multiple populations.
Multiple potential sources may explain the variability between feature tracking and HARP. In addition to measurement of deformation at different levels of the LV wall, there may be differences in ventricular level of the analyzed short-axis images. Tagged image analysis is restricted to the obtained short-axis images, whereas the entire short-axis stack is available for analysis by feature tracking. Circumferential and radial strain have been shown to vary by ventricular level on both cardiovascular MR and echocardiography [9, 17]. This may explain why bias was decreased when using the midventricular level alone, because a single midventricular slice is more likely to correspond when assigning planes. Differences in temporal resolution may also lead to differences in the two techniques, but sample size limitations did not allow subanalysis of agreement by degree of discrepancy in temporal resolution between HARP and feature tracking. Given the differences in technique, it would be understandable for feature tracking and HARP to result in different values, with different normative ranges.
Radial strain on speckle tracking echocardiography has been shown to have poor reproducibility, whether using the same software or using different vendor software [18]. Our poor reproducibility of radial strain by feature tracking is consistent with a prior report of poor reproducibility by both HARP and feature tracking [9]. It is thus not surprising to see the lack of agreement on cardiovascular MR using these different techniques in this population. Given the differences in technique in measurement at the endocardium by feature tracking and midwall by HARP, the lower feature tracking values could potentially reflect subclinical abnormalities in the subendocardium in this population, not seen in the midwall by HARP, with particular discrepancy in patients with normal values by HARP. However, because of the wide differences the amount of contribution of this potential effect is speculative. Circumferential strain by feature tracking warrants further evaluation, but radial strain measurements require further validation and refinement.
Multiple studies have reported global strain using a single midventricular slice [8, 19, 20]. Our data confirm that this correlates well with global strain in this population. However caution is necessary in other populations because the present cohort is at risk for global changes in systolic function rather than regional wall motion abnormalities. In other populations, such as patients with congenital heart disease or ischemic heart disease, it is unclear whether measurement at one slice is an appropriate surrogate for global measures.
This study is limited by the small sample size and cross-sectional nature. Although harmonic phase imaging is an accepted methodology, there is a lack of a true, independent gold standard for comparing these methods. Other methods have been used to evaluate myocardial deformation on cardiovascular MR, such as tissue phase mapping [21], strain-encoded magnetic resonance imaging [22], and displacement encoding with stimulated echoes [23], but these methods were not evaluated in this study. Longitudinal strain was not evaluated; because the dominant motion of the left ventricle is circumferential, longitudinal strain is less reproducible [24], and myocardial tagged images were not obtained in the long axis. However, longitudinal strain on echocardiography may be an important prognostic marker [5, 25], and validation on cardiovascular MR is necessary. Similarly, global rather than segmental strain was evaluated because of decreased reproducibility of segmental strain [24] and clinical suspicion for global rather than regional dysfunction. The use of two different cardiovascular MR systems with potentially different signal-to-noise ratios and temporal resolutions may have affected HARP analysis, but subanalysis by tagging technique was limited by sample size.
Conclusion
Circumferential strain can be reliably and reproducibly measured by feature tracking in patients status post anthracycline therapy. Measurement of midventricular circumferential strain appears to be the best method in this population because of its reliability, ease of use, and close correlation with global circumferential strain, while radial strain measurement has significant limitations.
References
Shankar SM, Marina N, Hudson MM et al (2008) Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics 121:e387–396
Mulrooney DA, Yeazel MW, Kawashima T et al (2009) Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. Brit Med J 339:4606
(2008) Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers, version 3.0. Children’s Oncology Group. http://www.survivorshipguidelines.org/. Accessed 20 March 2014
Armstrong GT, Plana JC, Zhang N et al (2012) Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol 30:2876–2884
Ganame J, Claus P, Uyttebroeck A et al (2007) Myocardial dysfunction late after low-dose anthracycline treatment in asymptomatic pediatric patients. J Am Soc Echocardiogr 20:1351–1358
Toro-Salazar OH, Gillan E, O’Loughlin MT et al (2013) Occult cardiotoxicity in childhood cancer survivors exposed to anthracycline therapy. Circ Cardiovasc Imaging 6:873–880
Hoit BD (2011) Strain and strain rate echocardiography and coronary artery disease. Circ Cardiovasc Imaging 4:179–190
Hor KN, Gottliebson WM, Carson C et al (2010) Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging 3:144–151
Augustine D, Lewandowski AJ, Lazdam M et al (2013) Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: comparison with tagging and relevance of gender. J Cardiovasc Magn Reson 15:8
Weber OM, Higgins CB (2006) MR evaluation of cardiovascular physiology in congenital heart disease: flow and function. J Cardiovasc Magn Reson 8:607–617
Hor KN, Wansapura J, Markham LW et al (2009) Circumferential strain analysis identifies strata of cardiomyopathy in Duchenne muscular dystrophy: a cardiac magnetic resonance tagging study. J Am Coll Cardiol 53:1204–1210
Castillo E, Osman NF, Rosen BD et al (2005) Quantitative assessment of regional myocardial function with MR-tagging in a multi-center study: interobserver and intraobserver agreement of fast strain analysis with harmonic phase (HARP) MRI. J Cardiovasc Magn Reson 7:783–791
Bansal M, Cho GY, Chan J et al (2008) Feasibility and accuracy of different techniques of two-dimensional speckle based strain and validation with harmonic phase magnetic resonance imaging. J Am Soc Echocardiogr 21:1318–1325
Cho GY, Chan J, Leano R et al (2006) Comparison of two-dimensional speckle and tissue velocity based strain and validation with harmonic phase magnetic resonance imaging. Am J Cardiol 97:1661–1666
Llesuy SF, Milei J, Gonzalez Flecha BS et al (1990) Myocardial damage induced by doxorubicins: hydroperoxide-initiated chemiluminescence and morphology. Free Radic Biol Med 8:259–264
Yu W, Li SN, Chan GC et al (2013) Transmural strain and rotation gradient in survivors of childhood cancers. Eur Heart J Cardiovasc Imaging 14:175–182
Mor-Avi V, Lang RM, Badano LP et al (2011) Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr 24:277–313
Koopman LP, Slorach C, Manlhiot C et al (2011) Assessment of myocardial deformation in children using Digital Imaging and Communications in Medicine (DICOM) data and vendor independent speckle tracking software. J Am Soc Echocardiogr 24:37–44
Kempny A, Fernandez-Jimenez R, Orwat S et al (2012) Quantification of biventricular myocardial function using cardiac magnetic resonance feature tracking, endocardial border delineation and echocardiographic speckle tracking in patients with repaired tetralogy of fallot and healthy controls. J Cardiovasc Magn Reson 14:32
Kutty S, Rangamani S, Venkataraman J et al (2013) Reduced global longitudinal and radial strain with normal left ventricular ejection fraction late after effective repair of aortic coarctation: a CMR feature tracking study. Int J Cardiovasc Imaging 29:141–150
Jung B, Markl M, Foll D et al (2006) Investigating myocardial motion by MRI using tissue phase mapping. Eur J Cardiothorac Surg 29:S150–157
Neizel M, Lossnitzer D, Korosoglou G et al (2009) Strain-encoded (SENC) magnetic resonance imaging to evaluate regional heterogeneity of myocardial strain in healthy volunteers: comparison with conventional tagging. J Magn Reson Imaging 29:99–105
Aletras AH, Ding S, Balaban RS et al (1999) DENSE: displacement encoding with stimulated echoes in cardiac functional MRI. J Magn Reson 137:247–252
Morton G, Schuster A, Jogiya R et al (2012) Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J Cardiovasc Magn Reson 14:43
Poterucha JT, Kutty S, Lindquist RK et al (2012) Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J Am Soc Echocardiogr 25:733–740
Acknowledgment
This study was supported by the Cancer Director’s Research Fund, through the University of Michigan Comprehensive Cancer Center.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, J.C., Connelly, J.A., Zhao, L. et al. Strain measurement by cardiovascular magnetic resonance in pediatric cancer survivors: validation of feature tracking against harmonic phase imaging. Pediatr Radiol 44, 1070–1076 (2014). https://doi.org/10.1007/s00247-014-2992-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-014-2992-2