Abstract
A variety of abnormal imaging findings of the petrous apex are encountered in children. Many petrous apex lesions are identified incidentally while images of the brain or head and neck are being obtained for indications unrelated to the temporal bone. Differential considerations of petrous apex lesions in children include “leave me alone” lesions, infectious or inflammatory lesions, fibro-osseous lesions, neoplasms and neoplasm-like lesions, as well as a few rare miscellaneous conditions. Some lesions are similar to those encountered in adults, and some are unique to children. Langerhans cell histiocytosis (LCH) and primary and metastatic pediatric malignancies such as neuroblastoma, rhabomyosarcoma and Ewing sarcoma are more likely to be encountered in children. Lesions such as petrous apex cholesterol granuloma, cholesteatoma and chondrosarcoma are more common in adults and are rarely a diagnostic consideration in children. We present a comprehensive pictorial review of CT and MRI appearances of pediatric petrous apex lesions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The apex of the petrous pyramid is an important portion of the skull base, in close relationship to the cranial nerves, auditory apparatus, internal carotid artery and the venous plexus of the skull base. The ability to arrive at an accurate differential diagnosis for imaging findings in the petrous apex is critical in management and surgical decision-making because this location is difficult to access for biopsy or surgery. Petrous apex lesions are frequently asymptomatic but can present with cranial nerve palsy, often involving the sixth or seventh cranial nerves, which can improve following treatment of non-neoplastic lesions, especially in children [1, 2].
A wide spectrum of lesions occurs in the petrous apex in children, including both benign and malignant etiologies. Some lesions are very similar to those identified in adults; however, several lesions are unique to children. The purpose of this article is to familiarize the reader with the anatomy of the petrous apex and the varied causes of imaging abnormalities involving the petrous apex in children. Categories of petrous apex lesions in children include “leave me alone” lesions, infectious or inflammatory lesions, fibro-osseous lesions, neoplasms and neoplasm-like lesions, as well as a few rare miscellaneous conditions.
Imaging
Imaging of the temporal bone can be accomplished with the complementary modalities CT and MRI. CT is ideal for evaluating osseous destruction or expansion, and MRI is ideal for evaluating extra-osseous extension of petrous apex lesions as well as lesions arising outside the petrous apex and secondarily invading it. CT imaging of the temporal bones should include thin section (<0.7-mm slice thickness) images, reviewed in standard soft-tissue and bone algorithm. Reconstructions should always include the coronal plane. Additional long- and short-axis oblique images can be quite helpful in further evaluating structures such as the semicircular canals, ossicles, facial nerve canal, cochlea, modiolus, vestibular aqueduct and oval window. These oblique reformations are performed in the longitudinal oblique and the transverse oblique orientations. Longitudinal oblique (Stenvers) reformations are created in a plane perpendicular to a line drawn through the superior semicircular canal on an axial image. Transverse oblique reformations (Poschl) are created in a plane parallel to a line drawn through the superior semicircular canal on an axial image. CT evaluation of children with suspected inflammatory or neoplastic lesions should be performed after contrast administration; however, children undergoing temporal bone CT for evaluation of congenital sensorineural hearing loss do not require contrast-enhanced CT images. CT images should be reviewed at a window width of 4,000 Hounsfield units to best evaluate the osseous structures and at a window width of 120 Hounsfield units to evaluate the extracranial and intracranial soft tissues.
MR imaging protocols should include heavily T2-weighted 3-D acquisitions and pre-contrast T1-weighted images of the temporal bones. MRI protocols should also include images through the brain to assess primarily the brainstem. For indications other than congenital sensorineural hearing loss, MR imaging should include diffusion-weighted images and post-contrast fat-suppressed T1-weighted images of the temporal bones. MR angiogram and MR venogram studies are also frequently complementary to conventional images, to evaluate associated vascular complications of petrous apex pathology.
Anatomy
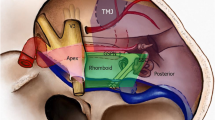
The petrous bone is not actually a separate bone; rather it is a pyramid-shaped portion of the temporal bone that serves as the posteromedial margin of the middle cranial fossa and as the anteromedial margin of the posterior fossa. Petrous apex refers to the anteromedial portion of the temporal bone that is anteromedial to the inner ear and internal auditory canal. The boundaries of the petrous apex are the greater wing of the sphenoid bone anteriorly, the occipital bone posteriorly, the foramen lacerum and clivus medially, and the nasopharynx inferiorly. The petrous bone is the hardest bone in the human body and contains the cochleovestibular organ and petrous internal carotid artery. It is closely associated with cranial nerves 5 through 8. Therefore patients with of the petrous apex can present with cranial neuropathies. The trigeminal ganglion (cranial nerve V3) lies along the superior margin of the petrous apex as it travels to the Meckel cave. Cranial nerve 6 also passes over the superior margin of the petrous apex before entering the Dorello canal. Cranial nerve 7 and cranial nerve 8 exit the brainstem and then enter the internal auditory canal, which marks the posterior margin of the petrous apex (Figs. 1, 2 and 3).
Normal CT anatomy. Axial thin-section bone CT slices through the petrous apices (*) in an 11-year-old boy demonstrate the relationship of the internal auditory canals (white arrows), petro-occipital fissures (white arrowheads), petrous carotid canals (CC), cochleas (open arrows), vestibules (black arrows) and jugular foramina (JF)
Normal MRI anatomy. Three nonconsecutive axial T2-weighted high-resolution MR images from superior to inferior in a 5-year-old girl. a Notice the close proximity of the non-pneumatized petrous apices (*) to the trigeminal nerves (black arrows), Meckel cave (white arrows) and the superior semicircular canals (open arrows). b The petrous apices (*) are posterior to the Meckel cave (white arrows) and anterior to the vestibulocochlear nerves (black arrows) within the internal auditory canals. c The non-pneumatized petrous apices (*) are anteriomedial to the cochleas (open arrows)
Development
The cartilaginous petrous apex progressively ossifies from an ossification center located in the arcuate eminence at 5–6 months of fetal life. Ossification spreads anterior and superior to the internal acoustic meatus and forms the medial wall of the cochlear aqueduct [3]. Ossification of the petrous apex can continue into early infancy as determined from a study of infant and pediatric skulls [4]. More precise information about the specific process and ossification centers involved in growth of the petrous apex is limited [4].
In infants younger than 4 months of age, a normal variant hypodense focus is sometimes recognized in the anterior otic capsule extending into the petrous apex, toward the petro-occipital fissure (Fig. 4). The hypoattenuated focus in the anterior otic capsule is sometimes associated with other foci of decreased attenuation in the middle otic layer and the fissula ante fenestram [4]. This is a normal developmental finding in infants that can represent incompletely mineralized bone or cartilage and should not be mistaken for an abnormality in young children. In an adult, low attenuation in any of these locations would be concerning for otosclerosis [4, 5]. Otosclerosis is rare in childhood.
Petrous apex aeration is variable and often parallels mastoid aeration [6]. Air cells arising from the medial mastoid antrum can extend into and pneumatize the petrous apex through multiple air cell tracts above and below the otic capsule [7].
‘Leave me alone’ lesions
“Leave me alone” lesions usually require no further imaging follow-up and include asymmetric pneumatization, trapped fluid and normal variant vestiges of cartilage in the very young, developing osseous petrous apex [4, 8].
Petrous apex asymmetric pneumatization (asymmetric fatty marrow)
Approximately 33% of petrous apices are pneumatized, and degree of pneumatization is asymmetrical in 5% to 10% of individuals [9–12]. Pneumatization of the petrous apex is from extension of air cells from the mastoid/middle ear cavity; these cells also provide pathways for disease [13]. When the petrous apices are asymmetrically pneumatized, the non-pneumatized side can be confused with a hyperintense T1 lesion. Asymmetric fatty marrow on the non-pneumatized side is hyperintense on T1- and T2-weighted MR images without fat suppression and is hypointense on fat-suppressed sequences, without osseous destruction or expansion (Fig. 5). Asymmetric pneumatization is more easily recognized on CT than it is on MRI, where preservation of bone marrow architecture is better recognized. Asymmetric pneumatization is an incidental finding and requires no further imaging or treatment.
Asymmetric pneumatization in a 12-year-old boy. a There is asymmetric hyperintense T2-W MR signal in the right petrous apex (arrow). b There is corresponding hyperintense T1-W MR signal (arrow) on the coronal image. c Axial CT image in the same child shows preserved osseous marrow trabeculation on the right and pneumatization of the left petrous apex
The main differential consideration when MRI shows a hyperintense T1-W signal petrous apex lesion is a nonexpansile cholesterol granuloma. Unlike asymmetric pneumatization, cholesterol granuloma maintains bright signal on fat-suppressed sequences. Cholesterol granulomas are also exceedingly rare in children. However, in the absence of fat-suppressed MR imaging or comparison CT images, follow-up imaging might be necessary to exclude a cholesterol granuloma [8].
Petrous apex effusion or trapped fluid
Petrous apex effusion or trapped fluid is thought to represent sterile, retained fluid in the petrous apex air cells after an episode of otomastoiditis caused by obstructed drainage from fibrosis along the communicating air channels [8]. However, opacification of previously pneumatized petrous apex air cells can also occur in the absence of a known clinical episode of otomastoiditis. This is presumably secondary to mucosal inflammation and fluid production, similar to the process that occurs in the paranasal sinuses and mastoid air cells in response to viral disease or allergens. The petrous apex air cells are filled with typical fluid signal on MRI (hyperintense T2, hypointense T1) and CT (without enhancement and without expansion, trabecular loss or bone destruction) (Fig. 6).
Trapped fluid in a previously pneumatized left petrous apex in a 10-year-old girl. a Multilobulated hyperintense T2-W MR signal is present in the left petrous apex (arrow), without expansion. This was hypointense on T1-weighted MR images (not shown). b Axial CT image shows opacification of petrous air cells with preserved air cell septations and no expansion (arrow). The right petrous apex and the mastoid air cells are well aerated. c On CT follow-up 2 years later, the left petrous apex air cells are clear
Trapped petrous apex fluid is a common incidental imaging finding and usually requires no further workup or treatment. Follow-up imaging, if performed for other reasons, typically shows a stable appearance of the imaging abnormality (Fig. 6). Rarely the petrous apex effusion has high protein content and has intermediate or hyperintense T1 signal on MRI. Differential considerations in these cases then include asymmetric pneumatization or small non-expansile cholesterol granuloma. Asymmetric fatty marrow would be hypointense with fat suppression, but distinguishing proteinaceous fluid from a small cholesterol granuloma can be difficult and follow-up imaging in 2–3 years should be considered [8]. On follow-up imaging, petrous apex effusions should be stable or the fluid should be resolved, whereas cholesterol granulomas can progress to become expansile over time.
Infections or inflammatory lesions
The most common infectious or inflammatory lesions involving the petrous apex in children include previously described trapped fluid, and involvement of petrous apex air cells in association with acute otomastoiditis and petrous apicitis. Petrous apex cholesterol granuloma is an uncommon lesion in children. Petrous apex mucocele occurring from expansion of an obstructed air cell with trapped respiratory epithelium is rare in adults and to our knowledge has not been described in children.
Petrous apicitis (apical petrositis)
Suppurative inflammation of the petrous apex can occur if there is extension of middle ear-mastoid infection into previously pneumatized petrous apex air cells. Although intracranial complications of otitis media are less frequent since the advent of antibiotics, petrous apicitis still occurs in children and adults. Early disease is confined to the petrous apex but advanced disease can spread to the adjacent skull base, the meninges and the cavernous sinus [14]. The trigeminal ganglion in the Meckel cave and the abducens nerve within the Dorello canal are both in close proximity to the petrous apex, being separated by only a thin layer of dura mater. Therefore, they are the most at-risk cranial nerves to be involved by petrous apex inflammation. Children with petrous apicitis are typically ill, presenting with part or all of the classic Gradenigo triad of otorrhea, lateral rectus palsy (abducens nerve) and deep facial/retro-orbital (trigeminal nerve) pain. Facial nerve paralysis and vertigo/sensorineural hearing loss from inflammation of the vestibulocochlear nerves can also occur. Involvement of cranial nerves 2, 3, 4, 9 and 10 has also been described secondary to inflammation extending into the cavernous sinus or the skull base [15]. In adults, petrous apicitis is often more indolent in nature, occurring secondary to chronic otitis media or after a mastoidectomy [16].
CT imaging in patients with petrous apicitis demonstrates petrous apex air cell opacification and destruction of the air cell septations (Fig. 7). On MR imaging, fluid signal is present in the petrous apex. Adjacent dural and cranial nerve enhancement indicates inflammatory involvement and can persist even after treatment (Fig. 7) [14]. Petrous apicitis can be complicated by intraosseous or intracranial abscesses, seen as classic rim enhancement and diffusion restriction on MRI [14, 17].
Otitis and right CN6 palsy secondary to right petrous apicitis in a 12-year-old girl. a On noncontrast CT there is a poorly marginated lytic area in the right petrous apex (arrow) in this girl, who had undergone mastoidectomy. b Axial post-contrast T1-weighted MR image shows abnormal enhancement involving the right petrous apex, middle ear and mastoids (arrowheads), as well as adjacent dural enhancement (arrow). c Axial post-contrast T1-weighted MR image at the level of the internal auditory canal shows enhancement of the 7th/8th CN complex (open arrow) and abnormal enhancement in the right cavernous sinus (arrow), surrounding a smaller-caliber cavernous carotid artery. d Coronal post-contrast T1-weighted MR image shows a small right internal carotid artery (arrow), consistent with arterial spasm
Historically treatment of petrous apicitis included aggressive surgical drainage. However, there has recently been increasing use of conservative measures in management of petrous apicitis, treating patients with antibiotics and possible myringotomy, reserving more invasive surgical drainage and ventilation procedures for patients who do not respond to conservative treatment [18].
Petrous apex osteomyelitis can also occur in a non-pneumatized petrous apex as a component of skull base osteomyelitis. This is typically seen in adults who have diabetes, with necrotizing otitis externa causing contiguous petrous apex involvement or involvement secondary to thrombophlebitis from the venous plexus of the petrous carotid canal. Petrous apex osteomyelitis occurring in non-pneumatized petrous apex is most commonly encountered in adults and is uncommon in children [9].
Cholesterol granuloma
Although cholesterol granuloma is the most common lesion in the petrous apex in adults, it is rare in children, especially before adolescence [8, 19]. Cholesterol granulomas are thought to be sequelae of chronic inflammatory disease, whereby a foreign-body giant-cell reaction to cholesterol deposits occurs. Obstructed air cells develop a vacuum phenomenon, resulting in episodes of repeated hemorrhage, chronic inflammation, fibrosis and vascular proliferation, all subsequently contained in a fibrous capsule [19]. An alternate mechanism proposed for development of cholesterol granulomas is a breakdown of bone between the aerated petrous apex and the adjacent marrow cavity, with the exposed marrow being a potential source of repeated hemorrhage [11]. Small lesions are asymptomatic, but these lesions are characterized by slow growth. Increase in lesion size can result in compressive effects on adjacent neurovascular structures so patients may present with headaches.
CT imaging shows an expansile osseous lesion causing cortical thinning and remodeling with central low attenuation and trabecular destruction. These lesions contain coarse septations or calcifications (Fig. 8) [16]. On MRI the lesions are sharply marginated, typically hyperintense on T1, T2 and fluid-attenuated inversion recovery (FLAIR) sequences, and often have a characteristic peripheral low signal T2 rim from hemosiderin deposition (Fig. 8). There is sometimes a thin rim of contrast enhancement; however the majority of the lesion does not enhance [16]. If cholesterol granuloma is in the differential diagnosis of a small asymptomatic lesion, periodic follow-up imaging is suggested because of the potential for expansion and mass effect on adjacent cranial nerves and the internal carotid artery. Generally serial follow-up imaging is performed every 2–3 years for smaller asymptomatic lesions to evaluate change in the lesion, and follow-up imaging can be performed earlier if the patient becomes symptomatic in the interval (Fig. 8) [8]. Because of the increasing awareness of the risks of radiation from CT scans and the classic appearance of cholesterol granuloma on MRI, MR imaging is the modality of choice to follow these lesions. Larger, symptomatic lesions causing mass effect on adjacent structures require surgical drainage for initial treatment, and follow-up postoperative imaging is recommended because these lesions can recur following drainage [19].
Cholesterol granuloma in a 16-year-old girl presenting with headache. a Axial T1-W and (b) T2-weighted MR images demonstrate a nonexpansile hyperintense signal within the right petrous apex. c On CT there is opacification of the right petrous apex air cells with preserved septations (arrow). Differential considerations at this point would include early cholesterol granuloma and atypical trapped fluid within petrous apex air cells. d Follow-up CT 3 years later demonstrates destruction of multiple septations (arrow), now consistent with cholesterol granuloma, which was confirmed at surgery
A cholesterol granuloma is invariably the initial consideration in an expansile osseous lesion at the petrous apex with hyperintense T1 and T2 MR signal. However, if the lesion is small and nonexpansile, then asymmetrical fatty marrow and proteinaceous petrous apex effusion are on the list of differential diagnoses. As described earlier, asymmetrical fatty marrow does not show trabecular destruction on CT and is hypointense on fat-suppressed MR sequences. Proteinaceous petrous apex effusion can be difficult to differentiate from a small cholesterol granuloma, so follow-up imaging to assess change in size or stability of the lesion is quite helpful.
Fibro-osseous lesions
Fibrous dysplasia is the most common fibro-osseous lesion involving the petrous bone in children and young adults, and Paget disease is more often seen in older adults. In both conditions, involvement of the petrous bone is often part of multifocal skull base involvement. Aneurysmal bone cysts have also been described at the petrous apex in children and young adults. Other fibro-osseous lesions such as ossifying fibroma and cemento-ossifying fibroma are extremely rare in the petrous apex.
Fibrous dysplasia
Fibrous dysplasia is a genetic disorder of bone proliferation and maturation. Fibrous dysplasia can be monostotic, polyostotic or part of the McCune-Albright syndrome (triad of fibrous dysplasia, café au lait spots and endocrine dysfunction with precocious puberty) [20]. The monostotic form is more prevalent, but involvement of the temporal bones is more common in the polyostotic form. The polyostotic form also presents in younger children compared to the monostotic form, which presents in older children and adults [20]. The most common presentation of temporal bone involvement is conductive hearing loss caused by external auditory canal stenosis [20]. Although temporal bone involvement is not uncommon, involvement of the petrous apex with fibrous dysplasia appears to be quite rare.
On MR imaging, the lesions are expansile, often with low signal intensity on T1- and heterogeneous hypointense or hyperintense signal on T2-weighted MR images. Occasionally there is a soft-tissue component as well [21]. The lesions often enhance avidly and can be easily mistaken for a malignant process on MRI. CT is almost always diagnostic, demonstrating the characteristic expansile lesion in the medullary space with ground-glass matrix (Fig. 9). The lesions can show mixed sclerotic and lucent areas or lucent areas with thin sclerotic margins on CT [20].
Most lesions of fibrous dysplasia spontaneously cease to grow past young adulthood. Complications of skull base involvement include narrowing of neural or vascular foramina, resulting in cranial neuropathies or vascular insufficiency and surgical curettage might be considered in such cases [11, 20].
Paget disease of the skull base can have an appearance similar to that of fibrous dysplasia, but this is a disease of older adults. Meningioma at the petrous apex, whether dural-based or intraosseous, can also cause expansion and sclerosis of the bone but is seen as an enhancing soft-tissue mass on MRI or CT. Meningiomas are more common in adults and are also exceedingly rare in children without neurofibromatosis type 2.
Aneurysmal bone cysts
Aneurysmal bone cysts involving the temporal bone are quite uncommon, and those involving the petrous apex are exceedingly rare, but are nevertheless more often described in children and young adults [22, 23]. Aneurysmal bone cysts can be found in isolation or associated with other fibro-osseous lesions, including fibrous dysplasia [20]. The imaging appearance of aneurysmal bones cysts involving the petrous apex is similar to that involving any other osseous structure, classically an expansile, multiloculated, “bubbly” lesion with fluid-fluid levels and peripheral and septal contrast enhancement (Fig. 10). An uncommon variant of aneurysmal bone cyst is a solid lesion that demonstrates diffuse contrast enhancement. Although imaging can be diagnostic of an aneurysmal bone cyst, these cysts can also occur as secondary lesions superimposed on a histologically different primary lesion, so histopathological examination might be needed to confirm the diagnosis [22].
Imaging in a 2-month-old boy with left facial palsy secondary to petrous apex aneurysmal bone cyst. a Axial CT demonstrates an expansile lytic lesion (arrows) with cortical disruption and otic capsule invasion, without aggressive periosteal reaction. b Axial fast imaging employing steady state acquisition (FIESTA) T2-weighted MR image demonstrates heterogeneous, primarily hypointense signal within the mass, with two associated fluid-fluid levels (arrows). c Axial post-contrast T1-weighted fat-suppressed MR image shows heterogeneous, predominantly septal enhancement within the lesion as well as the cochlea (asterisk). The presence of fluid-fluid levels raised the question of aneurysmal bone cyst, which was confirmed at surgery
Neoplasms and neoplasm-like lesions
In children malignant neoplasms of the petrous apex are more common than benign neoplasms. Benign petrous apex neoplasms such as meningiomas and nerve sheath tumors are almost exclusively seen in adults, except in the setting of neurofibromatosis. The most common primary malignant petrous apex lesions in children are rhabdomyosarcoma and Ewing sarcoma. The most common metastatic disease to involve the petrous apex in children is neuroblastoma. Conversely in adults the common malignant petrous apex lesions include chondrosarcoma, chordoma, plasmacytoma and hematogenous metastases from breast, lung, prostate, skin or renal malignancies. Most malignant petrous apex neoplasms are destructive lesions, isointense or hyperintense on T2-weighted images, with variable enhancement [24].
Rhabdomyosarcoma
Rhabdomyosarcoma is the most common soft-tissue malignancy in children and the most common primary malignancy of the temporal bone [25]. Half of rhabdomyosarcomas in children occur in the head and neck, and 7% of head and neck rhabdomyosarcomas involve the temporal bone [25]. CT and MRI are complementary in the evaluation of children with temporal bone rhabdomyosarcoma. CT best demonstrates aggressive osseous destruction and can show associated enhancing soft-tissue mass (Fig. 11). Temporal bone involvement is considered a parameningeal site, and up to 55% of patients with parameningeal rhabdomyosarcoma also have intracranial extension; MR imaging is ideal to assess associated intracranial extension. Temporal bone rhabdomyosarcoma is rarely completely resectable, therefore treatment consists of biopsy/debulking procedure followed by chemotherapy and radiation therapy.
Right petrous apex rhabdomyosarcoma in a 2-year-old boy. a Axial contrast-enhanced CT image demonstrates destruction of the right petrous apex by an enhancing soft-tissue mass (arrow). b On post-contrast T1-weighted MR imaging the mass shows heterogeneous enhancement (arrow). Biopsy revealed rhabdomyosarcoma
Ewing sarcoma
Ewing sarcoma is a small round blue cell group of neoplasms that primarily affects children and young adults. This neoplasm often involves the axial and appendicular skeleton, with less than 6% of reported lesions involving the skull [26]. On imaging, Ewing sarcoma involving the petrous apex is an aggressive-appearing, enhancing mass with osseous destruction and periosteal reaction (Fig. 12). It is important to delineate tumor extent into the adjacent structures to allow for surgical planning. Therefore, CT and MRI are often complementary studies used to evaluate children with petrous apex Ewing sarcoma. Because of the proximity to, and possible involvement of important adjacent skull base structures, treatment includes combinations of radiotherapy, chemotherapy and surgical debulking [26].
Metastatic neuroblastoma
Neuroblastoma is unique to children, and most patients have an abdominal, retroperitoneal primary tumor, with 3–5% occurring in the neck as primary site of origin [27]. Head and neck involvement is most often identified as metastatic disease to cervical lymph nodes and osseous metastases to the calvarium, orbits and skull base. Osseous metastases from neuroblastoma are frequently multiple and usually have imaging findings of a well-defined enhancing soft-tissue mass with osseous destruction on CT and MRI (Fig. 13). Characteristic aggressive periosteal reaction, best identified on CT, is frequently present. Treatment is multimodal and prognosis is poor in children with extensive metastatic disease.
Metastatic neuroblastoma in a 4-year-old boy. a Axial contrast-enhanced head CT shows a destructive lesion of the right petrous apex (large arrow). The lesion extends into the middle ear cavity (small arrow) and the jugular foramen (arrowheads). No periosteal reaction is present in this child, but this feature is frequently present in children with skull base metastatic neuroblastoma. b On axial T1-W post-contrast fat-saturated MR image there is enhancement of the large soft-tissue mass, consistent with metastasis. (Courtesy of Dr. Caroline Robson)
Langerhans cell histiocytosis
Langerhans cell histiocytosis is a disease of proliferating Langerhans-type histiocytes, with an undetermined etiology. The temporal bone is the most common site of skull base involvement. It can affect any portion of the temporal bone, with the mastoid portion and petrous apex most frequently affected [28]. On CT, Langerhans cell histiocytosis appears as an enhancing soft-tissue mass with well-circumscribed destruction of the temporal bone that sometimes extends to the bony labyrinth and middle ear ossicles (Fig. 14). On MRI, the lesions appear as focal enhancing masses surrounded by bone marrow and soft-tissue edema [28]. Unlike metastatic neuroblastoma, aggressive periosteal reaction is typically lacking in Langerhans cell histiocytosis.
Management is varied, including but not limited to watchful waiting, surgical curettage, radiation therapy, systemic steroid therapy and chemotherapy. Prognosis is variable, depending on extent of disease. Follow-up imaging can demonstrate healing lesions (Fig. 14).
Miscellaneous lesions
Cholesteatoma
Petrous apex cholesteatomas can be acquired or congenital and the two cannot be differentiated histologically [29]. These lesions are more common in young adults and less frequent in younger children. Akin to epidermoid cysts, congenital petrous apex cholesteatomas are proposed to arise from normal epidermoid cell rests in the petrous apex, or from sequestration of migrated epithelial remnants from the external auditory canal during the fetal period. Acquired petrous apex cholesteatomas arise in the tympanomastoid region and erode medially through or around the labyrinth into the petrous apex. Patients often present with symptoms of hearing loss, although recurrent meningitis, facial or abducens nerve palsy or trigeminal neuralgia can also be the initial presentation [29].
On CT imaging petrous apex cholesteatomas are well-defined expansile lesions, with central low attenuation (Fig. 15). On MRI, they have hypointense T1 and hyperintense T2 signal and might be nonenhancing or demonstrate subtle peripheral rim enhancement following contrast administration (Fig. 15). Restricted diffusion is characteristic of cholesteatomas, in any region of the temporal bone (Fig. 15).
Congenital petrous apex cholesteatoma in a 32-year-old man. a Axial CT demonstrates a well-defined, lobulated lytic lesion in the left petrous apex (arrow), with extension eroding the otic capsule adjacent to the cochlea. b Axial post-contrast T1-weighted MR image shows lack of contrast enhancement (arrows). c Axial diffusion-weighted MR image shows hyperintensity (arrow) consistent with diffusion restriction. These findings are typical of cholesteatoma. (Courtesy of Dr. H. Ric Harnsberger)
Cholesteatomas are slow-growing, locally aggressive lesions and are surgically managed. High-resolution temporal bone CT is necessary prior to surgery to evaluate the extent of osseous destruction and predict prognosis. MRI with diffusion-weighted imaging is increasingly being used to evaluate residual disease and recurrent disease [29].
Petrous apex meningocele
Petrous apex cephaloceles (most commonly meningoceles) are often considered incidental “leave me alone” lesions in adults. Although these lesions can be asymptomatic in adults, they are more commonly symptomatic in children presenting with cerebrospinal fluid (CSF) leak [30–32]. These lesions represent a protrusion of meninges and CSF into the petrous apex. These skull base meningoceles are proposed to occur when there is a congenitally thin petrous apex roof or small petrous apex roof dehiscence that enlarges over time from chronic increased CSF pulsations, often in obese individuals [30].
On CT and MRI, there is a lobulated, expansile cystic CSF-filled structure centered along the posterolateral margin of the Meckel cave containing the trigeminal ganglion (Fig. 16). Rarely an arachnoid cyst in this location is indistinguishable from petrous apex meningocele by imaging [30].
Right petrous apex meningocele in a 9-year-old boy. a Contrast-enhanced CT shows smooth destruction of the petrous apex. Expansion of the Meckel cave on the right (arrow) is not well seen on bone algorithm image. b Coronal T1-weighted and (c) axial T2-W fat-saturated MR images show cerebrospinal fluid signal intensity filling the petrous apex lesion and extending into the enlarged right Meckel cave (arrows). (Courtesy of Dr. Carolina Guimaraes)
Asymptomatic petrous apex meningoceles usually require no treatment, but the presence of a cerebrospinal fluid leak necessitates surgical repair.
Petrous internal carotid aneurysm
Petrous internal carotid artery aneurysms are extremely rare, particularly in children, but are sometimes incidentally identified on imaging. These aneurysms can be congenital, traumatic or mycotic in origin and can be asymptomatic or present with cranial nerve palsies, Horner syndrome, sensorineural hearing loss, epistaxis or otorrhagia [33, 34]. Petrous internal carotid aneurysms are often fusiform in configuration. On CT there is smooth expansion of the walls of the petrous carotid canals, or the walls might be dehiscent (Fig. 17). On MRI the lesions can have variable signal intensity depending on internal turbulence or the presence of thrombus [9]. Although CT angiography and MR angiography can both be used for identification and characterization of petrous carotid artery aneurysms, conventional catheter angiography is used for detailed evaluation of the lesion prior to intervention. Symptomatic lesions can be treated by endovascular techniques (obliteration or stents), or surgical trapping and high-flow bypass [33, 34].
Connective tissue disease and known petrous internal carotid artery aneurysms in a 19-year-old man. The aneurysms were identified on MR angiography (not shown) at an outside institution. Noncontrast head CT demonstrates massive, relatively smooth dilatation of the petrous carotid canals bilaterally (arrows)
Summary
A variety of lesions occur in the pediatric petrous apex. Many are asymptomatic and identified on imaging studies performed for clinical reasons unrelated to the petrous apex, and others are identified when the child presents with cranial nerve palsies, headaches or hearing loss. Imaging studies, especially CT and MRI, in association with the clinical presentation, are very important to characterize lesions involving the petrous apex. Imaging characteristics are quite helpful in both narrowing the differential diagnosis and planning clinical management (Table 1). Many of the lesions that involve the petrous apex in children are very similar to those identified in adults. These include entities such as cholesterol granuloma, cholesteatoma, trapped petrous apex fluid and asymmetrical pneumatization of the petrous apex. However, a few lesions are primarily identified in children, including a developmental lucency in infants, rhabdomyosarcoma, Langerhans cell histiocytosis and metastatic neuroblastoma.
References
Aroichane M, Repka MX (1995) Outcome of sixth nerve palsy or paresis in young children. J Pediatr Ophthalmol Strabismus 32:152–156
Shargorodsky J, Lin HW, Gopen Q (2010) Facial nerve palsy in the pediatric population. Clin Pediatr 49:411–417
Rich PM, Graham J, Phelps PD (2002) Hyrtl’s fissure. Otol Neurotol 23:476–482
Moser T, Veillon F, Sick H et al (2008) The hypodense focus in the petrous apex: a potential pitfall on multidetector CT imaging of the temporal bone. AJNR Am J Neuroradiol 29:35–39
Chadwell JB, Halsted MJ, Choo DI et al (2004) The cochlear cleft. AJNR Am J Neuroradiol 25:21–24
Yetiser S, Kertmen M, Taser M (2002) Abnormal petrous apex aeration. Review of 12 cases. Acta Otorhinolaryngol Belg 56:65–71
Curtin HD, Som PM (1995) The petrous apex. Otolaryngol Clin North Am 28:473–496
Moore KR, Harnsberger HR, Shelton C et al (1998) ‘Leave me alone’ lesions of the petrous apex. AJNR Am J Neuroradiol 19:733–738
Razek AA, Huang BY (2012) Lesions of the petrous apex: classification and findings at CT and MR imaging. Radiographics 32:151–173
Connor SE, Leung R, Natas S (2008) Imaging of the petrous apex: a pictorial review. Br J Radiol 81:427–435
Isaacson B, Kutz JW, Roland PS (2007) Lesions of the petrous apex: diagnosis and management. Otolaryngol Clin North Am 40:479–519
Chaljub G, Vrabec J, Hollingsworth C et al (1999) Magnetic resonance imaging of petrous tip lesions. Am J Otolaryngol 20:304–313
Virapongse C, Sarwar M, Bhimani S et al (1985) Computed tomography of temporal bone pneumatization: 1. Normal pattern and morphology. AJR Am J Roentgenol 145:473–481
Hardjasudarma M, Edwards RL, Ganley JP et al (1995) Magnetic resonance imaging features of Gradenigo’s syndrome. Am J Otolaryngol 16:247–250
Sherman SC, Buchanan A (2004) Gradenigo syndrome: a case report and review of a rare complication of otitis media. J Emerg Med 27:253–256
Chapman PR, Shah R, Cure JK et al (2011) Petrous apex lesions: pictorial review. AJR Am J Roentgenol 196:WS26–WS37
Murakami T, Tsubaki J, Tahara Y et al (1996) Gradenigo’s syndrome: CT and MRI findings. Pediatr Radiol 26:684–685
Kantas I, Papadopoulou A, Balatsouras DG et al (2010) Therapeutic approach to Gradenigo’s syndrome: a case report. J Med Case Rep 4:151
Charles D, Bluestone JOK (2007) Otitis media in infants and children, 4th edn. BC Decker Inc., Hamilton, pp 407–408
Lustig LR, Holliday MJ, McCarthy EF et al (2001) Fibrous dysplasia involving the skull base and temporal bone. Arch Otolaryngol Head Neck Surg 127:1239–1247
Jee WH, Choi KH, Choe BY et al (1996) Fibrous dysplasia: MR imaging characteristics with radiopathologic correlation. AJR Am J Roentgenol 167:1523–1527
Sayama CM, MacDonald JD (2010) Aneurysmal bone cyst of the petrous bone: case presentation and review of the literature. Pediatr Neurosurg 46:308–312
Lackmann GM, Tollner U (1993) Aneurysmal cyst of the petrosal bone. Arch Dis Child 69:241–242
Reid SR, Hetzel T, Losek J (2006) Temporal bone rhabdomyosarcoma presenting as acute peripheral facial nerve paralysis. Pediatr Emerg Care 22:743–745
Durve DV, Kanegaonkar RG, Albert D et al (2004) Paediatric rhabdomyosarcoma of the ear and temporal bone. Clin Otolaryngol Allied Sci 29:32–37
Kadar AA, Hearst MJ, Collins MH et al (2010) Ewing’s sarcoma of the petrous temporal bone: case report and literature review. Skull Base 20:213–217
Brown RJ, Szymula NJ, Lore JM Jr (1978) Neuroblastoma of the head and neck. Arch Otolaryngol 104:395–398
D’Ambrosio N, Soohoo S, Warshall C et al (2008) Craniofacial and intracranial manifestations of Langerhans cell histiocytosis: report of findings in 100 patients. AJR Am J Roentgenol 191:589–597
Moffat D, Jones S, Smith W (2008) Petrous temporal bone cholesteatoma: a new classification and long-term surgical outcomes. Skull Base 18:107–115
Moore KR, Fischbein NJ, Harnsberger HR et al (2001) Petrous apex cephaloceles. AJNR Am J Neuroradiol 22:1867–1871
Hervey-Jumper SL, Ghori AK, Quint DJ et al (2010) Cerebrospinal fluid leak with recurrent meningitis following tonsillectomy. J Neurosurg Pediatr 5:302–305
Schick B, Draf W, Kahle G et al (1997) Occult malformations of the skull base. Arch Otolaryngol Head Neck Surg 123:77–80
Liu JK, Gottfried ON, Amini A et al (2004) Aneurysms of the petrous internal carotid artery: anatomy, origins, and treatment. Neurosurg Focus 17:E13
Malikov S, Thomassin JM, Magnan PE et al (2010) Open surgical reconstruction of the internal carotid artery aneurysm at the base of the skull. J Vasc Surg 51:323–329
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
CME activity
This article has been selected as the CME activity for the current month. Please visit the SPR Web site at www.pedrad.org on the Education page and follow the instructions to complete this CME activity.
Rights and permissions
About this article
Cite this article
Radhakrishnan, R., Son, H.J. & Koch, B.L. Petrous apex lesions in the pediatric population. Pediatr Radiol 44, 325–339 (2014). https://doi.org/10.1007/s00247-013-2836-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-013-2836-5