Abstract
Background
The contribution of MRI in the prenatal evaluation of congenital lung abnormalities (CLA) has not been extensively investigated.
Objective
(1) To compare diagnostic accuracy and assessment of prognostic factors between US and MRI in CLA and (2) to assess the diagnosis agreement between prenatal imaging and postnatal diagnosis.
Materials and methods
We included 23 consecutive fetuses who underwent concomitant US and MRI during gestation as well as postnatal CT and surgery (n = 22).
Results
US-MRI sets were performed at median gestational age of 26 (n = 16) and 34 (n = 22) weeks. Postnatal diagnoses were 11 congenital pulmonary airway malformations (CPAM), 4 bronchopulmonary sequestrations (BPS), 6 hybrid lesions and 2 cysts. US and MRI agreement was significantly better during the second trimester than during the third one (P = 0.02). Disagreements were related to missed cysts (n = 5), mediastinal shift (n = 6) and vessels (n = 5). US and MRI diagnosis agreement was present in 20 cases, including 5 cases of misdiagnosis. US and MRI were concordant with postnatal diagnosis in 17 and 16 cases, respectively.
Conclusion
In our series, no clear superiority of MRI over US in the prenatal evaluation of CLA was demonstrated, but US better demonstrated systemic feeding vessels and MRI cysts and normal lung adjacent to the lesion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
US imaging has been used for a long time in routine practice for the detection and follow-up of CLA, classically known as CPAM, BPS, congenital lobar emphysema and bronchogenic cyst [1]. The contribution of US in the evaluation of CLA has been widely studied. Despite the possibility for US to evaluate poor prognostic factors such as mediastinal shift, pleural effusion and fetal hydrops [2, 3], some limitations of US have been reported. US might fail to successfully establish prenatal diagnosis when the systemic feeding artery of BPS remains overlooked [4] or in complex malformations (particularly when diaphragmatic hernia is associated) when the lesions are hard to assess [2]. Finally, it is acknowledged that US sometimes detects spontaneous lesion resolution [5] while lesions remain apparent on postnatal CT [6].
The role of MRI as a complementary diagnostic and prognostic tool in the prenatal evaluation of CLA has not been widely investigated. Sporadic cases and small series have been reported [7–14], but very few large series providing comparison of the diagnostic and prognostic contribution of US and MRI are available in the literature [15, 16]. Therefore, the relevance of MRI and its possible advantages over US in the investigation of CLA need to be further assessed.
We conducted a systematic study in fetuses with CLA (congenital diaphragmatic hernia excluded) and compared the results of prenatal imaging (US and MRI) with postnatal CT and surgery. Our aims were (1) to compare the accuracy of diagnostic findings and the assessment of prognostic factors between US and MRI and (2) to assess the diagnosis agreement between prenatal imaging and postnatal CT and surgery with histological findings.
Materials and methods
Patients
All consecutive fetuses with CLA seen between April 2007 and January 2011 were explored during the prenatal period with MRI and included in our database RespiRare software (elaborated by UMR-S 707 and 719, INSERM) of the national reference centre for rare lung diseases. Our institutional ethics review board approved the use of this database register (CCTIRS, n°08.015bis). The parents were informed by their physicians of the nature and goal of all investigations performed and all of them gave their informed consent.
Inclusion criteria in the study were (1) lung malformation detected with prenatal US, (2) at least one prenatal US-MRI examination performed concomitantly and (3) postnatal CT. Exclusion criteria were (1) diaphragmatic hernia without associated lung malformation and (2) unavailable prenatal imaging at the time of the study for investigator assessment.
Techniques
The mothers were usually referred following the detection, during US screening performed in the second trimester of pregnancy, of a lung abnormality. Because of the natural course of CLA during the second part of pregnancy, we performed two MRI scans to clarify the best timing for this imaging modality. The first MRI was performed on the same day as the US (US 1-MRI 1). The second MRI was performed during the third trimester on the same day as the US (US 2-MRI 2). When the malformation was detected during the third trimester, only one MRI was performed. After birth, high-resolution CT was performed at a variable age, according to the child’s respiratory symptoms. The children were operated on when surgery was deemed necessary and the specimens were analysed by pathologists.
Prenatal US exams were performed on a Toshiba Applio 80XV (Toshiba America Medical Systems, Tustin, CA) by radiologists working in our tertiary care centre. Prenatal MRI was performed on a 1.5-T Achieva (Philips Medical Systems, Best, The Netherlands) 30 min after maternal oral administration of flunitrazepam (1 mg) according to our routine protocol. T2-weighted images (single-shot turbo spin-echo sequences TR/TE 15,000 ms/120 ms; flip angle 90°; turbo SE factor 102; matrix 256 × 170; field of view 300 mm; section thickness 50% overlapped 4 mm; acquisition time 45 s) were acquired in the three planes of the space. Spiral chest CT was performed on a Philips unit MX8000 IDT16 (Philips, Best, The Netherlands) after administration of intravenous contrast agent at a dose of 2 ml/kg of body weight. Parameters used were set at 90 kV and 50 mAs. Multiplanar reconstructions were obtained. All US, MRI and CT images were retrospectively analysed by a senior radiologist with 25 years of experience in paediatric radiology (CG).
Classification
The lesions were described in terms of anatomical location, dimensions, presence of cysts, echogenicity or intensity, visibility of a feeding artery, mass effect (e.g. mediastinal shift, flattened or reversed diaphragm, hydrops). When cysts were observed, their size was graded as follows: I, < 5 mm; II, ≥ 5 mm [17]. Moreover, the size of the lesion was measured in three dimensions—height (H), lateral diameter (LD) and anteroposterior diameter (APD)—on both prenatal US and MRI and postnatal CT images. CPAM volume ratio was calculated as previously described in fetuses with prenatal suspicion of CPAM [18]. Through visual approximation, the volume of the lesion was scored as being inferior, equal or superior to 50% of the ipsilateral lung volume.
The suspected prenatal (US + MRI) and postnatal (CT) diagnosis was established on the basis of the presence of cysts within a solid lung lesion, a feeding artery, a solid lung lesion without cysts, or a single central cyst, these various patterns suggesting respectively type 1 or 2 CPAM, BPS, type 3 CPAM or emphysema, and bronchogenic cyst or oesophageal duplication. A hybrid lesion was defined as a combination of at least two of these lesions.
The diagnosis established by surgery and pathology was based on the visibility of systemic arterial supply by the surgeon for BPS, and the presence at pathology of cystic cavities lined by respiratory ciliated epithelium suggesting CPAM (type 1 for lesions greater than 2 cm, type 2 for lesions less than 2 cm, and type 3 for pulmonary macroscopically non-cystic hyperplasia) [19]. Emphysema, defined as airspace enlargement, could be associated with all types of lesions and located in an enlarged lobe (congenital lobar emphysema) or widespread in an otherwise not enlarged pulmonary tissue.
Statistical analysis
Quantitative variables are reported as median (interquartile range [IQR]) or mean (standard deviation [SD]) as adapted, and qualitative variables as number (percentage). Comparisons of continuous variables between US and MRI measurements were established using paired Wilcoxon signed rank test. Comparison between categorical variables used chi-squared test. All tests were two-tailed, and P values less than 0.05 were considered statistically significant. Statistical analyses were performed using StatView software (SAS Institute, Cary, NC, USA).
We defined agreement between US and MRI using (1) relevant items such as the presence of cysts, systemic feeding artery and mediastinal shift and (2) evaluation of the global size of the lesion (<, = or > 50% of the ipsilateral lung), the size of the largest cyst (< or ≥ 5 mm) and the presence of normal lung adjacent to the lesion. We defined agreement between US 2 or MRI 2 and CT according to the detection of cysts, systemic arterial supply (whatever the number of arteries), location of the lesion, and the presence of normal lung adjacent to the lesion. To investigate inter-techniques (US-MRI) agreement, we prepared Bland-Altman plots of the three measurements of the lesion (height, lateral diameter and anteroposterior diameter) (Fig. 1). US and MRI CPAM volume ratios were considered in agreement if there were both ≤ 1.6 or > 1.6 [18].
Dimensions of the lesions. a Bland-Altman plot of height of the lesion (H) measured using US and MRI (inter-techniques variability) in 31 US-MRI sets performed in 21 fetuses. Open triangles refer to US 1-MRI 1 and solid triangles to US 2-MRI 2. Lines show mean (solid line) ± 1.96 SD (dotted lines) of the difference between the height dimension values. b Changes in three dimensions between the two MRI scans of lesions in 17 fetuses. Solid circles refer to three fetuses with BPS that switched from MRI 1 hyperintensity to MRI 2 hypointensity. Stars refer to BPS that remained hyperintense on MRI 2. Open triangles refer to nine fetuses with no antenatal suspicion of BPS. LD lateral diameter, APD anteroposterior diameter
Results
All 23 fetuses (15 male and 8 female) seen in our centre during the study period were included. The study encompassed 38 US exams, 40 MRI exams (38 US-MRI sets all performed on the same day) and 23 CT exams (one per child) (Table 1). The median (range) gestational age at first and second MRI assessment was 26 (25–29) weeks and 34 (31–37) weeks, respectively. CT was performed at a median postnatal age of 1 month (from 1 day to 3 months). The lung imaging findings are summarised in Table 1. It is noteworthy that no extra pulmonary abnormalities were detected by MRI. The neonates were delivered at a median (IQR) (range) gestational age of 39.6 (38.1; 40) (33.6–42.3) weeks, with a median (IQR) interval between birth and the last prenatal imaging of 5.5 (3.4; 6.2) weeks. The only child who presented severe neonatal respiratory distress died at 20 days of age because of refractory respiratory distress despite early surgery (case 8, Table 1). One child had a short duration neonatal respiratory distress and another sustained pulmonary infection at 8 months of age and was operated on at 11.5 months. All but one child underwent postnatal surgery at a median (IQR) (range) age of 6.5 (5; 8) (0.2–11) months. The surgical and pathological findings are summarised in Table 1.
In one child, only US 1-MRI 1 was available and in seven children, only US 2-MRI 2 was available (Table 1). Because of poor contrast resolution between the lesion and the normal surrounding lung, it was impossible to measure properly the lesion in four US scans (Table 1); for the same reason, one of the three dimensions was not available in three US scans. Twenty CLA were located in lower lobes (ten right, ten left); two were diffuse to the whole left lung and one was present in the right upper lobe. There was no significant difference between height, lateral diameter and anteroposterior diameter dimensions in all US-MRI sets (P = 0.81 for height, P = 0.33 for lateral dimension, P = 0.46 for anteroposterior diameter). Bland-Altman plots established in 22 children (31 US-MRI sets for height (Fig. 1), 34 US-MRI sets for lateral diameter and anteroposterior diameter) showed a trend for the three dimensions to be higher when measured using MRI compared with US. However, the mean (SD) biases were 0.8 (7) mm, 1 (4.6) mm, 1 (5.7) mm for height, lateral diameter and anteroposterior diameter, respectively, of no clinical relevance. In 13 children, the CPAM volume ratio could be compared between US and MRI. In two children (cases 8 and 20, Table 1), CPAM volume ratio was discordant between US and MRI, each time with results close to 1.6 for one modality.
In three out of the seven fetuses who presented an imaging pattern in keeping with BPS and underwent a second MRI, the intensity of the lesion changed from hyperintensity to hypointensity (Fig. 1). The changes in the three dimensions of the lesions between MRI 1 and MRI 2 according to the presence of a systemic feeding artery and to the changes in signal intensity are also shown in Fig. 1.
There was a similar number of cases with agreements or disagreements between US and MRI in the 38 recorded sets, but agreement was significantly more frequent between US 1-MRI 1 sets than between US 2-MRI 2 sets (Table 2). In the 38 US-MRI sets, causes of disagreement between US and MRI (four sets encompassed two disagreements) were: (1) the presence of a systemic feeding vessel (n = 5 sets in four cases, two sets in one case, demonstrated a systemic supply by MRI and not by US) (Figs. 2 and 3); (2) a mediastinal shift (observed in n = 6 sets, in six cases, with US 2 but not with MRI 2, the latter being in agreement with postnatal CT in five cases); (3) the presence of cysts (observed in n = 5 sets, in four cases, with MRI but not with US in four sets (three cases), and in all cases cysts were present on postnatal CT); (4) largest cyst ≥ 5 mm (n = 2 cases, both considered smaller with MRI than with US and without cysts detected on CT); (5) the visibility of normal ipsilateral lung n = 3 sets in three cases, the whole lung was involved in two fetuses on MRI, which was not obvious on US (Fig. 4) and conversely in the remaining fetus, CT being concordant with MRI in all three cases, and (6) the size of the lesion with respect to the ipsilateral lung (n = 1, the lesion appeared larger with MRI than with US). The last two discrepancies can be ascribed to ill-defined borders of echogenic lesions during the third trimester of pregnancy, as mentioned in four US 2-MRI 2 sets in Table 1 (non-available size of the lesion).
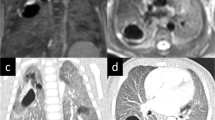
Prenatal and postnatal imaging in a child with CPAM type II of the right lower lobe (case 3). a, b Prenatal US. Axial (a) and sagittal (b) prenatal US performed at 34 weeks of gestation shows heterogeneous lesion (arrow) of the right lower lobe without a well-defined cystic structure. c, d MRI. Axial (c) and sagittal (d) prenatal MRI performed on the same day shows heterogeneous lesion of the right lower lobe (arrow) with markedly hyperintense structures consistent with cysts (arrowheads). e, f Postnatal CT performed at 1 month of age. Axial (e) and sagittal (f) CT images show multiple cysts in the right lower lobe. Compared with prenatal imaging, cysts have markedly increased in size. This child was asymptomatic at birth. CPAM type II was eventually confirmed by pathological examination
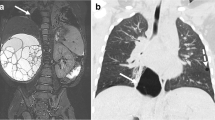
Prenatal imaging in a fetus with a hybrid lesion of the left lower lobe associating CPAM and BPS (case 9). Prenatal US (a) and MRI (b) performed at 34 weeks of gestation. The feeding artery is clearly visible with US and is not demonstrated on MRI. The lesion of the left lower lobe (dotted arrow) is non-homogeneous and cysts are clearly visible with both imaging modalities
Pre- and postnatal imaging in a child with CPAM of the left lower lobe (case 1). a-c Prenatal US and MRI performed at 26 weeks of gestation. a No normal lung is visible on US, where the lesion demonstrates multiple cysts and the mediastinum (star) is displaced towards the left. On coronal (b) and sagittal (c) T2-W MRI images, normal lung (arrow) is clearly visible. d Postnatal CT at 1 month of age. Sagittal reformatting confirms the location of the lesion in the lower left lobe
In 22 fetuses with late antenatal imaging, there was a similar number of cases with agreements and disagreements between US 2 or MRI 2 and CT (Tables 1 and 2). Disagreement between US 2 and CT was observed in ten cases (presence of cysts, n = 8; feeding artery, n = 1; location, n = 1; presence of normal lung, n = 1), and disagreement between MRI 2 and CT was observed in seven cases (presence of cysts, n = 5 all with US 2-CT disagreement too; feeding artery, n = 3 different from the cases with US 2-CT disagreement; location, n = 1 same as for US 2-CT disagreement).
The suspected diagnosis, based on all prenatal imaging data, was different according to the type of prenatal imaging modality in three (13%) fetuses (cases 5, 6 and 9) (Table 1), because of cysts or supplying artery being overlooked by one imaging modality. However, patient 11 was considered as a concordant case because of the detection of an abnormal vessel with US and of the presence of a triangular hypointense lesion of the left lower lobe with MRI, which is very suggestive of BPS [16]. Diagnosis based on second- and third-trimester imaging was concordant with postnatal findings in 12/17 and 15/20 cases, respectively (P = 0.56).
The suspected diagnosis based on postnatal imaging and the final diagnosis after surgery is displayed in Table 1. In three cases of BPS (numbers 4, 6 and 9), the systemic feeding artery was missed by either US or MRI in two fetuses and by both modalities in the third. In three other cases (numbers 7, 9 and 16), CPAM was associated with BPS, while only BPS was suggested by prenatal imaging (Fig. 5). Disagreements on the presence of cysts were mostly (n = 6) cases of cysts detected by CT while overlooked using US 2 and/or MRI 2. However, in two fetuses (case 9 with hybrid lesion encompassing CPAM on pathology and case 17 not operated on), cysts were visible at last prenatal imaging but not on CT. In another case (case 5, Fig. 6), emphysema was suspected on US imaging but not on CT because of numerous cysts favouring a CPAM. In retrospect, associated emphysema should have been suspected on CT. Finally, two cases (numbers 7 and 18) with a minima CPAM lesion on pathology were not recorded as discordant between antenatal imaging and final diagnosis because of the impossibility for any imaging modality (including CT) to detect such slight lesions.
Prenatal and postnatal imaging in a child with a hybrid lesion of the left lower lobe, associated CPAM and BPS (case 21). Both prenatal US (a) and MRI (b) performed at 34 weeks of gestation show the feeding artery (arrow) of the BPS. Cysts were not visible. c, d Postnatal CT at 1 month of age. The systemic artery (arrow) and numerous cysts (arrowheads) are well demonstrated, suggesting a hybrid lesion
Prenatal and postnatal imaging in a child with a hybrid lesion of the right upper lobe associating CPAM and emphysema (case 5). a US at 26 weeks of gestation shows a hyperechoic homogeneous lesion of the right lung without cyst and with a mediastinal shift towards the left. b MRI performed the same day shows several small posterior hyperintensities (arrow) consistent with cysts. Therefore, the diagnosis of CPAM was favoured. According to both modalities, the lesion was located in the right lower lobe. At birth, the child presented with immediate and transient respiratory distress. For this reason, CT was performed at 5 days of age. At CT (axial slice c and d) the lesion is located in the right upper lobe (which was in accordance with the surgical findings). Numerous cysts (arrow) are visible within the lower part of the lesion. In the upper part of the lesion, no cyst is visible. The lesion is hypodense with normal vessels (arrowheads) coursing through it, which in retrospect should have raised concern for emphysema
Discussion
CLA are relatively rare and classification and management are still controversial. It is generally accepted that both the timing of the embryological insult and the level in the tracheobronchial tree determine the type of lesion and histopathology [1, 20, 21]. Most of these malformations carry a good prognosis, with a mortality (intrauterine or neonatal) rate less than 5% of all antenatally detected lesions, with symptoms at birth in 17% of cases [22]. Although emergency surgery is required in severely symptomatic neonates, a conservative approach is advocated by some authors in infants with CPAM and BPS with few or no respiratory symptoms [23, 24]. However, other authors recommend elective surgery [22, 25, 26] because of the risk of infection, recurrent pneumonia and malignancy [1, 25, 27].
The prenatal natural history of CPAM and BPS has been described with US. CPAMs demonstrate a growth potential until 26-28 weeks of gestation and then usually decrease in size or even appear to vanish (microcystic types), while some CPAMs remain stable or grow larger (macrocystic types) [1–3, 5, 20, 28]. However, true antenatal resolution is exceptional [28] and even if early postnatal chest radiograph is normal, it is commonly accepted that CT must be performed [24, 29]. BPS can also regress markedly and the abnormal vascular supply might disappear [5]. In utero bronchial tree obstruction with retention of mucoid fluid might also be responsible for a transient hyperechoic lesion [3, 30]. In our series, we found that most of the lesions decreased in size between the two imaging evaluations (Fig. 1). However, no lesion completely disappeared at follow-up, and Fig. 1 shows that changes in size did not differ between lesion types. With US, CPAM appear as hyperechoic masses that are unilateral in the majority of cases (with only one lobe or segment involved) and are sometimes associated with cysts [3]. We used the classification based on US findings [17] in distinguishing between macrocysts (≥ 5 mm in diameter) and microcysts (< 5 mm). Poor prognostic factors such as mediastinal shift, polyhydramnios and hydrops have been more frequently described in association with the microcystic type [21], while large cysts responsible for hydrops sometimes require thoracoamniotic shunting [6]. Finally, CPAM volume ratio > 1.6 has been reported to be strongly associated with a poor outcome [18, 31]. In our study, we recorded all these prognostic factors and found that the only child with severe neonatal respiratory distress had a microcystic lesion extended to one entire lung with a CPAM volume ratio > 1.6 or borderline at both evaluations. BPS appear as echogenic, homogeneous masses, typically located in the left inferior lobe with a systemic arterial supply. Usually, intralobar and extralobar BPS cannot be differentiated by prenatal US [1].
Differentiating the microcystic type of CPAM from emphysema is challenging because both entities appear as solid echogenic masses without systemic arterial supply. However, normal pulmonary vessels coursing through the lesion are more likely to be observed in emphysema [32] (Fig. 6). Hybrid lesions are a combination of pathologies. The most common overlap is that of BPS and type II CPAM, suggested by the presence of cysts and a systemic arterial supply [1, 4, 33].
The contribution of fetal MRI has been reported in several isolated cases or series [7–16]. Most CPAM and BPS were T2 hyperintense and could become hypointense and inhomogeneous as part of the regression process [15, 16]. When CPAM was associated with BPS, the signal was heterogeneous. In BPS, the feeding artery was identified by MRI, whereas US failed to identify the vessel [9, 10, 16]; however, MRI could not help differentiate intralobar from extralobar BPS [16]. In our series, MRI missed the feeding vessel that was detected by US in three cases. In one study, emphysema was hyperintense and difficult to differentiate from microcystic CPAM, but stretching of the hilar vessels with otherwise relatively normal lung architecture was characteristic [16]. Hyperechoic bronchogenic cysts might be more easily visualised with MRI than with US [12]. Moreover, MRI could detect malformations such as diaphragmatic hernia associated with CLA [10] and determine the amount of normal lung adjacent to the CLA, both entailing a prognostic impact [34].
In our study, agreement between US and MRI in the description of the lesion was significantly better during the second trimester of pregnancy (Table 2). This finding favours late MRI scan (during the last trimester of pregnancy) to obtain additional information compared with US. In four cases, the arterial supply was missed either by US (one case of twin pregnancy) or by MRI (three cases, including one twin pregnancy). The mediastinal shift was not always properly evaluated by US but the main poor prognostic factor, hydrops, was not observed in our series. Our series showed good agreement between US and MRI in the evaluation of the lesion location and of the lesion and cyst dimensions, as well as in CPAM volume ratio measurements. In a few cases, the dimensions of the lesion and the visibility of adjacent normal lung could not be evaluated properly with US because of progressive maturation of normal lung and borders of the lesion getting blurred. The high contrast resolution of MRI enabled a better visibility of the lesion borders. In complex lesions involving the whole lung as in case 10, accurate diagnosis was not assessed either with US or with MRI or even postnatal CT, and it remained unclear after surgery.
US and MRI were similarly concordant with postnatal CT, which correctly diagnosed pulmonary lesions with respect to surgery and pathology findings. However, CT failed to diagnose hybrid lesions in two out of six cases, which is less than previously reported [4]. Discrepancies between US and MRI regarding the suspected diagnosis were related to disagreement in the detection of cysts, emphysema or systemic vessels. Finally, US antenatal diagnosis differed from MRI antenatal diagnosis in only three (13%) cases, and third-trimester imaging was similarly accurate to earlier imaging for diagnosis of the pulmonary lesions. Because CLA carry a good prognosis at birth in the absence of large lesions with mass effect (hydrops), there is time for performing postnatal CT. Elective surgery or conservative approach can then be decided depending on the child’s respiratory symptoms and according to the institution policy. MRI does not influence prenatal management more than US does, and we agree with other authors [4] that it should be used selectively. We suggest that MRI be performed during the third trimester of gestation either in complex lesions, when emergency surgery is likely to be required at birth (lesion involving the whole lung or with poor prognostic factors), or when the US study is hampered by maternal obesity or multiple pregnancy.
Our study has some limitations related to the relatively limited number of cases reviewed by a single investigator. No cases of hydrops and no cases of congenital lobar overinflation were included, limiting the comparison between US and MRI accuracy in these conditions. However, the inclusion criteria of the study were stringent because we selected only cases with complete prenatal assessment (US + concomitant MRI) and postnatal CT. Moreover, in a recent study, the interobserver reproducibility between two radiologists with a different level of experience in MRI reading was found to be perfect [16].
Conclusion
This series does not demonstrate clear superiority of MRI over US (performed in a tertiary centre) in the evaluation of CLA. The agreement between prenatal imaging and postnatal CT and surgery findings was good. Although we found that MRI was more reliable than US at detecting the presence of cysts or normal lung around the lesion, US proved better at detecting the presence of a systemic vessel.
References
Azizkhan RG, Crombleholme TM (2008) Congenital cystic lung disease: contemporary antenatal and postnatal management. Pediatr Surg Int 24:643–657
Illanes S, Hunter A, Evans M et al (2005) Prenatal diagnosis of echogenic lung: evolution and outcome. Ultrasound Obstet Gynecol 26:145–149
Cavoretto P, Molina F, Poggi S et al (2008) Prenatal diagnosis and outcome of echogenic fetal lung lesions. Ultrasound Obstet Gynecol 32:769–783
Farrugia MK, Raza SA, Gould S et al (2008) Congenital lung lesions: classification and concordance of radiological appearance and surgical pathology. Pediatr Surg Int 24:987–991
Hadchouel A, Benachi A, Revillon Y et al (2011) Factors associated with partial and complete regression of fetal lung lesions. Ultrasound Obstet Gynecol 38:88–93
Adzick NS (2009) Management of fetal lung lesions. Clin Perinatol 36:363–376
Hubbard AM, Adzick NS, Crombleholme TM et al (1999) Congenital chest lesions: diagnosis and characterization with prenatal MR imaging. Radiology 212:43–48
Olutoye OO, Coleman BG, Hubbard AM et al (2000) Prenatal diagnosis and management of congenital lobar emphysema. J Pediatr Surg 35:792–795
Chen CP, Liu YP, Lin SP et al (2005) Prenatal magnetic resonance imaging demonstration of the systemic feeding artery of a pulmonary sequestration associated with in utero regression. Prenat Diagn 25:721–723
Dhingsa R, Coakley FV, Albanese CT et al (2003) Prenatal sonography and MR imaging of pulmonary sequestration. AJR Am J Roentgenol 180:433–437
Kawamura M, Itoh H, Yamada S et al (2005) Spontaneous regression of congenital cystic adenomatoid malformation of the lung: longitudinal examinations by magnetic resonance imaging. Congenit Anom (Kyoto) 45:157–160
Levine D, Jennings R, Barnewolt C et al (2001) Progressive fetal bronchial obstruction caused by a bronchogenic cyst diagnosed using prenatal MR imaging. AJR Am J Roentgenol 176:49–52
Liu YP, Shih SL (2008) Congenital lobar emphysema: appearance on fetal MRI. Pediatr Radiol 38:1264
Quinn TM, Hubbard AM, Adzick NS (1998) Prenatal magnetic resonance imaging enhances fetal diagnosis. J Pediatr Surg 33:553–558
Levine D, Barnewolt CE, Mehta TS et al (2003) Fetal thoracic abnormalities: MR imaging. Radiology 228:379–388
Liu YP, Chen CP, Shih SL et al (2010) Fetal cystic lung lesions: evaluation with magnetic resonance imaging. Pediatr Pulmonol 45:592–600
Adzick NS, Harrison MR, Glick PL et al (1985) Fetal cystic adenomatoid malformation: prenatal diagnosis and natural history. J Pediatr Surg 20:483–488
Crombleholme TM, Coleman B, Hedrick H et al (2002) Cystic adenomatoid malformation volume ratio predicts outcome in prenatally diagnosed cystic adenomatoid malformation of the lung. J Pediatr Surg 37:331–338
Stocker JT, Madewell JE, Drake RM (1977) Congenital cystic adenomatoid malformation of the lung. Classification and morphologic spectrum. Hum Pathol 8:155–171
Griffin N, Devaraj A, Goldstraw P et al (2008) CT and histopathological correlation of congenital cystic pulmonary lesions: a common pathogenesis? Clin Radiol 63:995–1005
Correia-Pinto J, Gonzaga S, Huang Y et al (2010) Congenital lung lesions—underlying molecular mechanisms. Semin Pediatr Surg 19:171–179
Stanton M, de-Njere I, de-Ajayi N et al (2009) Systematic review and meta-analysis of the postnatal management of congenital cystic lung lesions. J Pediatr Surg 44:1027–1033
Aziz D, Langer JC, Tuuha SE et al (2004) Perinatally diagnosed asymptomatic congenital cystic adenomatoid malformation: to resect or not? J Pediatr Surg 39:329–334
Fitzgerald DA (2007) Congenital cyst adenomatoid malformations: resect some and observe all? Paediatr Respir Rev 8:67–76
Calvert JK, Lakhoo K (2007) Antenatally suspected congenital cystic adenomatoid malformation of the lung: postnatal investigation and timing of surgery. J Pediatr Surg 42:411–414
Tsai AY, Liechty KW, Hedrick HL et al (2008) Outcomes after postnatal resection of prenatally diagnosed asymptomatic cystic lung lesions. J Pediatr Surg 43:513–517
Conforti A, Aloi I, Trucchi A et al (2009) Asymptomatic congenital cystic adenomatoid malformation of the lung: is it time to operate? J Thorac Cardiovasc Surg 138:826–830
Davenport M, Warne SA, Cacciaguerra S et al (2004) Current outcome of antenally diagnosed cystic lung disease. J Pediatr Surg 39:549–556
Sauvat F, Michel JL, Benachi A et al (2003) Management of asymptomatic neonatal cystic adenomatoid malformations. J Pediatr Surg 38:548–552
Achiron R, Strauss S, Seidman DS et al (1995) Fetal lung hyperechogenicity: prenatal ultrasonographic diagnosis, natural history and neonatal outcome. Ultrasound Obstet Gynecol 6:40–42
Yong PJ, Von Dadelszen P, Carpara D et al (2012) Prediction of pediatric outcome after prenatal diagnosis and expectant antenatal management of congenital cystic adenomatoid malformation. Fetal Diagn Ther 31:94–102
Biyyam DR, Chapman T, Ferguson MR et al (2010) Congenital lung abnormalities: embryologic features, prenatal diagnosis, and postnatal radiologic-pathologic correlation. Radiographics 30:1721–1738
Newman B (2006) Congenital bronchopulmonary foregut malformations: concepts and controversies. Pediatr Radiol 36:773–791
Dietrich RB, Cohen I (2006) Fetal MR imaging. Magn Reson Imaging Clin N Am 14:503–522
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beydon, N., Larroquet, M., Coulomb, A. et al. Comparison between US and MRI in the prenatal assessment of lung malformations. Pediatr Radiol 43, 685–696 (2013). https://doi.org/10.1007/s00247-012-2596-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-012-2596-7