Abstract
Background
Lymphatic malformations (LMs) are congenital lesions of the lymphatic system and consist of lymphatic channels and cystic spaces of varying sizes. Recent evidence has shown that LMs respond well to intralesional sclerotherapy.
Objective
The purpose of this retrospective study is to evaluate the outcome and efficacy of using doxycycline in treating macrocystic, microcystic and combined macro- and microcystic LMs in a tertiary-care pediatric center.
Materials and methods
Fifty children (32 boys, 18 girls) underwent doxycycline sclerotherapy for treatment of LMs between January 2005 and October 2010. Demographics, imaging, doxycycline treatment details, complications and follow-up details were documented.
Results
The mean age at first treatment was 5.9 years (3 days–17.8 years), median 4.2 years. Cervicofacial (19/50 children) and truncal (16/50 children) locations were the most frequently affected. One hundred forty-six sclerotherapy sessions were performed in 50 children (mean 2.9/child). The mean doxycycline dose/kg body weight for 146 sessions was 15.3 mg/kg (0.6–85.7 mg/kg). Complications occurred in 4/146 procedures. Clinical follow-up showed excellent response in 14/16 children with macrocystic LMs, 21/27 children with combined LMs and 4/7 children with microcystic LMs.
Conclusion
Doxycycline is a safe and effective sclerosant agent for treating LMs in children, with a low complication rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lymphatic malformations (LMs) are a specific type of vascular malformation that arises because of errors of vascular morphogenesis during early embryonic life resulting in abnormal lymphatic channels and cystic spaces of varying size [1]. LMs can involve skin, soft tissue, bone and thoraco-abdominal or pelvic viscera. Histologically, LMs are a complex of multiple dilated cystic spaces lined with a single layer of vascular endothelium, supported by connective tissue with a matrix of fibrous tissue and smooth muscle with small feeding vessels and aggregates of lymphocytes [2]. The cysts sometimes communicate and can contain serous, proteinaceous or hemorrhagic fluid [3].
LMs are characterized as macrocystic (with minimum cyst diameter ≥1 cm), microcystic (maximum cyst diameter <1 cm), or combined macro- and microcystic [4]. They are most frequently located in the head and neck region (48%), truncal and extremity locations (42%) and intrathoracically or intra-abdominally (10%) [5]. Although the disease is often contiguous when affecting multiple anatomical sites (such as in the neck and mediastinum), multifocal non-contiguous disease can also be seen in some children. The presentation of LMs is dependent upon their location, size and functional impairment. Sudden expansion with skin discoloration can occur because of intralesional bleeding, infection or inflammation. This can compromise vital functions when the LMs are present in certain anatomical locations (e.g., near airway/orbit). Blue skin discoloration can sometimes be seen when the cysts contain bloody fluid.
Surgical resection was previously considered standard treatment but the infiltrative nature and ill-defined limits especially of microcystic LMs can make surgery difficult [6]. With incomplete excision, disease persistence is likely. Recent evidence has shown that LMs respond well to intralesional sclerotherapy, with macrocystic LMs showing a better response than microcystic LMs [7, 8]. Multiple sclerosant agents have been used in the treatment of LMs including ethanol, OK-432, ethibloc, sodium tetradecyl sulphate, bleomycin and doxycycline [9–14].
Doxycycline is an inexpensive and readily available antibiotic of the tetracycline group with a good safety profile and widespread usage. It has been extensively used in the treatment of postoperative lymphoceles, pleurodesis (to treat recurrent pneumothorax and malignant pleural effusions), benign lymphoepithelial parotid cysts and mediastinal/esophageal cysts [15–19].
The experience of using doxycycline as a sclerosant for the treatment of LMs is limited. In this study, we report our experience using doxycycline for treating pediatric macrocystic, microcystic and combined LMs in a tertiary-care pediatric center.
Materials and methods
Patients
Institutional Research Ethics Board approval was obtained before conducting this retrospective study. Informed consent was waived. We included 50 children (32 boys, 18 girls) who underwent sclerotherapy using doxycycline for treatment of LMs between January 2005 and October 2010 in a tertiary care paediatric centre. Children with a diagnosis of veno-lymphatic malformations and Klippel-Trenaunay syndrome were excluded.
Data collection
Data were collected from the hospital medical records (the Electronic Patient Chart [EPC] and the Interventional Radiology database). The imaging undertaken before treatment was variable and included MRI, US and CT. This was reviewed from the PACS imaging system.
Patient demographics, age at initial clinical presentation, clinical details, imaging modalities in the assessment and imaging details were documented. The lesion location was classified as cervicofacial (including orbit), truncal (including axilla, chest wall, mediastinum, abdomen) or extremity. After reviewing the images, the LMs were grouped according to the cyst diameters as macrocystic (≥1 cm), microcystic (<1 cm) or combined macro- and microcystic (Fig. 1). In children who had cross-sectional imaging pre-treatment, the greatest diameters of the lesion were measured and the total volume was calculated using the formula for volume of an ellipsoid = 0.523 × diameter1 × diameter2 × diameter3 [20]. Previous treatment data including surgical excision and prior sclerotherapy were documented.
Combined macro- and microcystic LM involving the subcutaneous tissue of the left thigh. a Clinical photograph of the child with a large swelling on the medial and anterior aspect of left thigh with overlying skin discolouration. b T1-W MRI shows multiple hypointense cystic spaces of varying sizes in the anteromedial aspect of left lower limb; the microcystic component of the LM is not very well defined on T1-W imaging. c T2-W STIR MRI shows multiple hyperintense cystic spaces ranging from a few millimeters to centimeters in size. d Post-contrast T1-W fat-suppressed MRI shows peripheral enhancing rim of the larger cysts (macrocystic element) and mild enhancement of remaining LM
Clinical treatment response of a large cervical macrocystic LM. a Clinical photograph of a child with a large neck swelling on the left side prior to sclerotherapy. b Contrast-enhanced axial CT image of the neck shows the presence of a large macrocystic LM causing swelling and displacing the airway (black arrow). c US image during sclerosant injection. Puncture needle tip (white arrow) shown prior to drain insertion. The macrocyst has expanded with echogenic fluid due to spontaneous bleeding into the cyst lumen. d Flouroscopic image of neck and chest. Three small 5-F drains were placed at time of sclerotherapy into the macrocystic LM. The cyst cavities are outlined with contrast agent. e Clinical photograph at 3-month follow-up (after the last treatment session) shows no residual swelling and normal appearance of the neck. f US image at 3-month follow-up shows almost complete resolution of the macrocyst with residual echogenic tissue lateral to left lobe of thyroid
Details regarding type of anesthesia, use of antibiotics, number of sclerotherapy sessions, time interval between treatment sessions, dose of doxycycline per session, bodyweight at each session and presence of any complications were noted. Complications were classified as minor or major according to the standards of practice of the Society of Interventional Radiology [21]. Minor complications required either no therapy or nominal therapy with an overnight hospital stay for observation only. Major complications were those requiring a hospital stay >48 h, major therapy or an unplanned increase in level of care, and having permanent adverse sequelae or resulting in death.
Clinical and in some cases imaging follow-up was done after 4–6 weeks of treatment to assess change in size of the lesions and response to treatment. Imaging follow-up was done from images available on PACS. When images were not available on PACS, notes from the last follow-up in the clinic were accessed using EPC and details of any limited US performed in the clinic and physical examination were noted. Overall clinical response was classified as (1) excellent (decrease in size of ≥95%), (2) satisfactory (decrease ≥50%) and (3) no change/poor (<50% decrease in the size of the lesion/unchanged) [9, 22]. Children with lesion persistence or cysts amenable to further treatment were offered more sessions of sclerotherapy.
Technique
All procedures were performed by a pediatric interventional radiologist. Most procedures, including all those requiring general anesthesia (GA), were done in the Interventional Radiology (IR) suite. Informed consent was obtained prior to the procedure from all of the patients or parents. The procedures were performed depending upon the type and size of the LMs under GA or under topical/local anesthesia (LA). LA in addition to GA was used sometimes when percutaneous drains were inserted. In cases where LA was used without GA, it was either topically applied to the skin or it was injected into the cyst via a preexisting drain prior to doxycycline injection. Antibiotic prophylaxis using Cefazolin (Novopharm, Toronto, Ontario, Canada) 30–40 mg/kg body weight and dexamethasone 0.1 mg/kg body weight were administered intravenously at the beginning of the procedure. Steroids were routinely given to children having sclerotherapy under GA when a larger volume of the LM was treated, to decrease the post-procedure swelling, pain and emesis.
US was used to confirm the location to be treated and the lesion characteristics. The skin was prepped and draped using sterile technique. All cysts were punctured under US guidance. Larger macrocysts were cannulated with a micropuncture set and drained with a 5 French Duan drain (Cook, Bloomington, IN) or a 6–8 French APD drain (Boston Scientific, Natick, MA). Fluid was aspirated until the cyst was emptied. Smaller macrocysts were directly cannulated using a 20- to 22-gauge angiocatheter. Microcysts were treated by direct needle puncture (22- to 27-gauge) and occasionally fluid was aspirated if possible. When cysts were aspirated, both the volume and color of cyst fluid were documented.
Doxycycline solution at a concentration of 10 mg/ml was prepared by mixing 100 mg of doxycycline powder (APP Pharmaceuticals, LCC Schaumburg, IL) with 5 ml of sterile water and 5 ml of water-soluble contrast (Omnipaque 300, GE Healthcare Canada, Mississauga, Canada). An arbitrary maximum dose of 300 mg was used for children ≤12 months of age and 1,200 mg for those older than 12 years. For children between 12 months and 12 years, we used a maximum dose of 300 mg–1,200 mg, depending on the age and weight of the child. The cysts were injected under US guidance with doxycycline solution. The injected volume approximated to half of the volume of fluid aspirated from the cyst. Only those microcysts visible on US were injected with doxycycline. Cysts not identified on US including solid echogenic tissue were not injected. In those microcysts where no fluid was aspirated, only a small volume (0.2–0.5 ml) of doxycycline solution was injected at the discretion of the interventional radiologist. Occasionally, intercommunication between cysts was identified on US, most frequently seen as passage of injected fluid between the cysts or early deflation of an injected cyst. When cysts inter-communicated, the increased capacitance of the cystic spaces allowed for more sclerosant to be injected. As many cysts as possible were treated at each session. Following the injection of sclerosant into each cyst, the needle/cannula was removed. If drains were used, these were switched off for 4 h following doxycycline injection and then re-opened to free drainage. Children were kept under observation for a minimum of 4 h and analgesics (morphine [0.05 mg/kg body weight] and acetaminophen [15 mg/kg body weight]) were administered if required on a 4-hourly PRN basis. Children without indwelling drains were usually discharged the same day when stable and satisfactory. In children with indwelling drains, a repeat treatment was performed the following day, with children remaining in hospital overnight.
During our study period we introduced some changes to our technique. Early on in our experience, we undertook an initial single-day treatment at each session followed if needed by repeat single-day treatments at 6-week intervals. The number of repeat treatments was determined by the clinical response. Usually no more than three treatment sessions were undertaken. In August 2006, we increased the number of treatments at each interval for selected patients. Children underwent 2–3 treatment sessions over 2–3 consecutive days to improve treatment outcome in children with large macrocystic LMs. In these interval treatment sessions, the first procedure was performed under GA in the IR suite. For those undergoing second-day treatment via existing drains, the procedures were carried out on the ward. Analgesia (i.v. morphine 0.05 mg/kg and p.o. acetaminophen 15 mg/kg) was administered prior to the injection of the LA lidocaine 1% (Astra Zeneca, Mississauga, Canada) and doxycycline via the drain. The amount of lidocaine 1% used depended on cyst size, and the maximum dose did not exceed 4.5 mg/kg of body weight. Lidocaine 1% was left in the cyst for 10 min and aspirated before injection of doxycycline. In most subsequent treatment sessions, no antibiotics or steroids were administered and GA was given only if more cysts were to be treated. In August 2007, we introduced sodium tetradecyl sulphate 3% (STS) foam (1:1 STS:air) as an adjunct sclerosing agent to irrigate the cysts (dwell time 10–20 min) prior to doxycycline injection. The volume of STS used depended upon the cyst size to a maximum of 1 ml/kg of STS foam.
Results
The children’s mean age at the first treatment session was 5.9 years (range 3 days to 17.8 years) with a median age of 4.2 years. The majority of LMs (27/50 children) were combined macro- and microcystic type (15 boys, 12 girls). The most common lesion location was the neck (14 children) followed by the chest wall (12 children). The lesions were on the left side in 26 children, right side in 22 children and bilateral in 2 children. The patient demographics and lesion characteristics are summarized in Table 1.
Thirty-two children had MRI as the primary imaging modality before treatment, 16 had US and 2 had CT. The mean pretreatment lesion volume was 181.3 ml (range 2.8–2,071 ml) in 40/50 children. In 10 children with extensive or non-contiguous disease, total lesion volume could not be accurately measured.
A total of 146 sclerotherapy treatment sessions were performed in 50 children with a mean of 2.9/child (range 1–7) (Table 2).
The mean doxycycline dose per kg body weight for all procedures was 15.3 mg/kg (range 0.6–85.7 mg/kg). In 38 children the total mean dose/kg was ≤20 mg/kg (mean 7.2 mg/kg) and in 12 children it was ≥20 mg/kg (mean 40.8 mg/kg).
Twenty-three children (46%) underwent interval single-day treatment, 12 (24%) underwent interval consecutive-day treatment. The mean treatment time interval was 15.1 weeks (106 days), range (21–1,036 days). The best responses were identified in children undergoing interval treatment every 6 weeks, whether single-day or consecutive-day treatments (Table 3).
Of the 50 children treated, 39 (78%) received doxycycline alone and 11 (22%) received doxycycline plus STS. STS 3% foam was used as an adjunct to wash macrocysts before injecting doxycycline in children who did not respond to doxycycline injection and in the more recent children not previously treated. These 11 children received doxycycline plus STS during 24/44 (55%) treatments (mean volume 8.9 ml; range 3–20 ml). In our patients the outcomes did not differ much between these groups (Table 4).
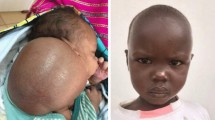
The mean follow-up between the last treatment and latest clinical follow-up was 1.2 years (range 42 days to 4.7 years). Three of the 50 children were lost to follow-up and therefore excluded from analysis. Imaging follow-up was available in 39/47 children (83%). In eight children (17%) the treatment outcome was based on clinical examination alone. Clinical follow-up showed improvement of pre-treatment symptoms in 45/50 (90%) patients (Fig. 2). See Fig. 3 for clinical outcomes following treatment of different types of LMs with doxycycline and doxycycline plus STS.
Complications were seen in 4/146 procedures (2 major and 2 minor) (Table 5). One child with a large mediastinal LM with neck extension of the disease developed Horner syndrome after the third-consecutive-day treatment, showing almost complete resolution of the Horner syndrome at 4-month clinical follow-up. The other major complication was severe pain seen in one child after the first treatment with doxycycline under local anesthesia. On serial treatments under GA no more complications were noted in this child.
Discussion
Doxycycline is a readily available antibiotic of the tetracycline group. It was first used in the treatment of LM by Molitch and associates [23]. Its use in sclerotherapy of LMs is based on its mechanism of inhibition of matrix metalloproteinase and cell proliferation, as well as suppression of vascular endothelial growth factor (VEGF) induced angiogenesis and lymphangiogenesis [24]. It causes collagen and fibrin deposition, leading to dense adhesions and fibrosis, and various studies have shown it to be effective as an agent for pleurodesis [25].
In our study, successful sclerotherapy was achieved in the majority (90%) of the children regardless of LM location. Successful sclerotherapy with doxycycline included children with an excellent (≥95% decrease in size) or satisfactory (<95% to ≥50% decrease in size) overall clinical response. Acute and chronic clinical symptoms (such as respiratory distress, pain, swelling leading to cosmetic deformity, and leakage of fluid from the skin) resolved in almost all children. The best response was seen in macrocystic LMs. Combined macro- and microcystic LMs also showed a favorable response. Microcystic LMs, however, showed a less favorable response. Other authors have also reported excellent clinical results when using doxycycline for the treatment of macrocystic LMs and good results in the treatment of microcystic LMs [6–8].
In our study MRI was the predominant imaging modality, used in 32/50 children, with US used in 16/50 children to assess the lesions before sclerotherapy. CT scanning was used in two children prior to referral to our service. In our practice we would recommend MRI as the imaging modality of choice to assess LMs because of its tissue-specificity and clear depiction of disease extent and tissue involvement. US, however, is extremely useful, not simply as a bedside tool but when disease is visible and involves the superficial tissues with no deep extension.
During the latter half of our study period we introduced some changes in the treatment protocol. We introduced consecutive-day treatments (i.e. 2–3 treatments over 2–3 consecutive days) and interval consecutive-day treatments (repeat consecutive-day treatments approximately every 6 weeks) in children with large or persistent LMs. The aim was to determine whether this improves treatment effectiveness. Our clinical observation is that improved results are seen with drainage and repeat sclerotherapy of large macrocysts within a shorter time period compared to those after single-day sessions. We also started using 3% sodium tetradecyl sulphate to wash the cysts, i.e. injecting then aspirating immediately before doxycycline injection in children with large or persistent LMs who did not show a good response to doxycycline injection alone. The mechanism of action of STS is unknown, but some studies have shown that its detergent action emulsifies the lipids within the cell membrane of LMs and increases membrane permeability to doxycycline thus enhancing its action toward cell death and fibrosis [14]. Pre-treatment with STS showed good success, especially with interval treatments, but we cannot ascribe this higher success rate to either consecutive-day treatments, interval treatments or to the use of STS. Given the heterogeneity of patient and cyst selection in those treated with STS in addition to doxycycline it is difficult to evaluate the effectiveness of STS. A randomized study in LM patients with similar disease distribution is needed to evaluate doxycycline effectiveness vs. doxycycline + STS.
As expected, lesions with larger volumes required multiple treatment sessions. A child who clinically presented with acute respiratory distress had a large LM involving the mediastinum; this was successfully treated using a combination of consecutive-day interval treatment sessions using doxycycline. However, in one child with a large intra-abdominal LM, excellent response following a single treatment session was confirmed on follow-up MRI. In two children the LMs were seen to increase in size post treatment after one and two treatment sessions, respectively. One of these children had a microcystic LM involving the extremity and chest wall. The other had an extensive macrocystic LM involving the cervicofacial location. Although it is known that bleeding can occur during sclerotherapy, which can cause an increase in the cyst size, we could not find a satisfactory explanation for cyst size increase in these children.
In our study group most of the children did not have significant pain or discomfort following sclerotherapy with doxycycline. Most of the procedures (118/146) were done under GA. Of the remaining 28 procedures without GA, two were associated with significant pain following doxycycline injection. In these two children excellent pain control was obtained with a bolus dose of intravenous morphine and in one of these children, aspirating the doxycycline through an existing drain also helped in reducing the pain. In children with existing drains without GA, doxycycline injection was tolerated relatively well with minimum discomfort. These children were given oral acetaminophen, intralesional 1% lidocaine and intravenous morphine.
Two major and two minor complications were seen. One child with an LM involving the cervicofacial region developed Horner syndrome after the third session of a consecutive-day treatment. Although the Horner syndrome most likely developed as a result of lesion swelling adjacent to the cervical sympathetic chain, interestingly doxycycline has been reported to cause demyelination of neural tissue [26]. Another child developed severe pain following the first treatment session, which was done under local anesthesia. On subsequent procedures under GA no similar complications were noted. This case highlights the importance of appropriate pain management, as doxycycline sclerotherapy can be very painful. The minor complications were skin blistering in one child and post-operative ileus in another, which can be seen in patients with intra-abdominal LMs. None of the cases in our study showed evidence of persistent pain, skin ulceration, airway obstruction, renal failure or scarring.
No conclusive data are available regarding the acceptable dose of doxycycline in the form of intralesional injections for treating LMs in children. In our study the average dose of doxycycline used was 213.7 mg (range 10–1,000 mg). Doxycycline was injected using a concentration of 10 mg doxycycline in 1 ml of sterile water. An arbitrary maximum dose of 300 mg was used for children ≤12 months of age and 1,200 mg for those older than 12 years. For children between 12 months and 12 years, we used a maximum dose of 300 mg–1,200 mg, depending on the age and weight of the child. The suggested empirical dose in the literature is 20 mg/kg [2, 27].
Regarding side effects, recent studies have shown that doxycycline is safe for use in children with little concern for staining of teeth [28]. Our study outcome was in accordance with other studies as we did not find this side effect in any of the children during the follow-up period (maximum being 4.7 years in one child) [7]. Our study data include cases where a significantly higher dose/body weight of doxycycline was used, as some of the children in our study group were newborns with extensive lesions necessitating a high dose/bodyweight. Longer follow-up is necessary to have more conclusive data on this aspect.
Defining end points in treating LMs is easier for macrocystic than microcystic disease because of the difficulty in eradicating microcystic disease. Many reports used end points of treatment such as achieving a decrease in swelling or size of the lesion or a cosmetic improvement on physical examination. Treatment end points for microcystic LMs are generally not well reported [6, 24]. According to literature, microcystic LMs include a spectrum of small cysts <1 cm in diameter and although some of these small cysts are visible on US, many are microscopic and appear only as solid tissue on US (which is echogenic) and MRI [4, 7]. When small sonographically visible microcysts are present and particularly when they are few in number in relation to the amount of echogenic solid-looking component, there remains debate as to the efficacy of sclerotherapy in treating such few microcysts. Surgical excision of microcystic LM is also problematic as it is well recognized that patients can be left with significant disfigurement, loss of function of the affected body part, non-healing scars and the possibility of recurrent disease [6, 7]. From our experience we would suggest treating those microcystic LMs in which multiple small injectable cysts are visible on US in an attempt to reduce lesion size. The risk from spontaneous bleeding and infection in macro- and microcystic LMs persists when the disease cannot be fully eradicated, irrespective of the treatment modality.
Our study highlights problems with post-treatment outcomes based on clinical evaluation alone without imaging. LMs can have deep extension of visible external disease and therefore follow-up after treatment, irrespective of the treatment modality (sclerotherapy, surgery or other) should include both an imaging and clinical evaluation. Imaging with appropriate modalities such as MRI is suggested because this can assess changes in the deep non-visible components of the disease, if and when present. This allows for a better assessment of treatment response, provides a potential window to compare response among treatment modalities, and allows us to view with caution claims for cures of microcystic LMs. Disease re-growth or recurrence might be missed if residual disease post treatment is located in deeper areas. The length of follow-up post treatment in our study varied between 42 days and 4.7 years. This relatively short follow-up for some children poses limitations in assessing disease recurrence, particularly for microcystic disease. However, most children in our practice are followed up in the clinic until 18 years of age.
This study is limited because of its observational and retrospective nature. Not all children had the same pre-procedure workup, and follow-up imaging was not available in all children. Nevertheless we have shown doxycycline to be an effective sclerosant in children with LMs.
Conclusion
Doxycycline is a safe and effective sclerosant for treatment of LM, with excellent clinical results seen in children with all types of LM. We recognise the limitation of sclerotherapy and other treatment modalities in those with extensive microcystic disease where very few cysts within the lesion can be injected percutaneously. Doxycycline has a low side-effect profile and low incidence of major complications. Selected patients can be offered staged treatment sessions, which can to some degree improve treatment outcome. Long-term follow-up is required for more conclusive data on disease persistence and recurrence.
References
Okazaki T, Iwatani S, Yanai T et al (2007) Treatment of lymphangioma in children: our experience of 128 cases. J Pediatr Surg 42:386–389
Shiels WE, Kenney BD, Caniano DA et al (2008) Definitive percutaneous treatment of lymphatic malformations of the trunk and extremities. J Pediatr Surg 43:136–139
Legiehn GM, Heran MK (2006) Classification, diagnosis, and interventional radiologic management of vascular malformations. Orthop Clin N Am 37:435–474
Giguère CM, Bauman NM, Smith RJ (2002) New treatment options for lymphangioma in infants and children. Ann Otol Rhinol Laryngol 111:1066–1075
Alqahtani A, Nguyen LT, Flageole H et al (1999) 25 years’ experience with lymphangiomas in children. J Pediatr Surg 34:1164–1168
Nehra D, Jacobson L, Barnes P et al (2008) Doxycycline sclerotherapy as primary treatment of head and neck lymphatic malformations in children. J Pediatr Surg 43:451–460
Burrows PE, Mitri RK, Alomari A et al (2008) Percutaneous sclerotherapy of lymphatic malformations with doxycycline. Lymphat Res Biol 6:209–216
Cordes BM, Seidel F, Sulek M et al (2007) Doxycycline sclerotherapy as the primary treatment for head and neck lymphatic malformations. Otolaryngol Head Neck Surg 137:962–964
Dubois J, Garel L, Abela A et al (1997) Lymphangiomas in children: percutaneous sclerotherapy with an alcoholic solution of zein. Radiology 204:651–654
Lee BB, Kim YW, Seo JM et al (2005) Current concepts in lymphatic malformation. Vasc Endovasc Surg 39:67–81
Ogita S, Tsuto T, Nakamura K et al (1994) OK-432 therapy in 64 patients with lymphangioma. J Pediatr Surg 29:784–785
Kim KH, Sung M, Roh J et al (2004) Sclerotherapy for congenital lesions in the head and neck. Otolaryngol Head Neck Surg 131:307–316
Niramis R, Watanatittan S, Rattanasuwan T (2010) Treatment of cystic hygroma by intralesional bleomycin injection: experience in 70 patients. Eur J Pediatr Surg 20:178–182
Shiels WE, Kang R, Murakami JW et al (2009) Percutaneous treatment of lymphatic malformations. Otolaryngol Head Neck Surg 141:219–224
Caliendo MV, Lee DE, Queiroz R et al (2001) Sclerotherapy with use of doxycycline after percutaneous drainage of postoperative lymphoceles. J Vasc Interv Radiol 12:73–77
Folk JJ, Musa AG (1995) Management of persistent lymphocele by sclerotherapy with doxycycline. Eur J Obstet Gynecol Reprod Biol 60:191–193
Robinson LA, Fleming WH, Galbraith TA (1993) Intrapleural doxycycline control of malignant pleural effusions. Ann Thorac Surg 55:1115–1121
Suskind DL, Tavill MA, Handler SD (2000) Doxycycline sclerotherapy of benign lymphoepithelial cysts of the parotid: a minimally invasive treatment. Int J Pediatr Otorhinolaryngol 52:157–161
Tang S, Sreenarasimhaiah J, Tang L et al (2007) Endoscopic injection sclerotherapy with doxycycline for mediastinal and esophageal lymphangiohemangioma. Gastrointest Endosc 66:1196–1200
Lass A, Brinsden P (1999) The role of ovarian volume in reproductive medicine. Hum Reprod Update 5:256–266
Sacks D, McClenny TE, Cardella JF et al (2003) Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol 14:S199–S202
Emran MA, Dubois J, Laberge L et al (2006) Alcoholic solution of zein (Ethibloc) sclerotherapy for treatment of lymphangiomas in children. J Pediatr Surg 41:975–979
Molitch HI, Unger EC, Witte CL et al (1995) Percutaneous sclerotherapy of lymphangiomas. Radiology 194:343–347
Alomari AI, Karian VE, Lord DJ et al (2006) Percutaneous sclerotherapy for lymphatic malformations: a retrospective analysis of patient-evaluated improvement. J Vasc Interv Radiol 17:1639–1648
Hurewitz AN, Lidonicci K, Wu CL et al (1994) Histologic changes of doxycycline pleurodesis in rabbits. Effect of concentration and pH. Chest 106:1241–1245
Kirse DJ, Suen JY, Stern SJ et al (1996) Histologic effect of doxycycline sclerotherapy on rat femoral nerve. Head Neck 18:506–511
Hoff DS, Gremmels DB, Hall KM et al (2007) Dosage and effectiveness of intrapleural doxycycline for pediatric postcardiotomy pleural effusions. Pharmacotherapy 27:995–1000
Volovitz B, Shkap R, Amir J et al (2007) Absence of tooth staining with doxycycline treatment in young children. Clin Pediatr (Phila) 46:121–126
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shergill, A., John, P. & Amaral, J.G. Doxycycline sclerotherapy in children with lymphatic malformations: outcomes, complications and clinical efficacy. Pediatr Radiol 42, 1080–1088 (2012). https://doi.org/10.1007/s00247-012-2406-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-012-2406-2