Abstract
We report a case of completely isolated enteric duplication in an 18-month-old boy in whom US revealed a reniform abdominal mass with a pseudokidney sign that had no connection to adjacent organs. Distinctive histopathological changes of the duplication account for these unusual imaging features. Our case represents a diagnostic challenge in this rare entity. To our knowledge, this is a unique case.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Completely isolated enteric duplications are a very rare variety of gastrointestinal duplications having their own blood supply and no discernible connection or communication with a normal bowel segment [1, 2]. They usually present as cystic structures in imaging studies. We report a case of completely isolated enteric duplication in an 18-month-old boy in whom US exhibited a renal-shape abdominal mass with a pseudokidney sign rather than a characteristic cystic lesion.
Case report
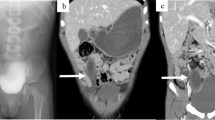
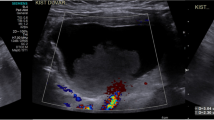
An 18-month-old boy was admitted to our hospital with a 3-day history of abdominal pain and vomiting. Physical examination and laboratory findings were normal. Because of his young age and the unclear findings, an abdominal US was arranged, which showed a 5-cm reniform mass with some internal echoes, located in the right lower abdomen. The mass consisted of a thick hypoechoic outer layer and a thin hyperechoic core layer compatible with a sonographic pseudokidney sign (Fig. 1). However, US revealed no continuity or connection between the mass and adjacent bowel loops. Subsequent CT demonstrated a reniform intraperitoneal mass located anterior to the right psoas muscle, medial to the ascending colon, and lateral to the inferior vena cava. The mass had a thick outer wall and some hypoattenuating content. After administration of intravenous contrast medium, concentric layers of different attenuation within the mass were demonstrated (Fig. 2). A preoperative diagnosis was difficult to make from these unusual imaging findings. The initial impression was that this might be a bowel-related lesion. At laparotomy, there was a 5 x 3 x 3 cm pear-shaped mass lying in the small bowel mesentery close to the terminal ileum. The blood supply appeared to arise from the ileocecal mesentery. There was no connection between the mass and hollow or solid viscera. The mass was excised without complications (Fig. 3). On cut section, the mass had a thick wall (1 cm) with a blind-ending narrow lumen containing scant mucoid material. Microscopic examination revealed the wall was lined by typical enteric glandular mucosa surrounded by an extremely thick submucosa and smooth muscle layer (Fig. 4). The histological features were compatible with an enteric duplication. The postoperative course of the child was uneventful and he has remained well.
Longitudinal sonogram using a multi-frequency (3.5–8 Hz) curvilinear probe in the right lower quadrant of the abdomen showed a well-defined reniform mass (between markers) with a central thin hyperechoic zone surrounded by a thick hypoechoic layer, a typical pseudokidney sign. There was no connection between the mass and adjacent bowel loops (B)
Contrast-enhanced CT (CTDI, 3.86 mGy) revealing a reniform intraperitoneal mass (M) located anterior to the right psoas muscle (P). The mass exhibited three concentric layers of different attenuation with enhancing inner and outer layers (arrowheads). The middle layer (arrow) shows little enhancement
Histologically, the mass consisted of markedly thickened (4 mm) submucosa (S) and a thick (5 mm) smooth muscle layer (SM) with enteric glandular mucosal lining (M, 1 mm thick). Dilated blood vessels were seen throughout the submucosa. (H&E staining, original magnification x 40). The congested submucosa was thought to contribute to the mural stratification on contrast-enhanced CT. Alongside the thickened muscular layer, it was thought to have produced the sonographical pseudokidney sign
Discussion
Gastrointestinal duplications are rare congenital anomalies that may occur anywhere in the alimentary tract. They are commonly found in the small intestine, with the ileum being the most common location [1]. The majority of these duplications are of sphero-cystic type, sharing a common wall (often the serosa) with the adjacent normal intestine, and also having a common blood supply [1]. Completely isolated enteric duplication is an extremely rare entity of gastrointestinal duplications, which do not share wall/layers or communicate with any part of adjacent bowel segment and which have their own blood supply [1, 2]. The diagnosis of enteric duplication is made on the characteristic histopathological features: a distinct smooth muscle wall layer and gastrointestinal tract lining epithelium [2]. The clinical presentation of enteric duplication is variable, and symptoms are dependent on size, with large lesions prone to cause abdominal pain, a palpable mass, intestinal obstruction and bleeding in cases of ectopic gastric and/or pancreatic mucosa [1, 3].
US is the most useful imaging tool in enteric duplications. A well-described sonographic feature is the double-wall or muscular rim signs, which refers to the appearance of a cyst resembling the gastrointestinal tract with an echogenic inner rim, corresponding to mucosa, surrounded by a hypoechoic rim reflecting the smooth muscle layer [3, 4]. This sign can be seen in more than 50 % of patients, but a false-positive double-wall sign is also present in other cystic lesions such as Meckel diverticulum, ovarian cyst and mesenteric cyst [3]. Sporadic reports have shown that the Y-configuration on US may be more specific than the double-wall sign for diagnosing an enteric duplication [4, 5]. On CT, enteric duplications can be recognized as smoothly rounded, fluid-filled cysts or tubular structures with thin, slightly enhancing walls in or adjacent to the wall of part of the alimentary tract [6].
Unlike previously reported cases, US in our case showed a well-circumscribed hypoechoic reniform mass with a thin hyperechoic inner rim, a typical pseudokidney sign. This sign was first described in colonic carcinoma and has also been seen in a variety of gastrointestinal diseases with thickened bowel wall such as in intussusception and in inflammatory bowel disease. It has not been described in enteric duplication [7, 8].
Histopathological examination of the specimen showed profound thickening of the submucosa and muscle layer. Such histological presentations are rare in enteric duplications and are believed to result from severe congestion and edema as dilated blood vessels were scattered throughout the submuscosa. Mural thickening probably led to complete circumferential loss of the typical gut wall layers resulting in a thick hypoechoic rim at US. Moreover, marked thickening of the enteric duplication caused apposition of luminal surfaces, which resulted in a hyperechoic, narrow lumen. These mechanisms were believed to contribute to the sonographic appearance of the pseudokidney sign. The histopathological features can also help to explain the mural stratification of the mass seen on contrast-enhanced CT. The mass was stratified into three concentric layers with different attenuation due to submucosal oedema separating the normally enhancing inner and outer layers (the mucosa and the thickened muscular wall).
Because isolated enteric duplication is extremely rare, there are few acknowledged radiological features, and this makes a preoperative diagnosis difficult.
In conclusion, our report expands the range of differential diagnoses for the sonographical pseudokidney sign. If there is no connection or continuity between a lesion and adjacent bowel loops, isolated enteric duplication may be an additional diagnostic possibility.
References
Srivastava P, Gangopadhyay AN, Kumar V et al (2009) Noncommunicating isolated enteric duplication cyst in childhood. J Pediatr Surg 44:e9–e10
Menon P, Na KL, Rao A et al (2004) Isolated enteric duplication cysts. J Pediatr Surg 39:e5–e7
Hur J, Yoon CS, Kim MJ et al (2007) Imaging features of gastrointestinal tract duplications in infants and children: from oesophagus to rectum. Pediatr Radiol 37:691–699
Cheng G, Soboleski D, Daneman A et al (2005) Sonographic pitfalls in the diagnosis of enteric duplication cysts. AJR 184:521–525
Kim YJ, Kim YK, Jeong YJ et al (2009) Ileal duplication cyst: Y-configuration on in vivo sonography. J Pediatr Surg 44:1462–1464
Kim SK, Lim HK, Lee SJ et al (2003) Completely isolated enteric duplication cysts: case report. Abdom Imaging 28:12–14
Ledermann HP, Borner N, Strunk H et al (2000) Bowel wall thickening on transabdominal sonography. AJR 174:107–117
Mumoli N, Niccoli G, Cei M et al (2008) The pseudokidney sign. JDMS 24:257–259
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, HL., Su, CT., Chang, CY. et al. Unusual imaging features of completely isolated enteric duplication in a child. Pediatr Radiol 42, 1142–1144 (2012). https://doi.org/10.1007/s00247-012-2380-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-012-2380-8