Abstract
To achieve diagnostic images during MRI examinations, small children need to lie still to avoid movement artefact. To reduce patient motion, obviate the need for voluntary immobilisation or breath-holding and therefore obtain high-quality images, MRI of infants is frequently carried out under sedation or general anaesthesia, but this is not without risk and expense. However, many other techniques are available for preparing children for MRI, which have not been fully evaluated. Here, we evaluate the advantages and disadvantage of sedation and anaesthesia for MRI. We then evaluate the alternatives, which include neonatal comforting techniques, sleep manipulation, and appropriate adaptation of the physical environment. We summarize the evidence for their use according to an established hierarchy. Lastly, we discuss several factors that will influence the choice of imaging preparation, including patient factors, imaging factors and service provision. The choice of approach to paediatric MRI is multi-factorial, with limited scientific evidence for many of the current approaches. These considerations may enable others to image children using MRI under different circumstances.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High-quality imaging is increasingly in demand in modern hospitals to aid diagnosis and treatment options and to monitor treatment response. Cross-sectional imaging such as CT and MRI has seen a huge increase in demand over recent years, led by the ability to create 3-dimensional imaging datasets in an ever-decreasing time frame. However, despite the impressive speed of CT, it is inherently limited in paediatric imaging by the ionising radiation dose administered to the patient.
MRI can provide excellent image quality, superior soft-tissue resolution to CT and does not impart ionising radiation, and is therefore well-suited to cross-sectional paediatric imaging. MR uses rapidly changing magnetic field gradients and electromagnetic radiofrequency pulses to provide high-resolution images predominantly based on proton spin characteristics within the body [1]. MR is now the imaging modality of choice in many paediatric circumstances, such as neurological and musculoskeletal imaging, and is becoming more widely used for thoracic and abdominal imaging. Traditional MRI has developed around imaging static or immobilised objects, such as the brain or joints, but chest or abdominal imaging requires patient co-operation (typically breath-holding or voluntary immobilisation), often for several minutes at a time.
Applying these techniques to children is hampered by the combination of patient co-operation, rapid or irregular respiratory rate, and small-scale anatomy, meaning that very small movements can create motion artefacts that render images non-diagnostic. A variety of methods have been developed to overcome this for young children; the conventional approach is to use patient sedation or anaesthesia, but this is not without risk and expense. The intimidating design of MR systems, long duration of an entire examination and the noisy atmosphere can make older children apprehensive, making it even more difficult for them to co-operate fully.
In this article, we discuss the risks of using sedation or anaesthesia and summarise the alternative methods available, including the current evidence for their effectiveness. Although double-blinded randomized controlled trials (DB-RCT) are the gold standard in research, according to a simple hierarchy (Table 1) [2], there are very few RCTs available from the imaging community regarding adequate infant examination preparation. We have therefore tried to summarise the level of evidence available regarding studies evaluating effectiveness during imaging, and if none is available, then studies evaluating the effectiveness in other clinical situations (Table 2). These considerations may enable others to image children using MRI under similar circumstances.
Sedation / anaesthesia
Childhood sedation or general anaesthesia (GA) is widely used around the world for many surgical and dental procedures as well as in the emergency department [3–5]. Diagnostic imaging may require sedation or anaesthesia where patient co-operation is required, particularly for a prolonged period of time, such as MRI, or where the radiation dose necessitates a single attempt, such as CT. The requirement for imaging during a particular phase of intravenous contrast medium administration may also dictate a single opportunity to perform optimal imaging. If awake imaging fails or is expected to fail, then general anaesthesia or sedation is used to manage the procedure.
There are several levels on a spectrum of drug-induced depressed consciousness. These range from minimal sedation (anxiolysis), moderate sedation (conscious sedation), deep sedation (analgesia) through to general anaesthesia, determined by patients’ levels of ability to respond to verbal commands, tactile and painful stimulation [6, 7]. Confusingly, several other descriptive terms have emerged for deeper levels such as sleep sedation, safe sleep, light anaesthesia and minimal anaesthesia, which will be avoided here [8]. Children generally require sedation levels deeper than moderate (conscious) sedation in an imaging setting [9], in which they may require assistance in maintaining an airway. Practitioners intending to produce a given level of sedation should be able to rescue patients (using MR-compatible resuscitation equipment) whose level of sedation becomes deeper than initially intended, i.e. individuals administering moderate sedation should be able to rescue patients who enter a state of deep sedation [6].
An extensive choice of pharmacological agents is available that have been reviewed fully elsewhere [8, 10, 11]. Ideally, a combined state of both amnesia and immobility is achieved with the lowest dose and fewest drugs possible. As sedation level is a spectrum, it is not always possible to predict how an individual patient will respond, and so the real skill of sedation / anaesthesia is finding the optimal tolerance level of suitable immobility whilst avoiding apnoea. Drugs used to induce sedation tend to be weak and have a wide margin of safety (and therefore do not always succeed), whereas those that induce anaesthesia are potent but short acting with a narrower margin of safety. Chloral hydrate or benzodiazepines are the most frequently used sedatives in diagnostic paediatric imaging with a low rate of side effects, although the highest relative MR examination failure rate [12]. This article compares the use of these drugs as a group versus other techniques.
There is no question that oral sedation for MRI in the hands of experienced paediatric anaesthetists and intensivists is safe and successful [13]. Insufficient doctors for imaging demand has led to several nurse-led sedation programs for paediatric MRI, also demonstrated to be largely safe and successful [8, 9, 14]. Even within sedation programs, modernising sedation practice such as the pre-sedation or drug administration route improves patient outcomes [15]. However, establishing high success rates largely depends upon the de-selection of children in whom sedation is unsafe or likely to be unsuccessful, the appropriate use and careful administration of drug doses, and the comprehensive training of experienced staff in immediate resuscitation [16]. Individual hospitals have typically developed their own policy on who performs sedation, and it is good safe practice to have written protocols concerning the management of patients, the indications of sedation or anaesthesia, the minimum staff requirement, and resuscitation / emergency procedures.
The main short-term risks of sedation / anaesthesia are those of under-sedation or over-sedation. Under-sedation is insufficient sedation to allow satisfactory imaging to take place, as judged by patient movement, repositioning or premature termination of the examination. Under-sedation is usually quoted in studies as the “failure rate” of the acquisition of a diagnostic series of MR images. Children who undergo sedation for MRI as opposed to GA frequently have a higher failure rate, i.e. patient movement leading to a non-diagnostic scan [17].
More concerning is the risk of over-sedation, respiratory depression requiring intervention, potentially resulting in the loss of protective reflexes and the ability to maintain an airway. Cardio-respiratory monitoring is mandatory in deep sedation / anaesthesia, but good clinical practice irrespective of conscious level. Adverse events typically occur when using multiple sedating agents, particularly nitrous oxide in combination with other medications, and when administered by non-medically trained personnel [18]. In younger infants or neonates, over-sedation is manifest in desaturations or apnoeas. Sedation is more likely to cause de-saturation in younger (preterm) infants, in those of lower body weight and those with a history of apnoea [19]. A sedation scoring tool, such as the Neonatal Pain, Agitation and Sedation Scale (N-PASS), can be used to evaluate an infant’s level of sedation and gestational-age-appropriate pain levels [20]; similarly there are several age-dependent scales available for older children [5]. Despite these advances, little is known about the clinical effectiveness, immediate toxicity or long-term effects after neonatal exposure to analgesics or sedatives [21]. Techniques for the induction of GA, mask or cannula, can also generate fear and anxiety in the child [22].
Very few studies have been conducted regarding medium to long-term adverse events after discharge following sedation for an imaging study. A study of 80 children sedated for imaging studies with chloral hydrate demonstrated sleepiness for more than 4 h after the procedure, which is to be expected [23]. However, 29% of children became hyperactive, 68% unsteady, and 15% vomited, with normal activity resuming after 4 h in only about half of the children studied [23]. A study of more than 300 children receiving chloral hydrate for MRI with a good success rate resulted in similar side effects including motor imbalance (30%), gastrointestinal effects (23%), agitation and restlessness (15%), and prolonged recovery [17]. Audits reveal that sedation results in approximately 20% of children feeling drowsy the following day [16], which may influence factors such as schooling.
There are clearly additional costs involved in safe sedation and anaesthesia, such as more advanced monitoring equipment, highly trained staff, access to inpatient facilities or hospital day beds, additional time in the scanner itself (or a preparation room) for anaesthetic induction, managing routine recovery and adverse reactions including resuscitation. Many studies have demonstrated that sedation and anaesthesia lengthen hospital stay and lead to increased costs in several different settings, but by how much? Using oral midazolam in an emergency department for laceration repair significantly increased both emergency department length of stay (by 20 min) and visit costs (by about USD 15), although this was acceptable to most parents [24]. During pediatric MRI, a recent study of 150 patients showed that an awake patient’s average visit duration was 2.4 h, compared to 3.6 sedated and 4.1 h anaesthetised [25]. Visit costs for sedated and anaesthetised patients were three and nine times higher than those for awake patients, respectively, which may be a significant factor for the paying patient [25]. Drugs that give faster induction may seem tempting (e.g. using propofol instead of chloral hydrate) but instead may result in longer recovery times [12].
Alternative techniques
Reducing the need for sedation for MRI would therefore improve patient safety and reduce costs within this environment. To avoid the dangers associated with sedation in infants, an obvious alternative is to conduct paediatric imaging examinations without sedation, but is this at the expense of diagnostic image yield?
Sucrose and pacifier
One of the most universal neonatal comforting techniques is non-nutritive sucking (NNS), whereby an infant is given a pacifier (dummy) with no fluid or nutrition being delivered. It is a simple, readily available and cost-effective way of reducing neonatal discomfort, and has been shown in several RCTs to reduce crying, grimacing and lowered behavioural pain scores in the newborn during painful procedures (e.g., blood sampling) [26–28]. Disposable latex-free pacifiers are commercially available specifically for hospital use (Fig. 1). One of the potential disadvantages of pacifier use in MR is that it may generate rocking motion artefact of the jaw or whole head during brain imaging, and so consideration may need to be given to the body part being imaged.
Oral sucrose solution is now becoming a routine adjunct to clinical paediatric care, and is used for its calming effects in distressed newborns, and apparent pain-relieving effects during invasive procedures (such as intravenous cannulation or heel-lance blood sampling) in term and preterm neonates. Its precise mode of action, whether mediated by opioid or non-opioid mechanisms, is unknown. Two main solutions are available: Sweet-Ease® (Inspiration-healthcare, Leicester, UK; Fig. 2) and TootSweet® (Natus Medical Inc., San Carlos, USA). Typically, 240–500 mg (1–2 ml) of 25% sucrose solution is given orally to term babies via a syringe, 2 min prior to a painful procedure, with a lower dose of 120 mg (up to 0.5 ml of 24%) in preterm infants [29, 30], although specific dosage guidelines and criteria may vary among hospitals [31].
There are several RCTs demonstrating that sucrose administration is useful to reduce pain and anxiety in children undergoing painful and non-painful procedures [32–34], but none in an imaging setting. Curiously, sucrose may not dampen nociceptive cortical activity (measured by electroencephalography) leading some authors to question whether the lack of clinical observational scores truly correspond to pain relief [35]. However, the other anxiolytic properties of sucrose may still be of benefit during non-painful radiological procedures. NNS can be more effective than sucrose alone [28, 36], although the combination of using pacifiers and sucrose together can be the most soothing to a distressed infant [26, 36, 37]. Sucrose may be a useful adjunct to other non-pharmacological techniques, as it has a short duration of action (likely a few minutes) and administration can be repeated. This means that it could be used to settle an infant both at the beginning of an MR procedure prior to intravenous cannulation (for contrast medium), as well as being administered during imaging itself, which makes the whole patient experience more tolerable.
Swaddling
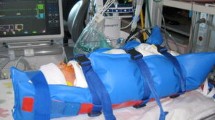
Historically, swaddling involves wrapping bands of tight material around an infant’s body to restrict movement [38]. More recently, gentle swaddling is widely used to reduce infant pain and distress during painful procedures [39, 40]. Swaddling restricts an infants’ motor activity, reduces crying and encourages sleep, although the effect diminishes with increasing age [41, 42]. Swaddling is used as an effective non-pharmacological technique to manage restless and irritable babies in non-imaging settings, e.g., neonatal abstinence syndrome [43] or during retinopathy of prematurity screening [44]. It is therefore widely used during routine paediatric MRI (Fig. 3), although the evidence for its effectiveness remains anecdotal.
Swaddling is sometimes used in conjunction with a swaddle or cradle board. These keep the legs forcibly extended and are popular in Native American, Far East and other communities as a form of infant carriage [42, 45]. However, they may lead to an increase in developmental hip dysplasia [46, 47]. Swaddling is therefore not recommended for infants with confirmed developmental dysplasia of the hip, and should be used with caution in infants at risk of developmental dysplasia, for instance breech presentation or family history [48, 49].
Feeding
“Feed and wrap” or “feed and sleep”, whereby sleep is induced in young children through feeding, warmth and comfort, is routinely used for MR examinations in infants younger than 3 months of age [50, 51]. Feed-and-sleep in conjunction with Vacuum fit beanbags enabled 32 out of 36 successful MR scans in infants younger than 12 weeks of age without the use of sedation or GA [52].
Adjusting a feed schedule to ensure that an infant will be fed at least 30 min prior to a scan can promote sleep [53] whereas older infants may require the scan appointment to be adjusted to fit in with their routine of feeding and sleep [54]. Careful attention is clearly required to details such as noise and light reduction, sensor placement to avoid stimulation, warmth, swaddling, and positioning to minimize movement.
Sleep manipulation
Sleep deprivation can reduce the need for sedation in young children and infants [10]. This can mean keeping infants awake during the night before the examination, as well as restricting morning naps. In a retrospective study of more than 1,000 patients younger than 2 years of age, sedation failure rates in CT and MR were marginally improved by sleep deprivation of less than 5 h sleep during the previous night (4.2% and 4.7%, respectively) [55]. However, this same study also demonstrated that sleep-deprived patients required significantly more nursing care hours than their non-sleep-deprived counterparts, which may have patient cost and throughput implications [55]. A smaller study of 41 children suggests that sleep deprivation resulted in a lower dose of sedation requirement [56], but the study population age group was unclear.
Just as pharmacological sedation can cause sleepiness, sleep deprivation may also lead to prolonged recovery and drowsiness on the day after imaging. Sleep deprivation prior to natural sleep in young infants may increase obstructive sleep apnoea and arousal threshold [57]. In older children, behavioural disturbances may persist for over 24 h.
The practicalities of inducing sleep deprivation in a young child may mean it is difficult to enforce. In-patient sleep deprivation prior to radiological procedures involves time-consuming preparation by health-care professionals, which may not be universally accessible [55, 56]. The obvious person to keep the child awake is the parent, although this could also impact on the parent’s sleep patterns. Outpatient examinations would require compliant parents who understand and appreciate the benefits of potentially avoiding sedation or reducing dosages of drugs used. As sleep deprivation requires significant parental or nursing effort and cooperation, and may decrease the efficiency of the imaging unit, some authors conclude that it is “not worth the effort” [55].
Sleep promotion could be an effective alternative to deprivation. Melatonin is a naturally occurring hormone that regulates the sleep-wake cycle, produced by the pineal gland during darkness [58], and thus artificial melatonin could be used to induce a “natural sleep” as an alternative or adjunct to sedation. In a study of 40 children (ages 14 months–17 years) who received oral melatonin, 65% went to sleep prior the MR examination, resulting in a 55% success rate [59]. Melatonin in conjunction with sleep deprivation in 17 children (ages 14 months–14 years) resulted in a higher proportion of children going to sleep prior to MR (76%) and a high success rate [59]. Interestingly, melatonin-induced sleep may result in more acceptable childhood behaviour when compared to sleep deprivation [60].
Melatonin prior to oral sedation has also been trialed in a randomized double-blind study of 98 children [61]. Melatonin did not significantly change the speed of onset or reliability of the sedation but did result in children taking slightly longer to be discharged home [61]. Melatonin and sleep deprivation may therefore increase the success rate of un-sedated paediatric MRI, whether used separately or in conjunction, but they have implications for parental and nursing care requirements.
Hypnosis and guided imagery have a debatable role in MRI in children. Hypnosis creates a state of focused concentration and reduction in peripheral awareness, but is highly variable among individuals [62]. Clearly, clinical trials of hypnosis or distraction cannot be blinded or placebo-controlled. However, hypnosis and alternative therapies such as distraction may reduce anxiety in children undergoing anaesthesia and painful-procedures [63, 64] and are widely used as alternative therapies in symptom management in children with cancer [65]. In a prospective RCT, hypnosis in conjunction with sedation reduced clinical time and costs during interventional radiological procedures, when compared to sedation alone [66]. “Guided imagery” is similar to hypnosis in that attention is focused upon pleasant images or sounds, referred to as a conscious state of distraction [67]. Several small RCTs suggest that post-operative pain and anxiety is lowered in children who are taught guided imagery [68, 69], but none pertains to an imaging setting.
Physical environment
The physical environment can make a significant difference to children and their behaviour with a bright, colourful room being more appealing than a standard clinical setting [70]. The environment should clearly be adapted to the age of the child: young infants may prefer a warm but darkened room to facilitate sleep, but older children may prefer a bright, lively environment thereby turning the MR examination into an adventure. Interacting with unsettled infants within the MR bore, using plastic toys, books and mirrored or reflective paper can enable them to be distracted from the imaging process. Projecting moving colour images can be equally effective at calming and distracting young children during CT and US imaging (e.g., Snoeszelen; FlagHouse, Hasbrouck Heights, NJ, USA) [71]. In a large retrospective study of young children (<4 years) undergoing CT, when children chose the environmental lighting, played with a model scanner and projected images onto the ceiling during the examination, there was a 28% reduction in sedation rates [72]. Projecting images is more difficult to achieve in MR due to the geometry of the bore, and typical projector devices are not MR compatible, but could project through the observation window.
Play therapy and practice MRI
Preparing a child for any procedure is vital to reduce anxiety and ensure co-operation, and so play therapy is used to aid the older child (typically over 4 years) to understand clinical procedures. Play specialists can help to inform and distract children and their parents prior to and during MR scans. DVDs, storybooks or information sheets can provide relevant information at an appropriate age, using cartoons to guide characters [73–76].
Retrospective studies show that adequate play specialist input prior to MR scanning can lead to very low failure rates (in children ages 4–8 years) [22, 71]. However, one randomized study evaluating educational materials prior to MRI in 52 school-age children (ages 7–12 years) and their parents, did not demonstrate a reduction in childhood or parental anxiety levels [77]. Conversely, this study suggested that preparatory material led to increased questioning by the child and therefore an inadvertent increase in overall anxiety [77].
Rehearsal of MR examination with a small-scale model of the MR scanner can help children and their family to understand and gain confidence about the procedure (Fig. 4). Children’s playgrounds often involve tunnels and cylindrical objects, and can be used as a reference for comparison. Visiting an actual or true-size mock scanner at a predetermined date prior to the appointment may be useful (Fig. 5). In a study of 90 children (ages 3–14 years) given a 5-min training session lying still in a full-size replica scanner without magnets enabled 85 to lie still and 81 (90%) to be imaged successfully [78]. A retrospective audit before and after the introduction of mock scanner preparation gave a 9% decrease in GA rates (from 27% to 18%) in children ages 3–14 years [79], with the biggest benefit (26% reduction) in the 4- to 5-year age group. However, GA rates in 3-year-olds in this study were still above 80% after the introduction of a mock scanner.
The mock MRI scanner room is decorated in a child-friendly manner, similar to the real scanner room, and if installed within the department then also encourages familiarity with the hospital and staff. As no magnetic coil is installed, MRI sounds can be played over a stereo system to accustom children to the noise. Reproduced with permission from [79]
The level of detail involved in MR rehearsal may determine its success. Practice MRI (PMRI) at one institution involved routine patients reading story books describing the procedure, experiencing the MRI procedure at the child’s own pace, using headphones, DVDs and the intended RF coil in a mock scanner, comfort positioning and breathing strategies. A total of 75% of 291 children older than 4 years of age passed an MR rehearsal and went on to have a clinical MRI without GA [80]. In another smaller group, there was a reduction in heart rate, and in self-reporting of distress, in children who had undergone a preparation session using a simulator [81].
Listening to the patient’s own CD or watching DVDs during imaging can now be used to distract and occupy the child, using equipment such as wall-mounted TV screens or in-bore MR-specific goggles. A large study of over 1,000 children demonstrated that the introduction of video goggles significantly decreased the need for sedation, cost and scanning time in children older than 3 years of age [82]. MR rehearsal kits enable children to become accustomed to wearing earplugs and headphones, whilst a CD replicating the acoustic noise generated during the imaging process can help prepare the child [22, 54]. Ideally, scanners or sequences with low acoustic noise would be used for children. For instance, several MR system vendors have produced systems with noise-reduction technology, such as vacuum-sealed gradient chambers to dampen the mechanical vibration, with evocative titles such as Pianissimo (Toshiba) and Whisper (Siemens). However, audiovisual distraction is nevertheless required to encourage an older child to remain still for long time periods.
Parental communications—before, during and after
Awake infants need to be relaxed and settled within a scanner to reduce both patient, parental and operator anxiety and enable good-quality images to be obtained. Infants take many of their cues from their immediate surroundings, typically parents [83]; therefore keeping parents relaxed and comfortable during the MRI of their child is not only good clinical practice but has beneficial implications for the child.
Family members or guardians frequently accompany paediatric patients into the MR environment. Informing and reassuring family members can have a positive effect on the child in the scanner, enabling individual comforting techniques to improve the MR experience and therefore image quality. Poor parent compliance may be one reason for failure in a mock scanner [79]. In certain groups where co-operation may be difficult to predict (e.g., autism) [54], parents can be crucial to the success of the examination, as they can devise plans outside of the hospital environment as well as comfort their child during the examination. Open (vertical) magnets allow parents to be in closer proximity to their child during the examination than a conventional closed-bore system, but open magnets tend to have lower static field strengths and may not provide optimal image quality.
Talking to parents between sequences can be highly beneficial, but requires communication equipment such as two-way headphones or microphones built into the scanner room. Explaining noise variations, duration of sequences and methods to settle or distract their child can be useful, in much the same way as if the adult were being imaged themselves. Parents are reassured that you can see them and their child through the observation window. Equally, adequate debriefing afterwards may enable better preparation for subsequent examinations.
Factors that influence decision-making
The risks of imaging preparation must be balanced against the need for high-quality diagnostic imaging. Many factors will influence the choice of approach to paediatric MRI, which are outlined here. Frequently, a single factor may outweigh all others, such as the urgency of the examination.
Patient factors
Age plays a significant role in MR planning. Some hospitals take the view that all children younger than 5 years will require additional MR intervention, and will have adopted a sedation or anaesthesia policy that is age-dependent [84]. Typically, this involves a “feed and wrap” technique for children younger than the age of 1 year, oral or intravenous sedation between 1 and 5 years, and distraction therapies for >6 year olds. However, these ages are arbitrary and individual assessment is required; many hospitals limit GA to those that may need extended examination time (e.g., oncology staging) and attempt un-sedated imaging in the first instance. There are several anecdotal cases of an adequately prepared 4-year-old undergoing successful MRI without sedation.
There are some clinical conditions in which sedation poses too great a risk to the child, i.e. either a full anaesthetic or an alternative technique should be used. An example of this may be Pierre Robin syndrome or other potential airway compromise, thus the need to maintain an airway in a difficult patient may override all other decisions. Some establishments would choose to channel these children towards an MRI under GA; others again may prefer to try the other techniques available taking the view that nothing is lost (from the child’s perspective), although there may be a financial cost to failed MR time. Other conditions would include the inability to understand the need to lie still or comply with instructions, severe developmental delay, learning or behavioural difficulties, and should be tailored to the individual establishments’ sedationist or paediatric anaesthetist skills. More careful preparation may be required in patients with attention-deficit-hyperactivity-disorder, for instance, with ultimate success dependent on adequate play therapy support. Clearly many other factors such as general anxiety, culture, gender, coping styles and previous medical experience may also play a role.
Imaging factors
The body part being imaged may also be a large determinant of the optimal approach. Limb and head imaging may not only be more rapid than body imaging in general, but it may also be easier for a child to keep a limb or their head in a fixed position for the required length of time (either voluntarily or with additional stabilisation) without significant movement artefact. Abdominal imaging that includes breath-holding or respiratory triggering may be more difficult, and raises the possibility of requiring further anaesthetic support earlier in the MR planning assessment.
Previous negative experiences with medical or imaging procedures may play a significant role. A previous negative experience with cannulation may preclude intravenous access for anaesthesia, although gaseous induction would be an option; equally vice-versa [22]. Both of these may deter the older child, who may be more inclined to undergo un-sedated imaging in order to avoid the negative preconceptions or experiences associated with anaesthesia. Equally, forward planning is needed, e.g., if the patient will require several cross-sectional imaging investigations during the course of disease treatment and follow-up, adequate evaluation should be given to the entire patient journey rather than considering each imaging investigation individually.
The urgency of examination may place undue pressure on giving sedation or anaesthesia, though this would be based on institutional availability. Baseline oncology imaging often involves a lengthy examination time (over several sequences, up to an hour) which may influence choice in this regard. If we consider the clinical scenario of a young child admitted one evening with respiratory compromise from an abdominal tumour, who could start urgency chemotherapy that night, some establishments may be encouraged to provide out-of-hours MR, sedation or anaesthetic cover for optimal MRI. Where this is not feasible, others may try an un-sedated MR in order to provide imaging within the required time-frame, or alternatively to try several other imaging modalities, e.g., US or CT, with their accepted limitations. The balance of failing sedation and waiting for the next sedation or anaesthesia slot in the timetable must be weighed up against the possibility of acquiring diagnostic imaging via other means, including other modalities.
Service factors
There is clearly a cost involved in GA / sedation, anaesthetist and recovery staff, hospital beds and imaging costs. However, this must be balanced against the cost of, for example, a mock scanner, educational play therapists, and the hidden costs of aborted scans due to patient un-cooperation. There is no easy way to compare all of these costs, and we have not attempted a cost-evaluation analysis here; decisions are likely to be made on a hospital-by-hospital basis.
In contrast to clinical and diagnostic MRI investigations, most ethics committees will not allow sedation to be used in research studies involving normally developing children, and so researchers may be encouraged to try other techniques before resorting to sedation.
Conclusion
We conclude that the choice of approach to paediatric imaging is multi-factorial, with limited scientific evidence for several of the discussed alternatives. Whilst some institutions may prefer to fall back on the use of sedation or anaesthesia because the advantages and disadvantages may be better understood, others may be encouraged to investigate other options on an individual or study basis. As magnet technology improves and imaging times come down, it would be useful to revisit this in the future to evaluate the effectiveness of new techniques, and the impact that they can have on patient care and imaging efficiency.
References
McRobbie DW, Moore EA, Graves MJ, Prince MR (2003) MRI: from picture to proton. Cambridge University Press, Cambridge
Muir Gray JA (1997) Evidence-based health care. Harcourt Publishers, London
Matharu LL, Ashley PF (2007) What is the evidence for paediatric dental sedation? Review. J Dent 35:2–20
Wengrower D, Gozal D, Gozal Y, Meiri Ch, Golan I, Granto E, Goldin E (2004) Complicated endoscopic pediatric procedures using deep sedation and general anesthesia are safe in the endoscopy suite. Scand J Gastroenterol 39:283–286
Atkinson P, Chesters A, Heinz P (2009) Pain management and sedation for children in the emergency department. BMJ 339:1074–1079
Gross JB (Chair), American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-anesthesiologists, et al. (2002) Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology 96:1004–1017
Sury M, Bullock I, Rabar S, DeMott K (2010) Sedation for diagnostic and therapeutic procedures in children and young people: summary of NICE guidance. BMJ 341:c6819
Sury MR, Smith JH (2008) Deep sedation and minimal anesthesia. Paediatr Anaesth 18:18–24
Sury MR, Hatch DJ, Deeley T, Dicks-Mireaux C, Chong WK (1999) Development of a nurse-led sedation service for paediatric magnetic resonance imaging. Lancet 353:1667–1671
Frush DP, Bissett GS, Hall SC (1996) Pediatric sedation in radiology: the practice of safe sleep. Am J Rad 167:1381–1387
Starkey E, Sammons HM (2010) Sedation for radiological imaging. Arch Dis Child Educ Pract Ed. doi:10.1136/dc.2008.153072
Dalal PG, Murray D, Cox T, McAllister J, Snider R (2006) Sedation and anesthesia protocols used for magnetic resonance imaging studies in infants: provider and pharmacological considerations. Anesth Analg 103:863–868
Volle E, Park W, Kaufmann HJ (1996) MRI examination and monitoring of pediatric patients under sedation. Pediatr Radiol 26:280–281
Beebe DS, Tran P, Bragg M, Stillman A, Truwitt C, Belani KG (2000) Trained nurses can provide safe and effective sedation for MRI in pediatric patients. Can J Anaesth 47:205–210
Karian VE, Burrows PE, Zurakowski D, Connor L, Poznauskis L, Mason KP (2002) The development of a pediatric radiology sedation program. Pediatr Radiol 32:348–353
Woodthorpe C, Trigg A, Alison G, Sury M (2007) Nurse led sedation for paediatric MRI: progress and issues. Paediatr Nurs 19:14–18
Malviya S, Voepel-Lewis T, Prochaska G, Tait AR (2000) Prolonged recovery and delayed side effects of sedation for diagnostic imaging studies in children. Pediatrics 105(E42):1–5
Coté CJ, Karl HW, Notterman DA, Weinberg JA, McCloskey C (2000) Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatr 106:633–644
Litman RS, Soin K, Salam A (2010) Chloral hydrate sedation in term and preterm infants: an analysis of efficacy and complications. Anesth Analg 1(110):739–746
Hummel P, Puchalski M, Creech SD, Weiss MG (2008) Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged pain. J Perinatol 28:58–60
Anand KJS, Hall RW (2006) Pharmacological therapy for analgesia and sedation in the newborn. Arch Dis Child Fetal Neonatal Ed 91:F448–F453
Pressdee D, May L, Eastman E, Grier D (1997) The use of play therapy in the preparation of children undergoing MR imaging. Clin Radiol 52:945–947
Kao SC, Adamson SD, Tatman LH, Berbaum KS (1999) A survey of post-discharge side effects of conscious sedation using chloral hydrate in pediatric CT and MR imaging. Pediatr Radiol 29:287–290
Nelson DS, Hoagland JR 3rd, Kunkel NC (2000) Costs of sedation using oral midazolam: money, time, and parental attitudes. Pediatr Emerg Care 16:80–84
Vanderby SA, Babyn PS, Carter MW, Jewell SM, McKeever PD (2010) Effect of anesthesia and sedation on pediatric MR imaging patient flow. Radiology 256:229–237
Carbajal R, Chauvet X, Couderc S, Olivier-Martin M (1999) Randomised trial of analgesic effects of sucrose, glucose, and pacifiers in term neonates. BMJ 319:1393–1397
Boyle EM, Freer Y, Khan-Orakzai Z, McIntosh N (2006) Sucrose and non-nutritive sucking for the relief of pain in screening for retinopathy of prematurity: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 91:F166–F168
Liu M-F, Lin K-C, Chou Y-H, Lee T-Y (2010) Using non-nutritive sucking and oral glucose solution with neonates to relieve pain: a randomised controlled trial. J Clin Nurs 19:1604–1611
Stevens B, Taddio A, Ohlsson A, Einarson T (1997) The efficacy of sucrose for relieving procedural pain in neonates – a systematic review and meta-analysis. Acta Paediatr 86:837–842
Anand KJS (2001) Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med 155:173–180
Stevens B, Yamada J, Ohlsson A (2010) Sucrose for analgesia in newborn infants undergoing painful procedures (Review). Cochrane Database of Systematic Reviews 1. doi:10.1002/14651858.CD001069.pub3
Harrison D, Johnston L, Loughnan P (2003) Oral sucrose for procedural pain in sick hospitalized infants: A randomized-controlled trial. J Paediatr Child Health 39:591–597
Taddio A, Shah V, Katz J (2009) Reduced infant response to a routine care procedure after sucrose analgesia. Pediatrics 123:e425–e429
Harrison D, Stevens B, Bueno M, Yamada J, Adams-Webber T, Beyene J, Ohlsson A (2010) Efficacy of sweet solutions for analgesia in infants between 1 and 12 months of age: a systematic review. Arch Dis Child 95: 406–413
Slater R, CorneLissen L, Fabrizni L, Patten D, Yoxen J, Worley A, Boyd S, Meek J, Fitzgerald M (2010) Oral sucrose as an analgesic drug for procedural pain in newborn infants: a randomised controlled trial. Lancet. doi:10.1016/S0140-6736(10)61303-7
Curtis SJ, Jou H, Ali S, Vandermeer B, Klassen T (2007) A randomized controlled trial of sucrose and/or pacifier as analgesia for infants receiving venipuncture in a pediatric emergency department. BMC Pediatrics. 7:27. doi:10.1186/1471-2431-7-27
Blass EM, Watt LB (1999) Sucking- and sucrose-induced analgesia in human newborns. Pain 83:611–623
Lipton EL, Steinschneider A, Richmond JB (1965) Swaddling, a child care practice: Historical, cultural, and experimental observation. Pediatrics 35:521–567
Fearon I, Kisilevsky BS, Mains SMJ, Muir DW, Tranmer J (1997) Swaddling after heel lance: age-specific effects on behavioural recovery in preterm infants. J Dev Behav Pediatr 18:222–232
Huang CM, Tung WS, Kuo LL, Ying-Ju C (2004) Comparison of pain responses of premature infants to the heelstick between containment and swaddling. J Nurs Res 12:31–40
van Sleuwen BE, L’hoir MP, Engelberts AC, Busschers WB, Westers P, Blom MA, Schulpen TWJ, Kuis W (2006) Comparison of behaviour modification with and without swaddling as interventions for excessive crying. J Pediatr e2:512–517
Franco P, Seret N, van Hees JN, Scaillet S, Groswasser J, Kahn A (2005) Influence of swaddling on sleep and arousal characteristics of healthy infants. Pediatrics 115:1307–1311
Kassim Z, Greenough A (2006) Neonatal abstinence syndrome: identification and management. Curr Paediatr 16:172–175
O’Sullivan A, O’Connor M, Brosnahan D, McCreery K, Dempsey EM (2010) Sweeten, soother and swaddle for retinopathy of prematurity screening: a randomised placebo controlled trial. Arch Dis Child Fetal Neonatal Ed 95:F419–F422
Yurdakok K, Yavuz T, Taylor CE (1990) Swaddling and acute respiratory infections. Am J Public Health 80:873–875
van Sleuwen BE, Engleberts AC, Boere-Boonekamp MM, Kuis W, Schulpen TWJ, L’Hoir MP (2007) Swaddling: a systematic review. Pediatrics 120:e1097–e1106
Kutlu A, Memik R, Mutlu M, Kutlu R, Arslan A (1992) Congenital dislocation of the hip and its relation to swaddling used in Turkey. J Paediatr Orthop 12:598–602
Mahan ST, Kasser JR (2008) Does swaddling influence developmental dysplasia of the hip? Pediatrics 12:177–178
Mahan ST, Kasser JR (2008) Safe swaddling and healthy hips: don’t toss the baby out with the bathwater: in reply. Pediatrics 121:1077
Saunders DE, Thompson C, Gunny R, Jones R, Cox T, Chong WK (2007) Magnetic resonance imaging protocols for paediatric neuroradiology. Pediatr Radiol 37:789–797
Sury MRJ, Harker H, Begent J, Chong WK (2005) The management of infants and children for painless imaging. Clin Radiol 60:731–741
Hansen SS (2009) Feed-and-sleep: a non-invasive and safe alternative to general anaesthesia when imaging very young children. Radiographer 56:5–8
Mathur AM, Neil JJ, McKinstry RC, Inder TE (2008) Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol 38:260–264
Nordahl CW, Simon TJ, Zierhut C, Soloman M, Rogers SJ, Amaral DG (2008) Brief reports: methods for acquiring structural MRI data in very young children with autism without the use of sedation. J Autism Dev Disord 38:1581–1590
Shields CH, Johnson S, Knoll J, Chess C, Goldberg D, Creamer K (2004) Sleep deprivation for pediatric sedated procedures: not worth the effort. Pediatrics 113:1204–1208
Kini K, Kini PG (2009) Sleep deprivation for radiological procedures in children. Pediatr Radiol 39:1255
Franco P, Seret N, Van Hees JN, Scaillet S, Vermeulen F, Groswasser J, Kahn A (2004) Decreased arousal among healthy infants after short-term sleep deprivation. Pediatrics 114:e192–e197
Richardson GS (2005) The human circadian system in normal and disordered sleep. J Clin Psychiatry 66:3–9
Johnson K, Page A, Williams H, Wassemer E, Whitehouse W (2002) The use of melatonin as an alternative to sedation in uncooperative children undergoing an MRI examination. Clin Radiol 57:502–506
Wassmer E, Carter PFB, Quinn E, McLean N, Welsh G, Seri S, Whitehouse WP (2001) Melatonin is useful for recording sleep EEGs: a prospective audit of outcome. Dev Med Child Neurol 43:735–738
Sury MR, Fairweather K (2006) The effect of melatonin on sedation of children undergoing magnetic resonance imaging. Br J Anaesth 97:220–225
Spiegel H, Spiegel D (2004) Trance and treatment: clinical uses of hypnosis, 2nd edn. American Psychiatric Press Inc, Arlington, Virginia
Yip P, Middleton P, Cyna AM, Carlyle AV (2011) Non-pharmocological interventions for assisting the induction of anaesthesia in children. Evid-Based Child Health 6:71–134
Accardi MC, Milling LS (2009) The effectiveness of hypnosis for reducing procedure-related pain in children and adolescents: a comprehensive methodological review. J Behav Med 32:328–339
Rheingans JI (2007) A systematic review of nonpharmalogical adjunctive therapies for symptom management in children with cancer. J Pediatr Oncol Nurs 24:81–94
Lang EV, Rosen MP (2002) Cost analysis of adjunct hypnosis with sedation during outpatient interventional radiological procedures. Radio 222:375–382
O’Donohue WT, Cummings NA (2008) Evidence-based adjunctive treatments. Elsevier, Burlington, Massachusetts
Lambert SA (1996) The effects of hypnosis/guided imagery on the postoperative course of children. J Dev Behav 17:307–310
Weydert JA, Shapiro DE, Acra SA, Monheim CJ, Chambers AS, Ball TM (2006) Evaluation of guided imagery as treatment for recurrent abdominal pain in children: a randomized controlled trial. BMC Pediatr 6:29
Roberton DM, South MJ (2007) Practical paediatrics, 6th edn. Longman, London
Khan JJ, Donnelly LF, Koch BL, Curlwright LA, Dickerson JM, Hardin JL, Hutchinson S, Wright J, Gessner KE (2007) A program to decrease the need for pediatric sedation for CT and MRI. Appl Radiol 36:30–33
Anastos JP (2007) The ambient experience in Pediatric Radiology. J Radiol Nurs 26:50–55
Bratton SC, Ray D, Rhine T (2005) The efficacy of play therapy with children: a meta-analytic review of treatment outcomes. Prof Psychol Res Pract 36:376–390
Jones EM, Landreth G (2002) The efficacy of intensive individual play therapy for chronically ill children. Int J Play Therapy 11:117–140
Diette GB, Lechtzin N, Haponik E, Devrotes A, Rubin HR (2003) Distraction therapy with nature sights and sounds reduces pain during flexible bronchoscopy. Chest 123:941–948
Russell C, Smart S (2007) Guided imagery and distraction therapy in paediatric hospice care. Paediatr Nurs 19:24–25
Hartman JH, Bena J, McIntyre S, Albert NM (2009) Does a photo diary decrease stress and anxiety in children undergoing magnetic resonance imaging? A randomized controlled study. J Radiol Nurs 28:122–128
de Bie HMA, Boersma M, Wattjese MP, Adriaanse S, Vermeulen RJ, Oostrom KJ, Huisman J, Veltman DJ, Delemarre-Van de Waal HA (2010) Preparing children with a mock scanner training protocol results in high quality structural and functional MRI scans. Eur J Pediatr 169:1079–1085
Carter AJ, Greer MLC, Gray SE, Ware RS (2010) Mock MRI: reducing the need for anaesthesia in children. Pediatr Radiol 40:1368–1374
Hallowell LM, Stewart SE, de Amorim ES, Ditchfield MR (2008) Reviewing the process of preparing children for MRI. Pediatr Radiol 38:271–279
Rosenberg DR, Sweeney JA, Gillen HS (1997) Magnetic resonance imaging of children without sedation: preparation with stimulation. J Am Acad Child Adolesc Psychiatry 36:853–859
Harned RK, Strain JD (2001) MRI-compatible audio/visual system: impact on pediatric sedation. Pediatr Radiol 31:247–250
Bowlby J (1969) Attachment. Hogarth Press, London
Shankar VR (2008) Sedating children for radiological procedures: an intensivist’s perspective. Pediatr Radiol 38:S213–217
Acknowledgements
Dr Arthurs is funded by a Medical Research Council / Royal College of Radiologists Clinical Research Training Fellowship. Both authors have received funding from the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB-PG-0807-14149). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The authors thank Addenbrooke’s Charitable Trust for infrastructure support, and the NIHR comprehensive Biomedical Research Centre award to Cambridge University Hospitals NHS Foundation Trust in partnership with the University of Cambridge.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Edwards, A.D., Arthurs, O.J. Paediatric MRI under sedation: is it necessary? What is the evidence for the alternatives?. Pediatr Radiol 41, 1353–1364 (2011). https://doi.org/10.1007/s00247-011-2147-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-011-2147-7