Abstract
Long-term right ventricular pacing is associated with left ventricular dysfunction and cardiomyopathy, particularly in pediatric patients and those with congenital heart disease (CHD). Research has shown that pacing-induced cardiomyopathy can be reversed with nonselective or selective His bundle pacing in adults, however, the information available about the use of this type of therapy in pediatrics and CHD is scarce. We performed a retrospective chart review of all the cases of His or left bundle pacing at the University of Minnesota, division of Pediatric Cardiology from January of 2019 to April of 2020. Parametric data are presented as mean ± standard deviation. Non-parametric data are presented as median value with interquartile ranges. Eight patients, ages 8 to 18 years (median of 11.5) and weight from 21.5 to 81.6 kg (median of 40 kg) underwent this procedure successfully. The most common structural heart disease was a repaired peri-membranous ventricular septal defect. Three patients (37.5%) had selective and three (37.5%) had nonselective His bundle pacing, and two patients (25%) had left bundle pacing. There were two cases of pacing-induced cardiomyopathy and each had a 14% and 16% improvement of the ejection fraction after nonselective His bundle pacing. There were no procedural complications. Selective and nonselective His bundle, as well as left bundle pacing may be a feasible procedure in pediatric patients with and without CHD. This procedure may improve pacing-induced cardiomyopathy in this population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pacing in the pediatric population requires thoughtful considerations regarding type of pacing, the consequences of long-term pacing, and how to best implement pacing. Long-term right ventricular pacing is associated with left ventricular dysfunction and cardiomyopathy in up to 13% of the cases with this type of pacing [1, 2]. Pediatric patients and those with congenital heart disease (CHD) are especially vulnerable to left ventricular dysfunction with chronic right ventricular pacing [3, 4]. His bundle pacing has been demonstrated to be feasible in adults that require long-term pacing, however, higher pacing threshold for His bundle capture, and high dislodgement rate have been important considerations [5].
Cardiac resynchronization therapy has been used for prevention and reversal of pacing-induced cardiomyopathy in adults [6]. Recent data suggest that selective or nonselective His bundle pacing can also reverse and prevent pacing-induced cardiomyopathy [7]. The 3830 transvenous lead (Medtronic, Minneapolis, MN, USA) was the first lead approved, with accompanying sheaths, for His bundle pacing. Due to their small size (4.1-French), these leads have demonstrated an excellent safety profile in pediatric right ventricular pacing [8]. Although there are some case reports documenting the feasibility of His bundle pacing in adult patients with congenitally corrected transposition (ccTGA), no case series describing the feasibility and outcomes of His bundle pacing in pediatric patients or those with other structural heart disease has been reported [9,10,11,12]. Furthermore, left bundle pacing in pediatrics has only recently been described in patients 26 kg or larger [13].
Aim
We aimed to describe our experience and demonstrate the feasibility of His bundle and left bundle pacing in pediatric patients with and without CHD.
Materials and Methods
After approval by the Institutional Review Board, a retrospective chart review of all cases of selective and nonselective His bundle and left bundle branch pacing was performed. Patients were captured from the electrophysiology database from the University of Minnesota, Division of Pediatric Cardiology. These procedures were performed by a single provider and included all cases done in our institution from January of 2019 to April of 2020.
All procedures were performed under general anesthesia with either intubation or laryngeal mask placement and without paralysis except during initial placement of LMA or ET tube. His bundle pacing and left bundle pacing were performed by standard method [14, 15]. However, given lack of pediatric description, we have detailed the procedure below.
Femoral venous access was obtained via the Seldinger technique and a 5-Fr sheath was placed in all patients except the patient with interrupted IVC. Through the 5Fr sheath, a 5Fr Livewire octopolar catheter (St Jude Medical, Saint Paul, MN, USA) was placed and positioned in the subclavian/axillary veins with AP fluoroscopy and saved for guidance during axillary venous access. The Livewire was then located to the level where the His bundle potential was largest at the distal bipolar (by intracardiac recording). Cine of the catheter position from RAO (− 30°) and LAO (+ 30°) were obtained for use later on during His bundle lead placement. The Livewire was then placed into the RV apex for backup pacing.
An incision was made 1 cm below the clavicle on either the right or left side and dissection down to the pectoralis major was performed. A device pocket was created within the muscular sheath and subsequent access was obtained with the Seldinger under fluoroscopy. Either ultrasound, saved fluoroscopy image after 10 ml of contrast, or saved fluoroscopy image of the Livewire in subclavian/axillary vein position were used as guidance for access into the axillary vein.
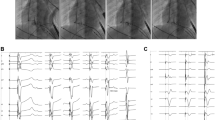
Two access points were obtained for the atrial and ventricular leads. The C315 guiding catheter (Medtronic, Minneapolis, USA) was deployed over a Glidewire (Terumo, Tokyo, Japan) close to the His bundle recording position. Subsequently, a 69 cm 3830 lead (Medtronic, Minneapolis, USA) was passed through the C315 (Medtronic, Minneapolis, USA) catheter and positioned at a point where the His signal was present and adequate sensing and threshold were obtained. Pacing at high output was performed to assess His bundle capture prior to coiling of the lead. Subsequently, the lead was coiled into the myocardium, typically just below the tricuspid valve or at the lower right atrium and then threshold/His bundle capture, sensing (including degree of injury), and impedance were assessed. The 3830 lead signal was projected into our Cardiolab system so that improved visualization of the His bundle signal could be seen before and after coiling the lead into the myocardium (Fig. 1) also similar to prior described procedure [14]. Sufficient slack was allowed in the right atrium to allow for growth of the patient, but slack was not allowed to accumulate below the tricuspid valve. For Left bundle pacing a similar approach to above was taken except the C315 delivery catheter was placed deeper in the right ventricle and at least 10 turns were given to the 3830 lead (instead of manufacturer-recommended 4 to 6 turns) and assessment of right bundle branch block pattern was noted to confirm left bundle activation during pacing. Left bundle pacing was performed when left bundle branch block was still apparent during His bundle pacing. Diaphragmatic stimulation was assessed during high output pacing.
A 49 cm 3830 lead was deployed with the J-curve atrial catheter to find a stable atrial position, (typically septal). The incisions were then closed with absorbable sutures and Steri strips applied along with a dressing. All patients were monitored overnight and underwent CXR and ECG within 2 h postoperatively and two-view chest x ray was obtained the following morning prior to discharge to home.
Following the new His bundle or left bundle pacing, any preexisting epicardial pacemakers were removed during the same procedure (4 patients). One patient had an ablation of intra-atrial re-entrant and typical flutter (via the internal jugular vein) just prior to her pacemaker placement/epicardial removal during the same procedure.
Statistics
Parametric and non-parametric data were presented. Parametric data are presented as mean ± standard deviation. Non-parametric data are presented as median value with total ranges reported due to low number of patients in study.
Results
A total of eight patients underwent selective or nonselective His bundle and/or left bundle branch pacing between January 2019 to April 2020. The median age was 11 years (range 9 to 14 years) and four (50%) were female. Six (75%) patients had CHD (Table 1). The median weight was 40 kg (range 21.5 to 80.7 kg). Median follow-up was 5 months (range 2 to 6 months). The four patients with a paced rhythm had a baseline bundle branch block (1 RBBB, 2 LBBB, 1 intraventricular conduction delay). The Median pre-implant QRS duration (QRSd) was 90 ms (range 78 to 190 ms). Mean decrease of QRSd was 12 ms with His bundle pacing. Four (50%) patients had right-sided device implants and four patients had left-sided implants. Average implant threshold was 0.5 ± 0.05 V at 0.4 ms with average impedance of 597 ± 120 ohms with median R-wave of 6.9 (mV) (range 2 to 19 mV). In the nonselective His bundle pacing patients, His capture ranged from 2 to 3.5 V at 0.4 ms, respectively.
Baseline echocardiogram demonstrated normal ejection fractions in six (75%) patients. Two (25%) patients had symptomatic pacing-induced cardiomyopathy (exertional dyspnea). Left ventricular ejection fraction increased by 14 and 16% for (cases 6 and 8, respectively) after His bundle pacing. Improvement of the left ventricular ejection fraction and symptoms occurred within 2 weeks after switching to His bundle pacing.
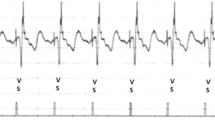
In regards to pacing location (Fig. 2), three (37.5%) patients had selective His bundle pacing whether unipolar or bipolar (Fig. 2a), three (37.5%) patients had nonselective His bundle pacing (2b) and 2 (25%) patients had left bundle pacing (2c).
Discussion
We describe our experience in eight patients who underwent His bundle and left bundle pacing. We found that selective and nonselective His bundle and left bundle pacing provided excellent outcomes without periprocedural complications in patients as small as 21.5 kg. Achieving physiologic ventricular activation promotes ventricular synchrony and may optimize the paced ventricular function. Our cohort seems to also demonstrate that pacemaker-induced cardiomyopathy may be reversed with transition from epicardial right ventricular basal or lateral wall right ventricular pacing to His bundle pacing. No dislodgements or other complications were noted during follow-up.
In our pediatric cohort, His bundle pacing required lower threshold than what has been typically reported in adult studies [5, 7]. In our cases, optimal lead pulse-width was 0.4 ms. Although we anticipate an improvement on battery longevity, the impact that this lower threshold might have in pediatric patients will be a matter for further research in the future.
Right-Sided System
His bundle pacing from the right side was mostly nonselective. This was likely due to inexperience in shaping the delivery catheter appropriately since it is designed for left-sided access. As we had an unusually high proportion of right-sided implants in our cohort (left-handed patients), a right-sided 3830 His bundle delivery catheter would have been helpful.
Cardiomyopathy Reversal
After demonstration of endocardial septal approach to His-Purkinje ventricular pacing feasibility, other studies in adults have demonstrated the ability to reverse pacing-induced cardiomyopathy with His bundle pacing with nonselective or selective His capture [5, 15]. Recently, in a pediatric series of patients without CHD, left bundle pacing was shown to reverse cardiomyopathy in a patient [13]. This study specifically shows that similar results can also be achieved with this pacing system in pediatric patients with CHD. Whether septal pacing would have been the same is unknown, however, given more physiological activation, His bundle pacing provides better clinical outcomes than RV pacing [16, 17]. We witnessed this intraoperatively with one of our cases where improvement in EF was noted when transitioning from epicardial pacing to His bundle pacing.
In comparison with traditional cardiac resynchronization therapy, given that His bundle or left bundle pacing requires only two leads, this type of pacing may be a more favorable long-term system in children with potential for fewer lead-related issues [6].
His Bundle Pacing in CHD
We found that nonselective, selective His bundle and left bundle pacing could be reasonably achieved in patients with CHD and even selective His bundle capture could be achieved, however with distortion of the conduction system, by large repaired ventricular septal defects, for example, difficulty was increased in obtaining His bundle pacing. Selective His bundle pacing was achieved in all patients without structural heart disease and one with a small muscular ventricular septal defect. Although case reports of patients with ccTGA undergoing His bundle pacing have been described, we are the first to describe patients with large peri-membranous ventricular septal defects undergoing His bundle pacing [9,10,11,12]. However, despite being damaged in those with large VSD’s, the His bundle was not anatomically displaced in any of our patients with or without CHD.
Conclusion
Selective and nonselective His bundle pacing, as well as left bundle pacing is feasible in pediatric patients with and without structural heart disease via left or right-sided access. His bundle pacing may reverse pacemaker-induced cardiomyopathy in a pediatric patient with CHD. More collaborative studies are needed to assess true outcomes over long-term follow-up, but short-term results seem promising.
References
Merchant FM, Mittal S (2018) Pacing-induced cardiomyopathy. Cardiac Electrophys Clin. 10:437–445
Gebauer RA, Tomek V, Salameh A, Marek K, Chloupecky V, Gebauer R, Metejka T, Vojtovic P, Janousek K (2009) Predictors of left ventricular remodeling and failure in right ventricular pacing in the young. Eur Heart J 30:1097–1104
Geldorp IE, Vanagt WY, Prinzen FW, Delhaas T (2011) Chronic ventricular pacing in children: toward prevention of pacing-induced heart disease. Heart Fail Rev 16:305–314
Kaltman J, Ro PS, Zimmerman F, Moak JP, Epstein M, Zetser IJ, Sha MJ et al (2008) Management ventricular pacing in pediatric patients and patients with congenital heart disease. Am J Cardiol 102:875–878
Patel B, Garg J, Chaudhary R, Sablani N, Gupta R, Shah M et al (2018) His bundle pacing: hemodynamics and clinical outcomes. Cardiol Rev. 26(4):201–206
Khurshid S, Obeng-Gyimah E, Supple GE et al (2018) Reversal of pacing-induced cardiomyopathy following cardiac resynchronization therapy. JACC Clin Electrophysiol. 4:168–177
Santosh K, Ellenbogen P, Ellenbogen KA (2019) Selective versus nonselective His bundle pacing-does it matter? JACC Clin Electrophysiol. 5:775–777
Khan A, Zelin K, Larpawich PP (2010) Performance of the lumenless 4.1-Fr diameter pacing lead implanted at alternative pacing sites in congenital heart: a chronic 5-year comparison. Pacing Clin Electrophys 33:1467–1474
Vijarayaman P, Mascarenhas V (2019) Three-dimensional mapping-guided permanent His bundle pacing in a patient with corrected transposition of great arteries. HeartRhythm Case Rep. 5:600–602
Mahata I, Macicek SL, Morin DP (2019) Direct His bundle pacing using retrograde mapping in complete heart block and L-transposition of the great arteries. HeartRhythm Case Rep. 5:291–293
Kean AC, Kay WA, Patel JK, Miller JM, Dandamudi G (2017) Permanent nonselective His bundle pacing in an adult with L-transposition and complete AV block. Pacing Clin Electrophysiol 40:1313–1317
Takemoto M, Nakashima A, Muneuchi K, Yamamura K, Shiokawa Y, Sunagawa K, Tominaga R (2010) Para-hisian pacing for a pediatric patient with congenitally corrected transposition of the great arteries (SLL). Pacing Clin Electrophysiol 33:e4–e7
Dai CC, Dai WL (2020) Guo BJ [Clinical observation on six children of left bundle branch area pacing]. Zhonghua Er Ke Za Zhi 58:107–112
Lyon S, Dandamudi G, Kean AC (2020) Permanent his-bundle pacing in pediatrics and congenital heart disease. J Innov Card Rhythm Manag. 11:4005–4012
Ponnusamy SS, Muthu G, Bopanna D (2019) Selective left bundle branch pacing for pediatric complete heart block. Indian Pacing Electrophysiol. 20:78–80
Karpawich PP, Gates J, Stokes KB (1992) Septal his-purkinje ventricular pacing in canines: a new endocardial electrode approach. Pacing Clin Electrophysiol 15:2011–2015
Sharma PS, Dandamudi G, Naperkowski A, Storm RH, Ellenbogen KA, Vijayaraman P (2014) Permanent His-bundle pacing is feasible, safe, and superior to right ventricular pacing in clinical practice. Heart Rhythm. 12:305–312
Funding
No funding to declare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
There are no conflicts of interest to declare.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jimenez, E., Zaban, N., Sharma, N. et al. His Bundle and Left Bundle Pacing in Pediatrics and Congenital Heart Disease: A Single Center Experience. Pediatr Cardiol 41, 1425–1431 (2020). https://doi.org/10.1007/s00246-020-02398-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-020-02398-9