Abstract
Taussig–Bing anomaly and aortic arch obstruction are two types of complex congenital cardiac malformations. Almost 50% of patients with Taussig–Bing anomaly have aortic arch obstruction. This report assesses the surgical outcomes of single-stage correction in neonates with both defects. Between November 2006 and November 2015, 39 neonates with Taussig–Bing anomaly and aortic arch obstruction (28 patients with coarctation of the aorta and 11 patients with interrupted aortic arch) underwent a one-stage arterial switch operation and aortic reconstruction. There were three in-hospital deaths and one late death (8 months after the surgery). The short-term survival rate was 92.3% (36/39), and the mid-term survival rate was 89.7% (35/39). Follow-up data were available for all patients who survived the operation (range 6–92 months). Echocardiology showed six cases of recoarctation, three cases of left ventricular outflow tract obstruction, three cases of right ventricular outflow tract obstruction, four cases of pulmonary artery stenosis, five cases of aortic regurgitation, and eight cases of pulmonary regurgitation. Eight patients required a reoperation during the follow-up period with no mortality. All survivors remained in good condition (New York Heart association functional class I or II). Single-stage correction of Taussig–Bing anomaly with aortic arch obstruction in neonates had favorable short- and mid-term outcomes in terms of mobility and reoperation rate. The optimal operative procedure should be chosen according to the position of the coronary arteries and the type of aortic anomaly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Taussig–Bing anomaly (TBA) was first described by Taussig and Bing in 1949 [1]. The original report described a side-by-side relationship of the great arteries, transposition of the aorta to the right ventricle, and a high ventricular septal defect that is partially overridden by the pulmonary artery (PA) (sub-PA VSD). The definition has since been broadened to include all types of double outlet right ventricle with sub-PA VSD [2]. TBA is the second most common type of double outlet right ventricle (24%) [3]. Additionally, 50% of subjects with TBA have been associated aortic arch obstruction [4]. Staged surgery is initially performed on these patients as follows: stage I involves reconstruction of the aortic arch with PA banding; stage II involves removal PA banding and correction of the cardiac defect, which is performed at a later date. Since the surgical techniques have been developed, single-stage correction has become the preferred treatment for TBA with aortic arch obstruction in many major centers. In this study, we aimed to evaluate the outcomes of single-stage correction and to investigate the risk factors for mortality.
Materials and Methods
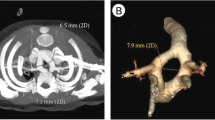
From November 2006 to November 2015, 39 patients with TBA and aortic arch obstruction were hospitalized at the Heart Center of Shanghai Children’s Medical Center. There were 25 boys and 14 girls with a mean age of 12.8 ± 4.6 days (range 2–25 days) and a mean weight of 3.1 ± 0.4 kg (range 2.3–4.5 kg). The diagnostic criteria for TBA in our center are double outlet right ventricle with sub-PA (more than 50% of the PA arising from the right ventricle) and without pulmonary stenosis. All patients were initially diagnosed by echocardiography. Additionally, 30 cases underwent cardiac CT to confirm the connections and development of the great arteries. Nine cases who were diagnosed as having severe pulmonary hypertension (>70 mmHg) by echocardiography underwent cardiac catheterization to evaluate the PA pressure. The preoperative clinical data are shown in Table 1.
Operative Technique
All patients underwent single-stage correction including (1) arterial switch operation (ASO) with baffling of the left ventricle to the neo-aorta to correct the TBA, and (2) resection and reconstruction of the coarctation by modified end-to-side anastomosis.
ASO was performed via a median sternotomy. The pericardium was opened, and the ascending aorta and right atrium were cannulated to establish a cardiopulmonary bypass (CPB). After a CPB had been established, a catheter was inserted into the left heart via the left superior pulmonary veins to reduce the pressure in the left atrium during the early steps. Deep hypothermic circulatory arrest or deep hypothermic low-flow techniques were used for CPB, and the rectal temperature was controlled between 20 and 24 °C. The patent ductus arteriosus and ligament were ligated and the left and right PAs were dissociated as required. After aortic clamping, an incision was made at the root of the PA trunk, and an autologous pericardial patch was used to repair the VSD and baffle the pulmonary orifice into the left ventricle. The ascending aorta was then transected 1 cm above the aortic valve to explore the coronary artery orifices and clip the aortic wall. Next, the PA was transected below the bifurcation and the coronary arteries were implanted to the new positions without distortion or traction. The ascending aorta and PA were then switched using the Lecompte technique: the ascending aorta was anastomosed to the neo-aorta, and the old coronary position was repaired with the distal PA and patches.
The success or failure of coronary transplantation is the crux of ASO. Transplantation techniques were chosen according to the type of coronary arteries as follows: (1) in patients with short coronary arteries with a bifurcate location close to their origin, the “bay window” technique was selected to increase the length and stability of the coronary arteries (2) in patients with coronary arteries penetrating the aortic wall that were difficult to flip in situ, the “open trap door” technique was used to scroll down the openings of the coronary arteries and expand them with a pericardial patch (3) in patients with a single coronary artery, the “pericardium sleeve” technique, which involves making a tunnel with a pericardial patch to extend the length of the coronary artery, was used (4) in patients with a right main coronary artery or large RV cone that passed anterior to the aortic root, the “double flapper extension” technique was chosen [5]. This technique involves clipping a coronary artery button from the aortic root as the first flapper, clipping another pedicle flapper from the position of the PA (new aortic position), making an equidistant extension with the first flapper, and then anastomosing both edges of these flappers to create an extension of the coronary artery tunnel.
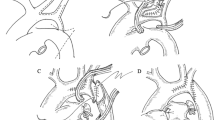
End-to-side anastomosis is the most commonly used operation for coarctation of the aorta (COA) or interrupt aortic arch (IAA). However, in patients with limited coarctation or interrupted aortic arch, normal end-to-side anastomosis results in high tension and poor growth potential, which can easily cause postoperative bleeding and recoarctation. In these cases, we performed a modified end-to-side anastomosis, and an anterior patch augmentation was performed. After resecting the coarctation, the aortic arch was directly anastomosed to the posterior wall of the descending aorta, and the anterior wall was expanded using a pericardial patch or a pulmonary patch. If the tension remained high, the tissue excised from the lower edge of the aortic arch was retained as a flapper to extend the length of the descending aorta. The posterior wall of the distal aorta was anastomosed to the flapper, and the inferior wall was expanded with an autograft patch (Fig. 1).
The mean CPB time was 195.39 ± 58.11 min (range 126–340 min) and the mean aortic cross-clamping time was 118.89 ± 26.81 min (range 68–168 min). In five cases, mild hypothermia (30–34 °C) was achieved, in eight cases, moderate hypothermia (25–30 °C) occured, and in 26 cases, deep hypothermia (<25 °C) was achieved. The myocardium was protected with 4:1 cold blood hyperkalemic cardioplegia (before 2011) and histidine-tryptophan-ketoglutarate solution (after 2011). The postoperative oxygen saturations were improved over preoperative values in all patients. Conventional indwelling pulmonary piezometer tubes were inserted in seven patients to prevent a pulmonary hypertension crisis. Right ventricle temporary pacing was implanted in five patients with arrhythmias after heart re-beating, and sternal closure was delayed in 19 patients for 2–4 days after surgery.
Intensive Care Unit (ICU) Management
Routine postoperative ECG monitoring, mechanical ventilation, and positive inotropic drugs were administered in the ICU. Moderate doses of dopamine (2.5–7.5 μg/kg/min) and milrinone (0.25–0.75 μg/kg/min) were administered to facilitate the recovery of heart function. Low doses of adrenaline (0.02–0.1 μg/kg/min) were optionally used to prevent low cardiac output syndrome. Nitroglycerin (0.3–0.5 μg/kg/min) was routinely administered to dilate the coronary arteries, and prophylactic antibiotics were used to prevent lung infection. Central venous pressure was monitored and adequate blood volumes were maintained. Chest radiography and echocardiography were regularly used to evaluate cardiopulmonary function.
When considering complications, 19 patients with low cardiac output syndrome recovered after receiving positive inotropic drugs for 3–4 days; extracorporeal membrane oxygenation (ECMO) was used in three patients with severe postoperative heart failure for cardiac support; 15 cases with lung infection were successfully treated with antibiotics; eight patients with hypoxemia required repeated intubation; six patients with pulmonary hypertension received aerosol inhalation of iloprost; six patients with cardiac tamponade underwent thoracotomy hemostasis; five patients with arrhythmias were treated with medical therapy; diaphragm folding procedures were performed on four patients with diaphragmatic paralysis; and three patients with acute renal failure received peritoneal dialysis.
Statistical Analysis
Data were analyzed with the statistical computing package SPSS 19.0. All results are expressed as the mean ± standard deviation. The survival rate and freedom from reoperation were estimated with Kaplan–Meier curves. P < 0.05 was considered significant.
Results
The three patients who died in the hospital had interrupted aortic arch conditions (two cases of A type and one case of B type) because of coronary perfusion inadequacy and severe LCOS. The one late death occured 8 months postoperatively and was mainly attributable to multiple organ failure in a patient who developed severe recoarctation of the aorta (narrowest diameter 0.16 m). The mean intubation time for all patients was 173.50 ± 169.35 h (range 25–722 h). The mean ICU stay was 11.46 ± 8.76 days (range 4–38 days), and the mean hospital stay was 27.06 ± 15.54 days (range 10–68 days).
The mean duration of postoperative follow-up was 38.5 ± 24.2 months (range 6–92 months) in the survivors. Cardiopulmonary function improved significantly in all survivors, and their ECGs showed sinus rhythm with no ST-T changes. Chest X-ray films showed smaller cardiac shadows and normal pulmonary flow. Echocardiography results are shown in Table 2. The comparison results of normal and modified groups are shown in Table 3.
Eight patients required reoperations during the mid-term follow-up (Fig. 2), and no death occured. Four recoarctation patients (>80 mmHg) underwent aortic arch reconstruction; two RVOTO patients and one patient with MPA stenosis had their condition enlarged by bovine pericardial patches; and one patient with PA branch stenosis was implanted with a PA stent. All survivors remained in good condition (New York Heart Association functional class I or II).
According to follow-up results, all aortic arch anastomoses experienced relative growth. Compared with the normal group, the modified group had certain advantages in the average growth rate (P < 0.05). The Kaplan–Meier curve showed a 5-year survival rate of 89.7% (35/39) (Fig. 3). The freedom from reoperation was 94.3, 85.7 and 77.1% at 1, 3, and 5 years, respectively (Fig. 4).
Discussion
The Anatomic Characteristics of TBA Associated With Aortic Arch Obstruction
A typical TBA comprises a double outlet right ventricle (aorta originating completely from the right ventricle with the PA overriding the ventricular septum), sub-PA VSD, and no pulmonary stenosis. The VSD is characteristically surrounded by the anterior and posterior branches of the septal bundle, and its superior edge is formed by a pulmonary cone. If this cone is absent, the superior edge would be formed by the pulmonary orifice. Because the infundibular septum is sagittal position, the region between the interventricular septum and anterior wall of the right ventricle is compromised. Variations in size of the infundibular septum and parietal bands would cause different degrees of subaortic stenosis [1]. Another mechanism for aortic arch obstruction is hemodynamic in nature, because the VSD is close to the pulmonary orifice in infants, the left ventricle ejects most of its output through the VSD to the PA, which could cause relative subaortic stenosis.
Development of the Operative Technique
Because pulmonary hypertension and occlusive disease develop early in patients with TBA, especially in those with aortic arch obstruction, the majority die in infancy [6, 7]. TBA with COA was first reported by Wedemeyer in 1970 [8]. The infant underwent patent ductus arteriosus ligation and repair of COA in stage I and complete physiological correction of the defect was achieved 8 months later by performing the Mustard procedure. Since the 1980s, staged surgery has experienced considerable growth and development. Some surgeons repaired the COA and performed PA banding or a modified Blalock–Taussig shunt in a stage I procedure. After 6 months, the patients could undergo a stage II procedure, including elimination of PA banding or B-T shunt and the Kawashima procedure or ASO [9, 10]. Brown and his coworkers reported acceptable outcomes of staged surgery in 20 years of clinical experience, achieving survival rates of 92% at 1 year, 81% at 5 years, and 76% at 10 and 15 years [11]. However, staged surgery has many long-term complications and a high reoperation rate because PA banding can cause pulmonary regurgitation and subaortic stenosis and also has a significant influence on cardiac function. Additionally, interventricular tunnel stenosis and left ventricular outflow tract obstruction can occur after the Kawashima procedure.
With increased experience and improved results in neonatal ASO, one-stage correction is currently advocated for almost all types of TBA [12, 13]. Some scholars have postulated that aortic arch obstruction is not a risk factor for patients with TBA undergoing ASO [14]. Thus in the past decades, single-stage correction has become the preferred choice for treatment of TBA with aortic arch obstruction in many major centers. Shanghai Children’s Medical Center has used ASO to treat TBA since 1999. In our experience, TBA with aortic arch obstruction should be corrected within 2 months of birth. After a definitive diagnosis had been made without any surgical contraindications, all 39 study subjects underwent single-stage correction. In our experience, the main advantages of single-stage correction are as follows: (1) the procedure is shorter and has a lower risk than staged surgery (2) unlike PA banding, it does not cause pulmonary hypertension or subaortic stenosis (3) median incisions provide wider access than lateral thoracic incisions for extending the aortic arch (4) there are fewer postoperative complications and a lower reoperation rate.
Outcomes and General Considerations Concerning the ASO Procedure
For decades, ASO has been used as the primary surgical procedure in infants with TBA and transposition of the great arteries. However, several studies have shown that ASO for TBA is associated with a higher morbidity and mortality than ASO for transposition of the great arteries [12, 15, 16]. In contrast to transposition of the great arteries, TBA is always associated with side-by-side arterial position and coronary artery malposition, which are the challenges for performing ASO [13]. To avoid compromising new coronary arteries, the Lecompte procedure is usually used to switch the positions of these arteries. However, this procedure destroys the normal form of the PA, especially for the side-by-side position. Some surgeons have proposed using the Jatene procedure without switching the artery positions for patients with a side-by-side anomaly [17]. While the forms of coronary arteries vary in patients with TBA, some left anterior descending and left circumflex arteries pass posterior to the PA. The PA is placed posteriorly in the Jatene procedure, which can compress the new left CA. To solve this problem, we have modified the Jatene method by extending the PA incision to the right PA, suturing part of the left incision, and anastomosing the right PA to the proximal part of the PA trunk. This modification retains the side-by-side position and places the PA on the right side, thus preserving the formation of the PA and avoiding coronary artery compression.
Coronary artery transplantation is the most difficult part of the ASO procedure. Traditionally, part of the myocardium is cut and the coronary arteries are dissociated extensively to reduce tension after transplantation. In patients with short coronary branches, the dissociated coronary arteries could experience tension or become distorted, or compressed, potentially causing myocardial ischemia, arrhythmia, or even death [18]. Because the “trap-door,” “bay window,” and “double-flaps” techniques have been used successively to improve coronary artery transplantation, the mortality of patients with coronary artery malformation has decreased significantly in the Shanghai Children’s Medical Center. According to several studies, the rate of success of ASO for TBA is 92%; mid- and long-term survival rates are both nearly 90% [18,19,20]. These results are consistent with the results of the present study, in which short- and mid-term survival rates were 92.3 and 89.7%, respectively.
The most common means of aortic arch repair are resection with end-to-side or end-to-end anastomosis, patch-graft aortoplasty, and subclavian flap aortoplasty. Dehaki and coworkers followed up 188 patients with COA for 10 years and reported that recoarctation occurred more frequently after patch-graft repair (12.7%) and end-to-end anastomosis (10.3%) than after the subclavian flap procedure (3.2%) [21]. However, because the left subclavian and vertebral arteries are ligated in the subclavian flap aortoplasty, long-term adverse effects include left arm sequelae and hypertension [22]. Several studies have shown no significant differences between these methods in recoarctation rate [22,23,24]. At Shanghai Children’s Medical Center, we perform modified end-to-side anastomosis when correcting aortic arch obstruction in patients with TBA [25]. Its advantages include the following: (1) the aortic anastomosis can be fully expanded without creating high tension (2) the patch extends the length of the aortic arch and maintains its geometric configuration as normal as possible to avoid postoperative hypertension (3) the anastomotic parts are not in the same plane, which could prevent recoarctation caused by anastomotic contracture (4) the incision flapper of the inferior edge of the aorta forms the anastomotic posterior wall and has some potential for growth.
Conclusions
In conclusion, single-stage correction of TBA with aortic arch obstruction in neonates requires less surgical time, has an acceptable survival rate and good mid-term outcomes. The optimal operative procedure should be chosen according to the position of the two great arteries and the coronary anomaly. Modified end-to-side anastomosis with patch augmentation is an effective procedure for neonates with aortic arch obstruction.
References
Walters HL III, Mavroudis C, Tchervenkov CI, Jacobs JP, Lacour-Gayet F, Jacobs ML (2000) Congenital heart surgery nomenclature and database project: double outlet right ventricle. Ann Thorac Surg 69(3):249–263
Taussig HB, Bing RJ (1949) Complete transposition of the aorta and a levoposition of the pulmonary artery: clinical, physiological, and pathological findings. Am Heart J 37(4):551–559
Wilkinson JL, Wilcox BR, Anderson RH (1983) The anatomy of double outlet right ventricle. Paediatr Cardiol 5:397–407
Alsoufi B, Cai S, Williams WG, Coles JG, Caldarone CA, Redington AM et al (2008) Improved results with single-stage total correction of Taussig–Bing anomaly. Eur J Cardiothorac Surg 33(2):244–250
Wang S, Xu Z, Liu J, Yan Q, Zhang H, Zhen J et al (2013) Coronary implantation using the autologous flap extension technique in complicated arterial switch operations. Pediatr Cardiol 34(4):795–801
Wedemeyer AL, Lucas RV, Castaneda AR (1970) Taussig–Bing malformation, coarctation of the aorta, and reversed patent ductus arteriosus operative correction in an infant. Circulation 42(6):1021–1027
Elgamal MA, McKenzie ED, Fraser CD Jr (2002) Aortic arch advancement: the optimal one-stage approach for surgical management of neonatal coarctation with arch hypoplasia. Ann Thorac Surg 73(4):1267–1273
Lim HG, Kim WH, Lee YT, Han JJ, Kim SC, Lim C et al (2003) Staged biventricular repair of Taussig–Bing anomaly with subaortic stenosis and coarctation of aorta. Ann Thorac Surg 76(4):1283–1286
Sadow SH, Synhorst DP, Pappas G (1985) Taussig–Bing anomaly and coarctation of the aorta in infancy: surgical options. Pediatr Cardiol 6(2):83–89
Brown JW, Ruzmetov M, Okada Y, Vijay P, Rodefeld MD, Turrentine MW (2006) Outcomes in patients with interrupted aortic arch and associated anomalies: a 20-year experience. Eur J Cardiothorac Surg 29(5):666–673
Wetter J, Sinzobahamvya N, Blaschczok HC, Cho MY, Brecher AM, Gravinghoff LM et al (2004) Results of arterial switch operation for primary total correction of the Taussig–Bing anomaly. Ann Thorac Surg 77(1):41–46
Griselli M, McGuirk SP, Ko CS, Clarke AJ, Barron DJ, Brawn WJ (2007) Arterial switch operation in patients with Taussig–Bing anomaly—influence of staged repair and coronary anatomy on outcome. Eur J Cardiothorac Surg 31(2):229–235
Comas JV, Mignosa C, Cochrane AD, Wilkinson JL, Karl TR (1996) Taussig–Bing anomaly and arterial switch: aortic arch obstruction does not influence outcome. Eur J Cardio-thoracic Surg 10:1114–1119
Wernovsky G, Mayer JE Jr, Jonas RA, Hanley FL, Blackstone EH, Kirklin JW et al (1995) Factors influencing early and late outcome of the arterial switch operation for transposition of the great arteries. J Thorac Cardiovasc Surg 109(2):289–302
Williams WG, Quaegebeur JM, Kirklin JW, Blackstone EH (1997) Outflow obstruction after the arterial switch operation: a multiinstitutional study. J Thorac Cardiovasc Surg 114(6):975–990
Losay J, Touchot A, Serraf A, Litvinova A, Lambert V, Piot JD et al (2001) Late outcome after arterial switch operation for transposition of the great arteries. Circulation 104(suppl 1):121–126
Masuda M, Kado H, Shiokawa Y, Fukae K, Kaneqae Y, Kawachi Y et al (1999) Clinical results of arterial switch operation for double-outlet right ventricle with subpulmonary VSD. Eur J Cardiothorac Surg 15(3):283–288
Zheng JH, Xu ZW, Liu JF, Su ZK, Ding WX (2008) Arterial switch operation with coronary arteries from a single sinus in infants. J Card Surg 23(6):606–610
Al-Muhaya MA, Ismail SR, Abu-Sulaiman RM, Kabbani MS (2012) NAJM H K. Short-and mid-term outcomes of total correction of Taussig–Bing anomaly. Pediatr Cardiol 33(2):258–263
Choi KH, Sung SC, Kim H, Lee HD, Ban GH, Kim G et al (2016) Transposition Complex with Aortic Arch Obstruction: outcomes of One-Stage Repair Over 10 Years. Pediatr Cardiol 37(1):160–166
Schwarz F, Blaschczok HC, Sinzobahamvya N, Sata S, Kom F, Weber A et al (2013) The Taussig–Bing anomaly: long-term results. Eur J Cardiothorac Surg 44:821–827
Dehaki MG, Ghavidel AA, Givtaj N, Omrani G, Salehi S (2010) Recurrence rate of different techniques for repair of coarctation of aorta: a 10 years experience. Ann Pediatr Cardiol 3(2):123–126
Walhout RJ, Lekkerkerker JC, Oron GH, Hitchcock FJ, Meijboom EJ, Bennink GB (2003) Comparison of polytetrafluoroethylene patch aortoplasty and end-to-end anastomosis for coarctation of the aorta. J Thorac Cardiovasc Surg 126(2):521–528
Adams EE, Davidson WR, Swallow NA, Nickolaus MJ, Myers JL, Clark JB (2013) Long-term results of the subclavian flap repair for coarctation of the aorta in infants. World J Pediatr Congenit Heart Surg 4(1):13–18
Shi G, Chen H, Jinghao Z, Zhang H, Zhu Z, Liu J (2014) Primary complete repair of interrupted aortic arch with associated lesions in infants. J Card Surg 29(5):686–691
Funding
The study was supported by grants 2012BAI04B05 (the National Science & Technology Pillar Program of China).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None declared.
Research Involving Human and Animal Participants
This article does not contain any studies with human participants performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Luo, K., Zheng, J., Wang, S. et al. Single-Stage Correction for Taussig–Bing Anomaly Associated With Aortic Arch Obstruction. Pediatr Cardiol 38, 1548–1555 (2017). https://doi.org/10.1007/s00246-017-1694-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-017-1694-6