Abstract
This study aimed to determine whether poor glycemic control in early pregnancy is associated with an increased risk of congenital heart disease (CHD) for infants of women with preexisting diabetes. A retrospective review examined two tertiary care centers of diabetic pregnancies that recorded early hemoglobin A1c (HbA1c) values (<20 weeks). The incidence of prenatally diagnosed CHD was calculated and stratified by HbA1c level. Poor glycemic control was defined as an HbA1c level of 8.5 % or higher. Fetal echocardiography was used to identify fetuses that resulted in infants with suspected CHD. Neonatal echocardiograms and pathology reports were reviewed for confirmation of the diagnosis. Of 535 patients, 30 (5.6 %) delivered an infant with confirmed CHD. Among the patients with poor glycemic control, 8.3 % (n = 17) delivered an infant with CHD, whereas 3.9 % (n = 13) of those with an HbA1c level lower than 8.5 % delivered an infant with CHD (p = 0.03). Poor glycemic control in early pregnancy is associated with an increased risk of CHD in offspring. The incidence of CHD in patients with adequate glycemic control still is sufficiently high to justify routine fetal echocardiography for all gravidas with preexisting diabetes regardless of HbA1c level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Congenital heart disease (CHD), one of the most common congenital malformations, complicates 5–8 of every 1,000 live births [5, 10, 17]. Poorly controlled diabetes early in pregnancy has been associated with an increased risk of fetal malformations, and elevated blood glucose levels during organogenesis appear to be teratogenic [4, 15].
Several authors have suggested that euglycemia in early pregnancy reduces the risk of congenital anomalies to near background levels [6, 11, 20], but some studies do not show that the risk for congenital malformations is related to diabetic control [16]. In pregnancies complicated by preexisting diabetes, the risk for CHD increases dramatically, with reported rates of 21–46 per 1,000 births [8, 12, 14, 19]. In addition, recent studies suggest that elevated hemoglobin A1c (HbA1c) is associated with a reduction in fetal long-axis cardiac function [7].
Although preconception glycemic control is widely recommended by physicians, few individuals achieve optimal control before conception or during the first 2 months of pregnancy. Hemoglobin A1c levels in the first half of pregnancy can be used as a marker for average blood glucose levels during the period of organogenesis. Some previous studies have suggested a critical HbA1c level of 8.5 %, above which rates of CHD increase dramatically [15], whereas other studies [8, 19] have failed to find such a critical HbA1c value.
Some authors advocate that fetal echocardiography should be offered to every diabetic woman regardless of glycemic control early in pregnancy [14]. Identifying the risk of CHD at various HbA1c levels would allow providers to counsel patients appropriately on the risk of CHD and other anomalies and could lead to improved allocation of resources by targeting appropriate pregnancies for expensive and time-intensive evaluations, such as fetal echocardiography. For patients with sufficiently low HbA1c levels, such interventions might be safely avoided.
This study aimed to assess whether offspring of diabetic gravidas with recorded HbA1c values of 8.5 % or more had a higher incidence of CHD than gravidas with HbA1c values lower than 8.5 %.
Materials and Methods
This retrospective study was conducted after institutional review board approval at both institutions. The medical records of women registered with the Diabetes in Pregnancy Program at Women and Infants Hospital of Rhode Island between June 2003 and June 2012 were identified, as well as the records of women who had been registered in the “A Sweeter Choice” program, a comprehensive diabetes in pregnancy program at the University of Hawaii, from January 2007 until March 2011. During this period, maternal diabetic control was routinely assessed via HbA1c levels at the first prenatal visit. Individuals with a pre-pregnancy diagnosis of diabetes and singleton gestation were included. If a patient had more than one pregnancy during the period studied, we examined only the most recent pregnancy for which information was available.
Demographic characteristics, ultrasound findings, and neonatal records were reviewed. Patients with preexisting diabetes were routinely referred for a level 2 (L2) ultrasound at 18–20 weeks of gestation. A fetal echocardiography typically was performed at the same time as the L2 ultrasound or several weeks afterward. Fetal echocardiography results were available for 491 of the 535 patients.
Maternal Characteristics
The maternal age at the Women and Infants Hospital was calculated at the time of the estimated date of confinement (EDC) by using date of birth (DOB) and EDC (http://www-users.med.cornell.edu/~spon/picu/calc/agecalc.htm). At the University of Hawaii, maternal age at the time of the first consultation appointment was recorded. Therefore, the average maternal age is reported separately for the two institutions. Maternal weight was recorded during the first prenatal appointment, and the EDC was based on the ultrasound and the first day of the patient’s last menstrual period (LMP), according to the American Congress of Obstetricians and Gynecologists (ACOG) recommendations [1].
If the EDC by the LMP and the first ultrasound examination were not consistent, then the ultrasound-obtained EDC was used. Gestational age at the time of the HbA1c measurement was calculated using the probable LMP (EDC minus 280 days). The difference between the date when the HbA1c was recorded and the probable LMP was divided by seven and reported as gestational age at the time of HbA1c measurement. For convenience, this then was rounded down to a whole number (e.g., 8 weeks and 2 days were recorded simply as 8 weeks). Gestational age at the time of fetal echocardiography was calculated using a similar method.
We also assessed the patients’ use of medications associated with CHD, such as angiotensin-converting enzyme (ACE) inhibitors, antiepileptic drugs (AED), and lithium, at the time of conception. Any personal or family history of CHD also was recorded at the time of the chart review.
Pre- and Postnatal Diagnosis of CHD
During the L2 ultrasound, the fetal heart was routinely assessed by demonstrating the position of the fetal heart within the chest, heart axis, M-mode, four-chamber view, and the left and right outflow tracts. The fetal echocardiography consisted of all these in addition to a real-time evaluation of the four-chamber heart view, wall motion, ventricular outflow tracts, aortic arch, ductal arch, valvular position and function, left pulmonary venous return, inferior and superior vena cava, Doppler velocimetry, and color flow mapping.
All fetal echocardiographies were performed by maternal fetal specialists at sites certified by the American Institute of Ultrasound in Medicine. For the purpose of this study, intracardiac echogenic foci, pericardial effusion of 3 mm or less, ventricular wall hypertrophy, arrhythmia, and cardiac axis deviation without cardiac anomaly were not considered to be consistent with CHD.
Congenital heart disease was subclassified into two categories: major (requiring referral for surgery immediately or early in childhood) or minor (not requiring any immediate surgical repair). Fetal echocardiography results were classified as normal, abnormal (major or minor CHD present), or limited (ventricular outflow tract and four-chamber views not obtainable by the provider after three separate attempts).
After delivery, we reviewed all neonatal charts and autopsy reports to confirm the diagnosis of CHD. Neonatal echocardiography was performed for infants with antenatal suspected cardiac anomalies or if clinically indicated, as determined by the pediatrician caring for the newborn. If the initial exam by the pediatrician did not show any abnormal cardiac findings and a neonatal echocardiography was not ordered, we assumed that the infant did not have CHD. For terminated pregnancies, the diagnosis of CHD was confirmed by reviewing the autopsy results. We excluded all infants with known major chromosomal abnormalities from our final analysis. We also excluded patients from our study if the initial HbA1c was drawn after 20 weeks.
Statistical Analysis
Sample size calculations were based on an a priori feasibility assessment that showed a 1:2 distribution between patients with an HbA1c level of 8.5 % or higher and those with a HbA1c level lower than 8.5 %. Using an alpha of 0.05 (two-sided), a power of 80 %, HbA1c level as an independent variable, and CHD as the outcome, we calculated a sample size using Chi square. Based on published data, we assumed that the incidence of CHD in patients with HbA1c levels lower than 8.5 % was ~1 % and that the incidence of CHD in patients with HbA1c levels of 8.5 % or higher approached 5 % [8, 15, 19]. Therefore, we calculated that a review of 579 charts would be needed to determine a statistically significant difference if one existed.
Statistical analysis was performed using SAS version 9.1 (SAS, Cary, NC, USA). Categorical variables were compared by Chi square or Fisher’s exact test. Continuous variables were compared using t tests for two groups and analysis of variance (ANOVA) for three or more groups for normally distributed data. Nonparametric data were evaluated using the Wilcoxon sum rank test for two groups and the Kruskal–Wallis test for three or more groups. Two-sided p values lower than 0.05 were considered statistically significant.
Results
The inclusion criteria were met by 535 patients. Table 1 summarizes the demographic and clinical characteristics of the patients included in the study. The mean gestational age was 10.2 ± 4.3 weeks for HbA1c measurements lower than 8.5 % and 8.8 ± 3.7 weeks for HbA1c values of 8.5 % or higher (p < 0.0001). Of the 535 patients, 164 had type 1 diabetes mellitus and 371 had type 2 diabetes mellitus. The distribution of patients with respect to HbA1c level is summarized in Fig. 1.
The study identified 30 confirmed cases of major and minor CHD. During the antenatal period, 15 fetuses had abnormal fetal echocardiographies, so they were suspected to have CHD. Neonatal echocardiography confirmed CHD in all 15 infants. In addition, neonatal echocardiography confirmed CHD in 14 infants who had either no fetal echocardiography or normal findings on fetal echocardiography. One newborn did not have neonatal echocardiography immediately after birth but was discharged after evaluation by a pediatric cardiologist, who documented a finding compatible with a small ventricular septal defect (VSD). The specific cardiac defects, fetal echocardiography results, maternal ages, and HbA1c levels are summarized in Table 2.
The sensitivity of fetal echocardiography for predicting major CHD in our study was 50 %, and the specificity of fetal echocardiography was 98.3 %. The positive predictive value of fetal echocardiography was 55.5 %, and the negative predictive value was 97.9 %. Table 2 indicates that normal fetal echocardiographies were associated with eight cases of postnatally diagnosed VSD, two bicuspid aortic valves, and one atrial septal defect (ASD). One infant with hemitruncus and one infant with tetralogy of Fallot had normal fetal echocardiographies. The prevalence of all CHDs and major CHDs in each of the two HbA1c categories are delineated in Table 3.
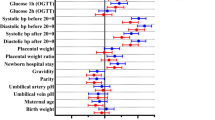
The overall incidence of CHD in our patient population was 5.6 %. The incidence of CHD in patients with an HbA1c level of 8.5 % or higher was 8.3 % compared with 3.9 % in patients with an HbA1c level lower than 8.5 % (p = 0.03). The crude odds ratio (OR) was 2.22 (95 % confidence interval [CI], 1.06–4.68) that patients with an HbA1c level of 8.5 % or higher would carry a fetus with CHD compared with those who had a lower HbA1c. When we adjusted for possible confounders such as age, body mass index (BMI), gestational age at the time the HbA1c was drawn, and diabetes type, the adjusted OR was 2.55 (95 % CI, 1.18–5.50), as shown in Table 4. The rate of major CHD for the patients with an HbA1c level of 8.5 % or more was almost three times higher than the rate for the patients with an HbA1c level lower than 8.5 % (6.4 vs 2.4 %; p = 0.02). As depicted in Table 4, the crude OR was 2.75 (95 % CI, 1.12–6.75) and the adjusted OR was 3.15 (95 % CI, 1.24–8.02).
When we analyzed rates of CHD, controlling for the use of teratogenic medications (lithium, ACE inhibitors, and antiepileptic drugs) during organogenesis and family history of CHD, the final results did not show a difference because these two variables were documented to be rare in our patient population. Two infants with CHD had a positive family history of CHD, and one infant with CHD had exposure to a potentially teratogenic medication. Because women with type 1 diabetes had statistically higher HbA1c values than women with type 2 diabetes, we performed an analysis comparing the risks for all CHDs and major CHDs by diabetes type. Our findings are summarized in Table 5. It appears that the risk for CHD is dependent on HbA1c value rather than diabetes type.
Discussion
Some studies have suggested a critical level of HbA1c above which the risk of fetal structural abnormalities (including CHD) is increased [15], but other studies have failed to find such a critical level [8, 12, 14, 19]. In our cohort of patients, the incidence of CHD was increased in those whose HbA1c level was 8.5 % or higher during the first half of the pregnancy. However, the 3.9 % incidence of CHD in those with an HbA1c lower than 8.5 % was approximately five times higher than the background rate of 0.5–0.8 % [5, 10, 17] and higher than the 1.9 % of live births reported by the Rhode Island Birth Defect Program between 2004 and 2008 [9]. For values of 8.5 % or higher, the rate of CHD (8.3 %) was more than 10 times higher than the background.
Our study confirms that the incidence of CHD rises as HbA1c levels increase. However, even at lower HbA1c levels, the incidence of CHD still is high, so referral of any patient with preexisting diabetes for fetal echocardiography would be appropriate regardless of HbA1c level. A similar conclusion can be drawn with regard to major CHD. The results of our study can be used to counsel patients preconceptionally and during early pregnancy on the risks of CHD in their offspring.
Our study had several limitations that should be taken into account when the results are interpreted. We assumed that neonates who underwent normal newborn cardiac examinations had no cardiac abnormalities, and a neonatal echocardiogram was not performed for every infant. Our study did not include internal controls to determine the incidence of CHD among nondiabetic pregnancies in our patient population. Our power analysis recommended that 579 charts be reviewed, but we included only 535 charts in our analysis after we had eliminated twins and patients whose HbA1c was collected after 20 weeks gestation. We also counted only the most recent pregnancy for each woman.
Nevertheless, after analysis of 535 charts, the results of our study still are significant. Our study was underpowered to determine whether individuals who meet strict criteria for optimal glycemic control, defined by an HbA1c level lower than 6.5 % (the value defined by ADA as the threshold for the diagnosis of preexisting diabetes in nonpregnant individuals and in the first trimester) [2], are at lower risk of having infants with CHD. By these criteria, 77.8 % of the patients in this study were not optimally controlled in the first half of pregnancy, similar to other studies [16, 19]. Notably, of 119 subjects with an HbA1c level lower than 6.5 %, 5 infants (4.2 %) had CHD, and this rate is similar to the 3.9 % risk of CHD in all cases with an HbA1c lower than 8.5 %.
It has been almost 20 years since the last study of CHD and HbA1c. The strength of our study is the large sample of 535 patients. The data were derived from diverse patient populations at one major tertiary care center on the East Coast and at the only tertiary care center for the state of Hawaii and other Pacific islands. In contrast, previous reports have involved fewer subjects: 175 subjects [19] and 286 subjects with 328 pregnancies [8].
Previous studies suggest that the four-chamber view and outflow tracts usually yield a CHD detection rate of 50–90 % [3, 13, 18, 21]. In our study, the CHD detection rate with fetal echocardiography was 50 %, but the majority of defects that fetal echocardiography failed to detect prenatally were VSDs, ASDs, and a bicuspid aortic valve. Small VSDs or ASDs can be challenging to diagnose prenatally. A bicuspid aortic valve can also be difficult to visualize with fetal echocardiography and is not part of the routine fetal echocardiography.
Patients with poor glycemic control are at increased risk for fetal defects including CHD. This evidence supports the goal of optimal glycemic control before conception and in early pregnancy during organogenesis. Our study also suggests that preexisting diabetes by itself may be an independent risk factor for CHD, but in the patients with the worst control (HbA1c > 8.5 %), the risk of CHD increases dramatically. Further studies are needed to determine whether optimally controlled patients with an HbA1c level lower than 6.5 % have a greater risk for CHD than the baseline population. Currently, based on our results, we recommend a screening fetal echocardiogram for all pregnancies complicated by pregestational diabetes regardless of glycemic control.
References
American College of Obstetricians and Gynecologists (2009) Ultrasonography in pregnancy. ACOG practice Bulletin no. 101. Obstet Gynecol 113:451–461
American Diabetes Association (2011) Standards of medical care in diabetes 2011. Diabetes Care 34:S11–S61
Carvalho JS, Mavrides E, Shinebourne EA, Campbell S, Thilaganothan B (2002) Improving the effectiveness of routine prenatal screening for major congenital heart defects. Heart 88:387–391
Chung CS, Myrianthopolos NC (1975) Effect of maternal diabetes on congenital malformations. Birth Defects 11:23–58
Ferencz C, Rubin JD, McCarter RJ, Brenner JI, Ca Neil, Perry LW, Hepner SE, Downing JW (1985) Congenital heart disease: prevalence at livebirth. The Baltimore-Washington Infant Study. Am J Epidemiol 121:31–36
Fuhrman K, Reiher H, Semmler K, Fisher F, Fisher M, Glockner E (1983) Prevention of congenital malformations in infants of diabetic mothers. Diabetes Care 6:219–223
Gardiner HM, Pasquini L, Wolferson J, Kulinskaya E, Li W, Henein M (2006) Increased periconceptual maternal glycated haemoglobin in diabetic mothers reduces fetal long axis cardiac function. Heart 92:1125–1130
Gladman G, McCridle BW, Boutin C, Smallhorn JF (1997) Fetal echocardiographic screening of diabetic pregnancies for congenital heart disease. Am J Perinatol 14:59–62
http://www.health.ri.gov/publications/databooks/2010BirthDefects.pdf. Accessed 27 December 2012
Jenkins T, Wapner R (2004) Prenatal diagnosis of congenital disorders. In: Creasy R, Resnik R (eds) Maternal-fetal medicine: principles and practice. WB Saunders Co., Philadelphia, pp 235–280
Kitzmiller JL, Gavin LA, Gin GD, Jovanovic-Peterson L, Main EK, Zigrang WD (1991) Preconception care of diabetes: glycemic control prevents congenital anomalies. JAMA 265:731–736
Lisowski LA, Verheijen PM, Copel JA, Kleiman CS, Wassink S, Visser GHA, Meijboom EJ (2010) Congenital heart disease in pregnancies complicated by maternal diabetes mellitus. An international clinical collaboration, literature review, and meta-analysis. Herz 35:19–26
Marek J, Tomek V, Skovranek J, Povysilava V, Samanek M (2011) Prenatal ultrasound screening of congenital heart disease in an unselected national population: a 21-year experience. Heart 97:124–130
Meyer-Wittkopt M, Simpson M, Sharland GK (1996) Incidence of congenital heart defects in fetuses of diabetic mothers: a retrospective study of 326 cases. Utrasound Obstet Gynocol 8:8–10
Miller E, Hare JW, Cloherty JP, Dunn PJ, Gleason RE, Soeldner JS, Kitzmiller JL (1981) Elevated maternal hemoglobin A1c in early pregnancy and major congenital abnormalities in infants of diabetic mothers. N Engl J Med 304:1331–1334
Mills JL, Knopp Rh, Simpson JL, Jovanovic-Peterson L, Metzger BE, Holmes LB, Aarons JH, Brown Z, Reed GF, Bieber FR, Allen M, Holzman I, Ober C, Peterson CM, Withiam MJ, Duckles A, Mueller-Heuback E, Polk BF (1988) Lack of relation of increased malformation rates in infants of diabetic mothers to glycemic control during organogenesis. N Engl J Med 318:671–676
Moller J (1990) Incidence of cardial malformations. In: Moller J, Neal W (eds) Fetal, neonatal, and infant cardiac disease. Appleton and Lange, Norwalk, pp 361–369
Ogge G, Galioti P, Maccanti S, Faggiano F, Todros T (2006) Prenatal screening for congenital heart disease with four-chamber and outflow-tract views: a multicenter study. Ultrasound Obstet Gynecol 28:779–784
Shields LE, Gan EA, Murphy HF, Sahn DJ, Moore TR (1993) The prognostic value of hemoglobin A1c in predicting fetal heart disease in diabetic pregnancies. Obstet Gynecol 81:954–957
Steel JM, Johnstone FD, Hepburn DA, Smith AF (1990) Can pregnancy care of diabetic women reduce the risk of abnormal babies? Br Med J 301:1070–1074
Tegnander E, Williams W, Johansen OJ, Blaas HG, Eik-Nes SH (2006) Prenatal detection of heart defects in a nonselected population if 30,149 fetuses: detection rates and outcome. Ultrasound Obstet Gynecol 27:252–265
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Starikov, R., Bohrer, J., Goh, W. et al. Hemoglobin A1c in Pregestational Diabetic Gravidas and the Risk of Congenital Heart Disease in the Fetus. Pediatr Cardiol 34, 1716–1722 (2013). https://doi.org/10.1007/s00246-013-0704-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-013-0704-6