Abstract
Although a previous metaanalysis indicated that maternal smoking during pregnancy increased the risk of congenital heart defects (CHD) in offspring, the effect of smoking on individual CHD subtypes was not determined. Because CHDs are anatomically, clinically, epidemiologically, and developmentally heterogeneous, the authors conducted a systematic review and metaanalysis of the association between maternal smoking during pregnancy and the risk of CHDs, including CHD subtypes among offspring. Two types of summary relative risk (RR) estimates (any smoking vs no smoking and increasing categories of smoking, i.e., light, medium, and heavy) were calculated for CHDs as a group and for a number of CHD subtypes using both fixed- and random-effects models. Random effects estimates were reported if there was evidence of heterogeneity among the studies. Consistent with the previous metaanalysis, the authors observed a positive association between maternal smoking during pregnancy and the risk of CHDs as a group (RR, 1.11; 95 % confidence interval [CI], 1.02–1.21; number of cases [n] = 18,282). Additionally, women who smoked during pregnancy were more likely to have a child with 12 (71 %) of 17 CHD subtypes analyzed compared with women who did not smoke. The highest risk was for septal defects as a group (RR, 1.44; 95 % CI, 1.16–1.79; n = 2977). The evidence of dose response was observed for septal defects as a group, atrial septal defects, and atrioventricular septal defects. This systematic review and metaanalysis suggests that maternal smoking is modestly associated with an increased risk of CHDs and some CHD subtypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Congenital heart defects (CHDs) are the most common group of congenital malformations in the United States, with a birth prevalence of ~1 in 100 [5, 6, 8]. Due to congenital malformations, CHDs also are the leading cause of infant mortality, and those children who survive often require lifelong medical treatments. They may experience physical, developmental, and cognitive problems as well as reduced survival rates into adulthood [15, 32].
Despite the prevalence and clinical importance of CHDs, the etiology for ~70 % of cases remains unknown [8, 20]. One maternal exposure, long a suspected risk factor for CHDs, is smoking. This is important because in the United States, ~22 % of women of reproductive age smoke, and an estimated 12 % of women continue to smoke during their pregnancies [42].
A study by Fedrick et al. [14] was one of the first to report the association between maternal smoking and CHDs. However, the evidence since then has been mixed, with some studies showing positive associations and others providing null results [2, 9, 27]. The inconsistency in findings across studies likely is due to variability in case ascertainment and exposure assessment. Additionally, many studies had small numbers of cases and failed to control for potential confounders.
Finally, because CHDs include several distinct subtypes (e.g., conotruncal defects, left ventricular outflow track defects), there is a potential for etiologic heterogeneity, which may obscure findings when subtypes are “lumped” into a common phenotype to increase study power [8].
The equivocal evidence in the existing literature calls for a systematic assessment of currently available studies evaluating the association between maternal cigarette smoking during pregnancy and the risk of CHDs in offspring. A previous metaanalysis by Hackshaw et al. [17] estimated the effects of maternal smoking across a spectrum of birth defects including heart defects. However, the study did not evaluate the effects of maternal smoking on CHD subtypes, and dose–response relationships (i.e., increasing levels of smoking) were not assessed. Therefore, this study aimed to calculate summary relative risk (RR) estimates assessing the association between maternal cigarette smoking during pregnancy and CHDs overall as well as CHD subtypes, to examine dose–response relationships (i.e., light, medium, and heavy smoking), and to evaluate evidence of heterogeneity across studies.
Methods
Search Strategy
We searched the US National Library of Medicine Medline database for published articles in English from 1947 to July 2011 using Ovid and PubMed. Regular search terms and the Medical Subject Headings (MeSH) were used. The selected search terms included “tobacco,” “smoking,” “nicotine,” “periconceptional,” “maternal,” “mother,” “pregnant,” “gestation,” “birth defect,” “congenital,” “congenital heart defect,” “cardiovascular,” “heart,” “conotruncal,” “arteriosus,” “atrioventricular,” “pulmonary,” “ventricular,” and “septal.” The MeSH terms included: “tobacco,” “smoking,” “nicotine,” “tobacco smoke pollution,” “pregnancy complications,” “pregnancy,” “pregnancy outcome,” “mothers,” “congenital abnormalities,” “heart defect (congenital),” “cardiovascular abnormalities,” and “cardiovascular diseases.” Some terms not specific to the exposure and subtypes were included to identify studies that had examined several adverse birth outcomes or exposures including CHDs and smoking but may not have mentioned them in their titles or abstracts. Reference lists of articles were reviewed to identify additional articles.
Eligibility Criteria
We selected articles that (1) were original epidemiologic studies (i.e., case–control, cohort, or cross-sectional studies), (2) were published in the English language, (3) examined the association between maternal cigarette smoking anytime during pregnancy and CHDs overall or any one of the CHD subtypes in infants, (4) reported RRs (i.e., risk ratios or odds ratios) and associated 95 % confidence intervals (CIs) or had raw data available, (5) defined CHDs or one of the CHD subtypes as an outcome, and (6) provided exposure information.
Studies that examined only the effects of paternal or environmental tobacco smoke (i.e., secondhand smoke) and studies that assessed the association of interest in certain subgroups (e.g., mothers with CHDs, mothers with diabetes, or infants with Down syndrome) were not included in the review. In the case of multiple publications using the same data, we selected the study that contained the most comprehensive information (e.g., longest study periods or most CHD subtypes analyzed).
Data Extraction
One study author (L.J.L.) first screened studies by title and by abstract and made exclusions based on the eligibility criteria. The studies meeting the inclusion criteria were independently reviewed by two authors (L.J.L., P.J.L) to retrieve information of interest including study characteristics (i.e., authors, year of publication, geographic region, periods of data collection, study design, case classification, control definition, sample size, source of exposure data, smoking status, levels of smoking, exposure period during pregnancy, and adjusted/matched variables) and to record reported effect estimates and associated 95 % CIs as well as raw data if effect estimates were not available. Discrepancies between the authors were resolved by discussion.
When available, RR estimates and 95 % CIs were extracted from each study for CHDs overall and CHD subtypes. We selected the main confounder-adjusted RRs whenever possible. Otherwise, unadjusted effect estimates were extracted from each study. We conducted metaanalyses for specific CHD subtypes (i.e., subanalyses) if at least two studies had available data. In some cases, multiple publications using the same data source were used if those publications reported RRs for different CHD subtypes [1, 36, 38–41, 46]. Specifically, three studies, namely, Adams et al. [1] (conotruncal defects), Botto et al. [7] (any CHDs), and Williams et al. [46] (septal defects) identified cases from the Atlanta birth defects registry in overlapping periods, but they analyzed different subtypes of heart defects. Thus, their effect estimates were entered separately for our CHD subtype analyses.
Furthermore, six published studies used the Finnish birth defects registry for overlapping periods. For those studies, the report by Tikkanen and Heinonen [37] was included in the main analysis (i.e., CHDs overall), and the remaining five studies were included separately for subanalyses because the CHD subtypes assessed were different across those studies [36, 38–41]. For our analysis of atrioventricular septal defects (AVSDs), we extracted AVSD cases without Down syndrome from the Alverson et al. [2] and Malik et al. [27] studies.
Most studies presented risk estimates and raw data for maternal smoking using a dichotomous definition of exposure (i.e., yes vs no) [1, 4, 7, 14, 18, 19, 21–25, 28, 31, 33, 34, 41, 43, 47, 48]. For those studies in which smokers were only separated into more than two categories (i.e., light, medium, and heavy) [2, 9, 13, 16, 27, 29, 30, 36–40, 45, 46], we combined the categories to calculate a summary RR for dichotomous exposure [9, 27, 29, 30, 38–40, 45, 46]. However, we also evaluated additional categories of smoking in our analysis (i.e., light, medium, and heavy) when this information was available to evaluate the dose–response relationship of maternal smoking to CHDs as a group and to CHD subtypes.
Statistical Analysis
Based on the exposure definitions reported, we computed two types of summary effect estimates: any cigarette smoking and increasing categories of cigarette smoking (i.e., light, medium, and heavy). We used nonsmokers as the reference category in all analyses. We calculated summary RR estimates and 95 % CIs using both fixed- and random-effects models for the CHDs overall and for the CHD subtypes.
We first tested for heterogeneity across studies using Cochran’s Q-test [10]. If there was an evidence of heterogeneity (P < 0.1), we used a random-effects model, which provided a more appropriate summary effect estimate between heterogeneous study-specific estimates, applying the DerSimonian and Laird method [11]. If the Q-test showed no evidence of heterogeneity, we used a fixed-effects analysis, applying inverse variance weighting to calculate summary RR estimates.
All analyses pertaining to summary RR estimates were calculated using the Stata 12.0 (StataCorp, College Station, TX, USA) command “meta.” Because this command required values for standard errors (SEs) and none of the studies reported SEs, we calculated SEs using the following formula:
Forest plots were constructed to show study-specific RR estimates and a summary RR estimate, with a different size of box representing the relative weight of an individual study in calculating the summary RR estimate. Additionally, the presence of publication bias was evaluated by Egger’s test (P < 0.05) and by visual inspection of the symmetry in funnel plots (Stata “metabias” and “metafunnel” commands).
In the main analysis examining the association between maternal cigarette smoking and the risk of CHDs overall, we performed a sensitivity analysis by calculating a summary effect estimate for CHDs overall limited to the studies that examined the association between maternal periconceptional smoking (i.e., 3 months before pregnancy through the first trimester) and CHDs.
Results
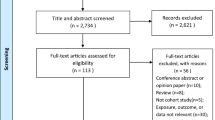
The study selection process identified 33 studies published between 1971 and 2011 for the metaanalysis (Fig. 1). The main study characteristics of included studies are shown in Table 1. As shown, 17 studies were conducted in the United States, 14 in Europe, and 2 in other regions (Canada and China). There were 23 case–control studies [1, 2, 4, 7, 16, 19, 22–25, 27, 30, 31, 34, 36–41, 43, 45, 46], 5 cohort studies [9, 21, 33, 47, 48], and 5 cross-sectional studies [13, 14, 18, 28, 29].
The studies derived their cases from various birth defects registries [1, 2, 7, 21, 27, 30, 31, 36–41, 45, 46], and the control subjects were randomly selected from birth certificates or hospital records or matched to cases by birth region or birth month [1, 4, 7, 25, 46]. For some studies (n = 11), cases were derived from a sample of live-born infants, whereas for other studies (n = 12), cases also were identified from stillbirths, neonatal deaths, and elective termination [7, 9, 14, 16, 19, 36–41, 46]. Although CHDs were diagnosed mainly within the first year after birth, diagnoses of CHDs were performed up to 7 years of age in the Chinese study [25] via echocardiography, cardiac catheterization, surgery, or autopsy. The specific inclusion and exclusion criteria used to identify and classify cases in each study are listed in the Online Resource.
Some studies (n = 6) collected information on maternal smoking and other variables using self-administered questionnaires [13, 14, 18, 33, 34, 48], whereas others (n = 23) collected maternal exposure information via telephone or in-person interviews [1, 2, 7, 16, 19, 21–25, 27, 29–31, 36–41, 45–47] after delivery (the time between delivery and the interview ranged up to 4 years [30]). In three studies, exposure information was collected from birth certificates [4, 28, 43], whereas one study used records from prenatal visits [9].
A wide range of exposure periods was examined, with 15 studies reporting the mother’s smoking status or level of smoking during the first trimester of pregnancy, including 1 to 3 months before conception [1, 2, 7, 16, 19, 22, 25, 27, 30, 31, 33, 36–41, 45, 46]. However, 14 studies did not specify the months of exposure during pregnancy.
Only one study examined the effects of maternal smoking during the late pregnancy period (months 4–10) [14]. Whereas 12 studies provided estimates on the association between maternal smoking and CHDs adjusted for a range of covariates [2, 19, 21–24, 27–30, 43, 47], 16 studies reported only unadjusted estimates (Table 1) [9, 13, 14, 16, 18, 31, 33, 34, 36–41, 45, 48].
Overall, 19 studies evaluated the association between maternal smoking during pregnancy and CHDs as a group in a total of 18,282 CHD cases (Table 2). There was evidence of heterogeneity across studies (P < 0.001) for CHDs overall. Thus, the random-effect estimate was reported. The summary RR estimate for CHDs overall was 1.11 (95 % CI, 1.02–1.21) among women who smoked during pregnancy compared with women who did not smoke during pregnancy (Table 2; Fig. 2).
We observed positive associations between maternal smoking during pregnancy and 12 (71 %) of 17 CHD subtypes analyzed. These positive associations ranged from 1.02 (fixed effects) for double-outlet right ventricle (95 % CI, 0.72–1.46; n cases = 179) to 1.44 (random effects) for septal defects as a group (95 % CI, 1.16–1.79; n cases = 2,977). There was no evidence of heterogeneity for most of the CHD subtypes evaluated (n = 13) (e.g., conotruncal defects, double-outlet right ventricle, hypoplastic left heart syndrome, and right ventricular outflow tract obstructions).
Based on the results of the Egger’s test (Table 2) and funnel plot (data not shown) for CHDs overall, there was no evidence of publication bias. Additionally, Egger’s test showed no evidence of publication bias for the CHD subtypes (Table 2). Funnel plots for the CHD subtypes also indicated that publication bias was not present (data not shown).
Summary RR estimates for CHDs overall and CHD subtypes were calculated by three different levels of smoking (light, medium, and heavy) (Table 3). The summary RR estimates for CHDs overall were 0.99 (95 % CI, 0.92–1.06; n = 10,126) for light smokers, 1.04 (95 % CI, 0.95–1.13; n = 10,126) for medium smokers, and 1.04 (95 % CI, 0.86–1.26; n = 3207) for heavy smokers compared with women who did not smoke during pregnancy.
Strong associations (RR, ≥1.99) were found for right ventricular outflow tract obstructions, pulmonary valve stenosis, and AVSD in infants prenatally exposed to heavy smoking compared with no smoking. The increase in summary RR estimates in response to increasing levels of maternal smoking were observed for only three CHD subtypes (septal defects overall, atrial septal defects [ASDs], and AVSDs). The dose response did not increase monotonically for other subtypes of CHDs.
Discussion
Overall, there was evidence that maternal smoking during pregnancy modestly increased the risk of CHDs in offspring. Although the studies included in our analysis varied in terms of case definition, control selection, and exposure assessment, the associations were largely consistent in the subanalyses and sensitivity analyses. The effect of maternal smoking was observed for CHDs overall and for several CHD subtypes. The strongest association was seen for septal defects overall. Specifically, women who smoked during pregnancy were 44 % more likely to have a child with a septal defect than women who did not smoke during pregnancy.
There was no evidence of a dose response between maternal smoking and CHDs overall in offspring. This also was the case for most of the CHD subtypes (12 of 15 subtypes, 80 %). This may have been attributable to the small sample sizes in the high-exposure groups or to differences in how increasing smoking status was defined across studies. We extracted exposure categories as they were reported in each study, and the studies assigned exposure level using cutoffs based on the number or packs of cigarettes smoked per day. The only evidence of a dose–response effect was for septal defects overall, ASDs, and AVSDs.
The mechanisms by which smoking may result in CHDs still remain unknown. Findings have shown that maternal smoking has adverse effects on the developing fetus, including hypoxia caused by carbon monoxide, nicotine, and reduction in the supply of essential nutrients to the embryonic tissues [2, 44]. Additionally, polycyclic aromatic hydrocarbons, common components of cigarette smoke, are suspected teratogens in laboratory animals and humans [3, 26].
In the previous metaanalysis of smoking and birth defects, including heart defects [17], the effect estimate for CHDs overall (RR, 1.09; 95 % CI, 1.00–2.18; 19 studies) was similar to ours, but our study selection criteria differed. For example, some studies [1, 4, 16, 27, 33] that they included in their analysis of CHDs overall were included in our subanalyses. Moreover, they did not evaluate CHD subtypes. Additionally, our metaanalysis included findings from the Baltimore-Washington Infant Study (BWIS), one of the a largest population-based case–control studies of CHDs, published in 2011 [2].
Our study must be considered in the light of certain limitations. For instance, our analysis was limited to studies published in English. However, we found no evidence of publication bias. Another limitation of our analysis was a lack of studies that examined effects of environmental tobacco smoke, which may have been an important component in the overall maternal smoking exposure during pregnancy. Furthermore, we derived most of our data from case–control studies, which may be more prone to information bias than cohort studies. However, the estimated effect of smoking was similar across different study designs (data not shown).
Our study had several strengths, including the large sample (n = 18,282) used to estimate the effect of maternal smoking on CHDs as a group. Additionally, due to the suspected heterogeneous etiologies, CHD subtypes were analyzed separately, and we were able to estimate a range of risks for CHD subtypes (RR, 1.02–1.44). We also evaluated increasing levels of smoking during pregnancy, and the evidence of dose response was observed for some CHD subtypes (i.e., septal defects overall, ASDs, and AVSDs).
Furthermore, we conducted a sensitivity analysis, restricting our analysis to studies with available information on exposure during the periconceptional period. Because heart anomalies develop during weeks 2–7 of gestation [35], we suspected that inclusion of studies that assessed exposure beyond the “critical period” may have biased our result toward the null. However, our sensitivity analysis showed no significant difference in the summary effect estimates (data not shown).
In conclusion, we found that mothers of offspring with CHDs were 11 % more likely to smoke during pregnancy than mothers of unaffected children. Although the effects were modest, smoking is a relatively common exposure among women of reproductive age and could have important public health consequences. Young women continue to smoke although the adverse effects of smoking on reproductive health are known, and more than a half of women smokers continue to smoke even after they learn that they are pregnant [12].
The demonstration of an association between smoking during pregnancy and CHDs can be used in the development of population-based prevention strategies to reduce the burden of CHDs and other birth defects. A decrease in maternal smoking during pregnancy would result in improved reproductive outcomes and may contribute to a reduction in infant mortality and morbidity.
References
Adams MM, Mulinare J, Dooley K (1989) Risk factors for conotruncal cardiac defects in Atlanta. J Am Coll Cardiol 14:432–442
Alverson CJ, Strickland MJ, Gilboa SM, Correa A (2011) Maternal smoking and congenital heart defects in the Baltimore-Washington Infant Study. Pediatrics 127:e647–e653
Barbieri O, Ognio E, Rossi O, Astigiano S, Rossi L (1986) Embryotoxicity of benzo(a)pyrene and some of its synthetic derivatives in Swiss mice. Cancer Res 46:94–98
Batra M, Heike CL, Phillips RC, Weiss NS (2007) Geographic and occupational risk factors for ventricular septal defects: Washington State, 1987–2003. Arch Pediatr Adolesc Med 161:89–95
Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD (2001) Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation 103:2376–2381
Botto LD, Correa A, Erickson JD (2001) Racial and temporal variations in the prevalence of heart defects. Pediatrics 107:e32
Botto LD, Lynberg MC, Erickson JD (2001) Congenital heart defects, maternal febrile illness, and multivitamin use: a population-based study. Epidemiology 12:485–490
Botto LD, Lin AE, Riehle-Colarusso T, Malik S, Correa A (2007) Seeking causes: classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res A Clin Mol Teratol 79:714–727
Cedergren MI, Kallen BA (2006) Obstetric outcome of 6,346 pregnancies with infants affected by congenital heart defects. Eur J Obstet Gynecol Reprod Biol 125:211–216
Cochran WG (1954) The combination of estimates from different experiments. Biometrics 10:101–129
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Ebrahim SH, Floyd RL, Merritt RK, Decoufle P, Holtzman D (2000) Trends in pregnancy-related smoking rates in the United States, 1987–1996. JAMA 283:361–366
Evans DR, Newcombe RG, Campbell H (1979) Maternal smoking habits and congenital malformations: a population study. BMJ 2:171–173
Fedrick J, Alberman ED, Goldstein H (1971) Possible teratogenic effect of cigarette smoking. Nature 231:529–530
Gilboa SM, Salemi JL, Nembhard WN, Fixler DE, Correa A (2010) Mortality resulting from congenital heart disease among children and adults in the United States, 1999 to 2006. Circulation 122:2254–2263
Grewal J, Carmichael SL, Ma C, Lammer EJ, Shaw GM (2008) Maternal periconceptional smoking and alcohol consumption and risk for select congenital anomalies. Birth Defects Res A Clin Mol Teratol 82:519–526
Hackshaw A, Rodeck C, Boniface S (2011) Maternal smoking in pregnancy and birth defects: a systematic review based on 173,687 malformed cases and 11.7 million controls. Hum Reprod Update 17:589–604
Himmelberger DU, Brown BW Jr, Cohen EN (1978) Cigarette smoking during pregnancy and the occurrence of spontaneous abortion and congenital abnormality. Am J Epidemiol 108:470–479
Hobbs CA, James SJ, Jernigan S, Melnyk S, Lu Y, Malik S et al (2006) Congenital heart defects, maternal homocysteine, smoking, and the 677 C>T polymorphism in the methylenetetrahydrofolate reductase gene: evaluating gene–environment interactions. Am J Obstet Gynecol 194:218–224
Jenkins KJ, Correa A, Feinstein JA, Botto L, Britt AE, Daniels SR et al (2007) Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation 115:2995–3014
Kallen K (1999) Maternal smoking and congenital heart defects. Eur J Epidemiol 15:731–737
Karatza AA, Giannakopoulos I, Dassios TG, Belavgenis G, Mantagos SP, Varvarigou AA (2011) Periconceptional tobacco smoking and isolated congenital heart defects in the neonatal period. Int J Cardiol 148:295–299
Kuciene R, Dulskiene V (2009) Maternal socioeconomic and lifestyle factors during pregnancy and the risk of congenital heart defects. Medicina Kaunas 45:904–909
Kuciene R, Dulskiene V (2010) Parental cigarette smoking and the risk of congenital heart septal defects. Medicina Kaunas 46:635–641
Liu S, Liu J, Tang J, Ji J, Chen J, Liu C (2009) Environmental risk factors for congenital heart disease in the Shandong Peninsula, China: a hospital-based case–control study. J Epidemiol 19:122–130
Lupo PJ, Langlois PH, Reefhuis J, Lawson CC, Symanski E, Desrosiers TA et al (2012) Maternal occupational exposure to polycyclic aromatic hydrocarbons and gastroschisis among offspring in the National Birth Defects Prevention Study. Environ Health Perspect 120:910–915
Malik S, Cleves MA, Honein MA, Romitti PA, Botto LD, Yang S et al (2008) Maternal smoking and congenital heart defects. Pediatrics 121:e810–e816
Malloy MH, Kleinman JC, Bakewell JM, Schramm WF, Land GH (1989) Maternal smoking during pregnancy: no association with congenital malformations in Missouri 1980–1983. Am J Public Health 79:1243–1246
McDonald AD, Armstrong BG, Sloan M (1992) Cigarette, alcohol, and coffee consumption and congenital defects. Am J Public Health 82:91–93
Shaw GM, Malcoe LH, Swan SH, Cummins SK, Schulman J (1992) Congenital cardiac anomalies relative to selected maternal exposures and conditions during early pregnancy. Eur J Epidemiol 8:757–760
Shaw GM, Iovannisci DM, Yang W, Finnell RH, Carmichael SL, Cheng S et al (2005) Risks of human conotruncal heart defects associated with 32 single nucleotide polymorphisms of selected cardiovascular disease-related genes. Am J Med Genet A 138A:21–26
Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G (2008) Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics 121:e759–e767
Shiono PH, Klebanoff MA, Berendes HW (1986) Congenital malformations and maternal smoking during pregnancy. Teratology 34:65–71
Smedts HP, de Vries JH, Rakhshandehroo M, Wildhagen MF, Verkleij-Hagoort AC, Steegers EA et al (2009) High maternal vitamin E intake by diet or supplements is associated with congenital heart defects in the offspring. BJOG 116:416–423
Srivastava D (2001) Genetic assembly of the heart: implications for congenital heart disease. Annu Rev Physiol 63:451
Tikkanen J, Heinonen OP (1991) Risk factors for ventricular septal defect in Finland. Public Health 105:99–112
Tikkanen J, Heinonen OP (1991) Maternal exposure to chemical and physical factors during pregnancy and cardiovascular malformations in the offspring. Teratology 43:591–600
Tikkanen J, Heinonen OP (1992) Risk factors for atrial septal defect. Eur J Epidemiol 8:509–515
Tikkanen J, Heinonen OP (1992) Risk factors for conal malformations of the heart. Eur J Epidemiol 8:48–57
Tikkanen J, Heinonen OP (1993) Risk factors for coarctation of the aorta. Teratology 47:565–572
Tikkanen J, Heinonen OP (1994) Risk factors for hypoplastic left heart syndrome. Teratology 50:112–117
Tong VT, Jones JR, Dietz PM, D’Angelo D, Bombard JM (2009) Trends in smoking before, during, and after pregnancy: pregnancy risk assessment monitoring system (PRAMS), United States, 31 Sites, 2000–2005. MMWR Surveill Summ 58:1–29
Van den Eeden SK, Karagas MR, Daling JR, Vaughan TL (1990) A case–control study of maternal smoking and congenital malformations. Paediatr Perinat Epidemiol 4:147–155
van Rooij IA, Wegerif MJ, Roelofs HM, Peters WH, Kuijpers-Jagtman AM, Zielhuis GA et al (2001) Smoking, genetic polymorphisms in biotransformation enzymes, and nonsyndromic oral clefting: a gene–environment interaction. Epidemiology 12:502–507
Wasserman CR, Shaw GM, O’Malley CD, Tolarova MM, Lammer EJ (1996) Parental cigarette smoking and risk for congenital anomalies of the heart, neural tube, or limb. Teratology 53:261–267
Williams LJ, Correa A, Rasmussen S (2004) Maternal lifestyle factors and risk for ventricular septal defects. Birth Defects Res A Clin Mol Teratol 70:59–64
Woods SE, Raju U (2001) Maternal smoking and the risk of congenital birth defects: a cohort study. J Am Board Fam Pract 14:330–334
Yerushalmy J (1973) Congenital heart disease and maternal smoking habits. Nature 242:262–263
Acknowledgments
The authors thank A. J. Agopian, Laura Mitchell, and Darryl Nousome for their assistance in the preparation of this article. This project was supported by the American Heart Association (P. J. Lupo, #10BGIA3060022). It also was supported, in part, by the NIOSH Southwest Center for Occupational & Environmental Health Training Grant #T42OH008421.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, L.J., Lupo, P.J. Maternal Smoking During Pregnancy and the Risk of Congenital Heart Defects in Offspring: A Systematic Review and Metaanalysis. Pediatr Cardiol 34, 398–407 (2013). https://doi.org/10.1007/s00246-012-0470-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-012-0470-x