Abstract
Patients who have had the Fontan procedure report poor exercise performance. Fontan subjects can tolerate a higher level of sub maximal activity than might be anticipated from Vo 2, suggesting a different mechanism of exercise limitation. Near-infrared spectroscopy (NIRS) provides a non-invasive, continuous method to monitor regional tissue oxygenation (rSO2) and thereby a window into regional oxygen supply–demand relationships. We hypothesized that Fontan patients would have altered rSO2 trends from normal population that might reflect the mechanisms of exercise limitation. All the patients without structural or acquired heart disease and Fontan patients were eligible for inclusion if they were ordered to undergo cardiopulmonary exercise testing (CPET). Four-site regional rSO2 were recorded continuously during exercise. The difference between the oxyhemoglobin saturation measured by pulse oximetry (Spo 2) and NIRS (rSO2) was computed as the regional arterial–venous saturation difference (AVDO2). A total of 33 normal subjects and five Fontan subjects scheduled for CPET were recruited. None of the Fontan subjects had a fenestration of the conduit. In the cerebral circulation, the Fontan patients have a significantly higher initial slope of increasing AVDO2 compared with normals. After vAT, the AVDO2 slope is flat for Fontan patients (p = 0.02). There is also a substantially larger rebound of cerebral rSO2 than in normal subjects after QT (p < 0.0001). Reduced anaerobic exercise capacity in Fontan patients may be secondary to limitation of cerebral blood flow, secondary to low systemic venous compliance due to absence of a sub-pulmonary ventricle, and augmented hyperventilatory response during exercise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients who have undergone the Fontan procedure report poor exercise performance with measured reduction in peak oxygen consumption (Vo 2) and exercise intensity [4–6, 10, 14, 17, 26, 28, 33]. Fontan subjects can tolerate a higher level of submaximal activity than might be anticipated from measurement of Vo 2. Chronotropic incompetence and arterial desaturation are minimally responsible for the variance in aerobic performance and physical working capacity [21]. Exercise capacity is not altered by closure of fenestration [15]. However, Fontan subjects can tolerate a higher level of submaximal activity than might be anticipated from Vo 2, suggesting a different mechanism of exercise limitation than whole-body oxygen transport.
Near-infrared spectroscopy (NIRS) provides a noninvasive, continuous method to monitor regional tissue oxygenation (rSO2) [13, 31]. Clinically useful NIRS techniques rely on adaptations of the Beer-Lambert law for measurement of the concentration of a substance according to its absorption of light while accounting for scatter in biologic tissue [20]. NIRS devices measure the venous weighted oxyhemoglobin saturation in a field of tissue, rather than in arteries, and thus the rSO2 parameter provides a window into regional oxygen supply–demand relations. The use of multisite NIRS monitoring during cardiopulmonary exercise testing (CPET) for the purpose of studying global cardiac output distribution trends through the patterning of visceral, muscular, and cerebral rSO2 data has been reported in normal populations [23].We hypothesized that Fontan patients would have altered rSO2 trends compared with a normal population that might reflect the mechanisms of exercise limitation. Our aim was to study the regional blood flow distribution patterns in Fontan patients with incremental exercise.
Methods
This study was initiated with funding assistance from the Children’s Research Institute, a division of Children’s Hospital and Health System, and the Medical College of Wisconsin, Milwaukee, WI, and had Institutional Review Board approval. All patients without structural or acquired heart disease and previous Fontan procedure were eligible for inclusion if they were ordered to undergo CPET by a cardiologist at the Herma Heart Center’s Exercise Physiology Laboratory for evaluation of non-life-threatening symptoms. Patients without structural heart disease who were referred for CPET for a variety of indications and who had normal stress test result formed the control group. The patients underwent a routine physical examination before initiation of exercise assessment. Consent from parent and assent from subject was obtained before enrollment.
The CPET protocol began with application of 12-lead electrocardiogram leads, an automated oscillometric blood pressure cuff on the left arm, and a pulse oximeter on the right index finger (Spo 2; GE-Marquette, Waukesha, WI). Four NIRS probes with 4-cm source-detector spacing and shallow-field rejection (Adult Somasensor, INVOS 5100C; Somanetics Corp, Troy, MI) were placed on the midline forehead, below the 12th rib in the left para vertebral space, on the vastus lateralis, and on the deltoid muscle (rSO2 C, rSO2 R, rSO2 L, and rSO2 A), respectively. Four site regional rSO2 and Spo 2 were measured and recorded continuously at six-second intervals at rest, during exercise, and throughout a 5-min recovery period. Baseline spirometry using a forced expiratory maneuver was performed following the standards of the American Thoracic Society. Patients were introduced to the treadmill and given specific instructions as to what to expect during the exercise portion of the study. Immediately before the initiation of exercise, a snorkel-style mouthpiece was placed in the child’s mouth for the measurement of breath-by-breath oxygen consumption (Vo 2), carbon dioxide production (VCO2), and instantaneous respiratory quotient (RQ). After 1 min of baseline data collection, a ramping treadmill protocol was initiated. At set intervals throughout the test, the workload was progressively increased. The progression was terminated when the child reached voluntary or symptom-limited exhaustion (quitting time [QT]). Throughout the exercise portion of the test, 12-lead electrocardiograph machine, blood pressure, and O2 saturation, in addition to breath-by-breath ventilatory measures, were recorded on a minute-by-minute basis. Immediately after exercise, the child was allowed to cool down for 3 min by walking slowly, with the remainder of the 5-min recovery being performed in a seated position. All Spo 2, heart rate, blood pressure, Vo 2, VCO2, RQ, and NIRS data were synchronously aggregated. Subjects who were unable to complete the CPET protocol or who had incomplete metabolic cart data acquisition were excluded.
The whole-body anaerobic threshold (vAT) was computed on VCO2 and RQ post-hoc using the v-slope method [25]. The difference between the oxyhemoglobin saturation measured by pulse oximetry (Spo 2) and NIRS (rSO2) was computed as the regional arterial–venous saturation difference (AVDO2). Regional AVDO2 measurements in the arm, leg, brain, and kidney, as well as heart rate measurements, were modeled using a three-part change-point model with mixed effects, i.e., a linear increase until vAT, followed by a linear increase with a potentially different slope until QT, and finally an exponential decrease to a level that could be different from the initial starting value.
Results
Thirty-three normal subjects and 5 Fontan subjects scheduled for CPET were recruited. Only 1 normal subject was excluded for not completing the CPET protocol due to anxiety. None of the Fontan subjects had a fenestration of the conduit.
The typical trends of the four-site rSO2 with progressive exercise during CPET in a control and Fontan subject is shown in Figs. 1 and 2. In the cerebral circulation in normal subjects, AVDO2 increases at a clinically insignificant rate of 0.2 percentage points/min until vAT (Table 1). After vAT, the rate of increase rises to 1.5 percentage points/min. There is a rebound return of the rSO2 to baseline AVDO2 after exercise termination (QT). In contrast, the Fontan patients differ in multiple ways. They have a significantly higher initial slope of increasing AVDO2. After vAT, the AVDO2 slope is flat for Fontan patients (p = 0.02). There is also a substantially larger rebound of cerebral rSO2 by 10.8 percentage points compared with normal subjects after QT (p < 0.0001). In the arm, among normal patients, AVDO2 increases at a rate of 1 percentage point/min until vAT, then the rate of increase rises by an additional 2.5 percentage points/min (Table 2). There is no substantial rebound of rSO2 after QT. The trends are not statistically significant in the arm tissue bed in Fontan patients. In the leg, there are no statistical differences in the AVDO2 slopes among the Fontan patients and normal subjects (Table 3). At the kidney, in normal subjects, AVDO2 increases at a rate of 1 percentage point/min until vAT (p < .0001), after which the rate increase rises by an additional 2.1 percentage points/min (p < .0001) (Table 4). There is a brisk return to baseline after QT. The Fontan patients had a higher baseline AVDO2. The trends with exercise were not different for the normal subjects at the renal site. Finally, predicted trajectories with 95% confidence pointwise confidence bands for two hypothetical patients with typical values of vAT and QT were as follows: for the Fontan patient vAT = 7 min and QT = 10 min, whereas for the normal patient vAT = 9 min and QT = 14 min (Fig. 3).
Discussion
Implementation of the Fontan procedure [7], and its modifications has resulted in improved survival for a diverse group of patients with complex congenital heart disease and single-ventricle physiology. Separation of the systemic and pulmonary circulations in these patients reduces systemic ventricular volume loading and provides normal or near-normal systemic arterial O2 saturation. Fenestration of the Fontan pathway was introduced in 1990 as a means to ameliorate the perioperative course in high-risk patients [3]. Previous studies in patients with the Fontan procedure report poor exercise performance, decreased peak oxygen consumption (Vo 2), and decreased Vo 2 at ventilatory anaerobic threshold (vAT) [4–6, 10, 14, 17, 26, 28, 33]. A large cohort of Fontan survivors participated in a multicenter cross-sectional study to determine the association between defined clinical factors and exercise performance [21]. In most cases peak exercise capacity was significantly depressed. A unique finding in this study was that vAT was in the normal range for the majority of subjects. This finding suggested that Fontan subjects can tolerate a higher level of submaximal activity than might be anticipated from measurement of peak Vo 2. Absence of a subpulmonary ventricle might limit Fontan patients’ ability to accommodate hemodynamic burdens associated with levels of exercise above the anaerobic threshold. Chronotropic incompetence and arterial desaturation were minimally responsible for the variance in aerobic performance (both at peak Vo 2 and at vAT) and the physical working capacity in the subgroup of subjects who achieved maximum effort. In this patient population, in subjects with Fontan fenestration closure, the exercise capacity remained unchanged [15]. The concept of multisite NIRS monitoring to characterize changes in integrative circulatory physiology has been described and has been extensively evaluated in the cerebral [11, 12, 19, 29, 31, 32], splanchnic [8, 22], and quasi global circulations [16, 24, 30] Hoffman et al. used frontal cerebral (rSO2-C) and dorsolateral T10-L2 (rSO2-R) renal probe sites to reflect changes in regional oxygenation in circulations presumably under different physiologic control and found distinct changes in cerebral and somatic oxygenation during different phases of operation with full-flow bypass and selective cerebral perfusion, thus demonstrating the regional nature of rSO2 measures. NIRS can be used to monitor cerebral and somatic oxygenation in various clinical situations—including during cardiopulmonary bypass, during deep hypothermic circulatory arrest [12, 18], and in other high-risk newborns [1, 8, 27, 30, 31]—and has been found to be helpful in predicting cerebrovascular dysfunction [18, 27] and splanchnic ischemia [8]. Multisite NIRS monitoring during exercise has been described as a tool to monitor blood flow distribution in different visceral organ beds and reliably predict vAT during ramping exercise [23]. Multisite NIRS monitoring shows differential desaturation patterns in the exercising muscle, brain, somatic and renal vascular beds during CPET and demonstrates that these patterns underlie the systemic oxygen consumption-to-flow-coupling dynamics observed during increasing levels of exercise [23]. Progressive sympathetic nervous system activation during exercise serves to match cardiac output to exercising muscle. Because blood flow distribution during exercise depends on the interaction of regional, autonomic, and humoral mechanisms affecting vascular resistance, regional blood flow-metabolism relations should change during activation of autonomic and behavioral responses to progressively intense exercise. This hierarchical redistribution of flow is known to be affected by individual differences, drug effects, and disease states. Individual variations in regional patterns of blood flow are likely to emerge under different pathologic conditions. Recent study documented that during cycle exercise at 360 W performed to exhaustion, left and right cerebral artery mean flow and velocity measured as by Doppler ultrasound declined continuously from the onset of exercise. However, cardiac output and mean arterial pressure demonstrated an increase at the onset of exercise that reached a peak value after approximately 3–5 min and then declined slightly before exhaustion [9]. The findings by Bhambhani et al. suggested that decreases in cerebral oxygenation and cerebral blood volume evident just beyond the vAT were associated with a significant reduction in PETCO2 (an indirect estimate of PaCO2) that occurred at this threshold [2]. It has been theorized that the acute appearance of metabolic acidosis with anaerobic work induces respiratory alkalosis, which acutely elevates cerebrovascular resistance, thus resulting in reduction of cerebral blood flow [2]. Cerebral desaturation from blood flow distribution in anaerobic exercise triggers its termination for self-preservation.
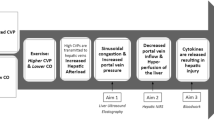
In the cerebral circulation, the Fontan patients have a higher initial slope of AVDO2, whereas post-vAT AVDO2 remains constant compared with normal subjects (p = 0.02). This is in the face of progressive cerebral vasoconstriction after vAT. There is a significantly larger rebound of rSO2C after QT (p < 0.0001). One can postulate that maximal oxygen extraction was achieved around vAT followed by cerebral oxygen debt, which resulted in rebound hyperemia in the recovery phase. This physiology suggests that reduced anaerobic exercise capacity in Fontan patients is probably secondary to impaired cerebral auto regulatory mechanisms, which is in turn secondary to low systemic venous compliance. One can speculate that low venous compliance from absence of a subpulmonary ventricle might result in progressive increase in central venous pressure (CVP) with escalating cardiac output with exercise. We hypothesize that during anaerobic exercise, increasing CVP coupled with hypocapnia mediated increased cerebrovascular resistance; both contribute to a reduction in cerebral blood flow with cerebral ischemia-mediated exercise termination. This might also explain the unchanged anaerobic exercise capacity in Fontan patients after fenestration closure [15]. Paridon et al. found that of the three factors responsible for O2 delivery during exercise (HR, arterial O2 content, and stroke volume), superior O2 pulse (O2 pulse is equal to stroke volume times the arterial-venous O2 content difference)/stroke volume at peak exercise; probably due to higher stroke volume at peak exercise) seems to be the most important factor that distinguishes the high-functioning Fontan patient [21]. Higher stroke volume might offset the effects of increasing CVP by providing a better driving pressure to the cerebral circulation.
Limitations of this study include the small number of patients tested and the lack of direct CVP measures. Actual CVP measurements are lacking due to the unavailability of noninvasive CVP monitors. This was a pilot study, and a larger prospective study is presently underway and is currently recruiting normal subjects and Fontan patients with varying intracardiac anatomy.
Conclusion
Reduced anaerobic exercise capacity in Fontan patients may be secondary to limitation of cerebral blood flow, which is secondary to low systemic venous compliance due to absence of a subpulmonary ventricle and augmented hyperventilatory response during exercise.
References
Berens RJ, Stuth EA, Robertson FA, Jaquiss RD, Hoffman GM, Troshynski TJ et al (2006) Near infrared spectroscopy monitoring during pediatric aortic coarctation repair. Paediatr Anaesth 16:777–781
Bhambhani Y, Malik R, Mookerjee S (2007) Cerebral oxygenation declines at exercise intensities above the respiratory compensation threshold. Respir Physiol Neurobiol 156:196–202
Bridges ND, Lock JE, Castaneda AR (1990) Baffle fenestration with subsequent transcatheter closure. Modification of the Fontan operation for patients at increased risk. Circulation 82:1681–1689
Diller GP, Dimopoulos K, Okonko D, Li W, Babu-Narayan SV, Broberg CS et al (2005) Exercise intolerance in adult congenital heart disease: Comparative severity, correlates, and prognostic implication. Circulation 112:828–835
Dimopoulos K, Okonko DO, Diller GP, Broberg CS, Salukhe TV, Babu-Narayan SV et al (2006) Abnormal ventilatory response to exercise in adults with congenital heart disease relates to cyanosis and predicts survival. Circulation 113:2796–2802
Durongpisitkul K, Driscoll DJ, Mahoney DW, Wollan PC, Mottram CD, Puga FJ et al (1997) Cardiorespiratory response to exercise after modified Fontan operation: determinants of performance. J Am Coll Cardiol 29:785–790
Fontan F, Baudet E (1971) Surgical repair of tricuspid atresia. Thorax 26:240–248
Fortune PM, Wagstaff M, Petros AJ (2001) Cerebro-splanchnic oxygenation ratio (CSOR) using near infrared spectroscopy may be able to predict splanchnic ischaemia in neonates. Intensive Care Med 27:1401–1407
Gonzalez-Alonso J, Dalsgaard MK, Osada T, Volianitis S, Dawson EA, Yoshiga CC et al (2004) Brain and central haemodynamics and oxygenation during maximal exercise in humans. J Physiol 557:331–342
Harrison DA, Liu P, Walters JE, Goodman JM, Siu SC, Webb GD et al (1995) Cardiopulmonary function in adult patients late after Fontan repair. J Am Coll Cardiol 26:1016–1021
Hayashida M, Kin N, Tomioka T, Orii R, Sekiyama H, Usui H et al (2004) Cerebral ischaemia during cardiac surgery in children detected by combined monitoring of BIS and near-infrared spectroscopy. Br J Anaesth 92:662–669
Hoffman GM, Stuth EA, Jaquiss RD, Vanderwal PL, Staudt SR, Troshynski TJ et al (2004) Changes in cerebral and somatic oxygenation during stage 1 palliation of hypoplastic left heart syndrome using continuous regional cerebral perfusion. J Thorac Cardiovasc Surg 127:223–233
Jobsis FF (1977) Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science 198:1264–1267
Mahle WT, Wernovsky G, Bridges ND, Linton AB, Paridon SM (1999) Impact of early ventricular unloading on exercise performance in preadolescents with single ventricle Fontan physiology. J Am Coll Cardiol 34:1637–1643
Meadows J, Lang P, Marx G, Rhodes J (2008) Fontan fenestration closure has no acute effect on exercise capacity but improves ventilatory response to exercise. J Am Coll Cardiol 52:108–113
Nagdyman N, Fleck T, Barth S, Abdul-Khaliq H, Stiller B, Ewert P et al (2004) Relation of cerebral tissue oxygenation index to central venous oxygen saturation in children. Intensive Care Med 30:468–471
Nir A, Driscoll DJ, Mottram CD, Offord KP, Puga FJ, Schaff HV et al (1993) Cardiorespiratory response to exercise after the Fontan operation: a serial study. J Am Coll Cardiol 22:216–220
Nollert G, Mohnle P, Tassani-Prell P, Uttner I, Borasio GD, Schmoeckel M et al (1995) Postoperative neuropsychological dysfunction and cerebral oxygenation during cardiac surgery. Thorac Cardiovasc Surg 43:260–264
Nollert G, Jonas RA, Reichart B (2000) Optimizing cerebral oxygenation during cardiac surgery: a review of experimental and clinical investigations with near infrared spectrophotometry. Thorac Cardiovasc Surg 48:247–253
Owen-Reece H, Smith M, Elwell CE, Goldstone JC (1999) Near infrared spectroscopy. Br J Anaesth 82:418–426
Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS et al (2008) A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol 52:99–107
Petros AJ, Heys R, Tasker RC, Fortune PM, Roberts I, Kiely E (1999) Near infrared spectroscopy can detect changes in splanchnic oxygen delivery in neonates during apnoeic episodes. Eur J Pediatr 158:173–174
Rao RP, Danduran MJ, Frommelt PC, Ghanayem NS, Berger S, Simpson PM et al (2009) Measurement of regional tissue bed venous weighted oximetric trends during exercise by near infrared spectroscopy. Pediatr Cardiol 30:465–471
Schulz G, Weiss M, Bauersfeld U, Teller J, Haensse D, Bucher HU et al (2002) Liver tissue oxygenation as measured by near-infrared spectroscopy in the critically ill child in correlation with central venous oxygen saturation. Intensive Care Med 28:184–189
Svedahl K, MacIntosh BR (2003) Anaerobic threshold: the concept and methods of measurement. Can J Appl Physiol 28:299–323
Troutman WB, Barstow TJ, Galindo AJ, Cooper DM (1998) Abnormal dynamic cardiorespiratory responses to exercise in pediatric patients after Fontan procedure. J Am Coll Cardiol 31:668–673
Tsuji M, Saul JP, du Plessis A, Eichenwald E, Sobh J, Crocker R et al (2000) Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 106:625–632
van den Bosch AE, Roos-Hesselink JW, Van Domburg R, Bogers AJ, Simoons ML, Meijboom FJ (2004) Long-term outcome and quality of life in adult patients after the Fontan operation. Am J Cardiol 93:1141–1145
Watzman HM, Kurth CD, Montenegro LM, Rome J, Steven JM, Nicolson SC (2000) Arterial and venous contributions to near-infrared cerebral oximetry. Anesthesiology 93:947–953
Weiss M, Dullenkopf A, Kolarova A, Schulz G, Frey B, Baenziger O (2005) Near-infrared spectroscopic cerebral oxygenation reading in neonates and infants is associated with central venous oxygen saturation. Paediatr Anaesth 15:102–109
Wyatt JS, Cope M, Delpy DT, Wray S, Reynolds EO (1986) Quantification of cerebral oxygenation and haemodynamics in sick newborn infants by near infrared spectrophotometry. Lancet 2:1063–1066
Yoshitani K, Kawaguchi M, Iwata M, Sasaoka N, Inoue S, Kurumatani N et al (2005) Comparison of changes in jugular venous bulb oxygen saturation and cerebral oxygen saturation during variations of haemoglobin concentration under propofol and sevoflurane anaesthesia. Br J Anaesth 94:341–346
Zajac A, Tomkiewicz L, Podolec P, Tracz W, Malec E (2002) Cardiorespiratory response to exercise in children after modified Fontan operation. Scand Cardiovasc J 36:80–85
Acknowledgments
We acknowledge the invaluable logistical support and coordination of this research provided by Mary M. Krolikowski. We also thank Aniko Szabo and Scott Jackson for statistical analysis. Clinical trial registration information: Registration No. NCT00556231 (available at: http://www.clinicaltrials.gov/ct2/show/NCT00556231?term=NIRS&rank=5). Grant support came from a Pilot Innovative Research Award, Children’s Research Institute, Children’s Health System, Milwaukee, WI.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rao, R.P., Danduran, M.J., Hoffman, G.M. et al. Cerebral Hemodynamics in the Presence of Decreased Systemic Venous Compliance in Patients with Fontan Physiology May Limit Anaerobic Exercise Capacity. Pediatr Cardiol 31, 208–214 (2010). https://doi.org/10.1007/s00246-009-9585-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-009-9585-0