Abstract
This study aimed to assess critically the role of 64-slice multidetector-row computed tomographic (MDCT) angiography for evaluating congenital heart disease. The study enrolled 60 consecutive patients (median age, 4.7 years; median weight, 16.5 kg) with congenital heart disease who underwent 64-slice MDCT angiography during the period June 2006 through September 2007. The results were classified as diagnostic categories, and the impact of the procedure on strategizing management was critically analyzed. In each of the groups, the current technique offered a clear advantage over conventional imaging and provided specific clues for surgical/interventional management. A management algorithm was evolved based on questions frequently asked about pulmonary artery anatomy. The correlation with surgical anatomy in all cases that involved surgery was excellent. Early results suggest that 64-slice MDCT angiography is a major breakthrough in cardiovascular imaging with an important diagnostic and decision-aiding role. Diagnostic cardiac catheterization, especially for evaluating great vessel anomalies, could be largely replaced by the described technique for congenital heart disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Traditionally, cardiologists have relied on echocardiography and conventional angiography to establish the diagnosis of congenital heart disease. Both techniques have potential limitations [12]. Echocardiographic study is operator dependent and limited by an acoustic window, especially in older children and adults [14]. Lung disease further complicates the quality of the echocardiographic image. Conventional angiography is an invasive procedure with its inherent risks [6]. During angiography, overlapping of pulmonary and systemic circulations may confuse the picture of a complex anatomy.
The pediatric cardiology community has been in constant search for an imaging method that is noninvasive with high precision and no complications. The emergence of magnetic resonance angiography (MRA) and 64-slice multidetector computed tomographic (MDCT) angiography has been a major technologic breakthrough in this direction. The enhanced preoperative understanding of congenital heart disease provided by MRA and MDCT angiography simplifies surgical decision making and consequently may improve outcome [7]. Faster acquisition and higher spatial resolution enables 64-slice MDCT to compete with MRA as the preferred imaging technology for congenital heart disease. The two methods may be complementary. The intracardiac anatomy is well depicted by MRA, whereas MDCT provides exquisite images of the great vessels [2].
The current technology of 64-slice MDCT with three-dimensional (3D) software has opened new vistas for noninvasive evaluation of congenital heart disease, providing image quality equal to conventional angiography or even better. Three-dimensional imaging is particularly useful for diagnostic investigation of complex forms of congenital heart disease, preparation for complex interventional procedures, and postoperative assessment of surgical reconstruction [1]. When coupled with electrocardiography gating, MDCT angiography can be used in functional evaluation of ventricular wall motion, ventricular ejection fraction, and motion of cardiac valves. It also allows the performance of high-quality coronary MDCT angiography [5, 8].
Currently, magnetic resonance imaging (MRI) is limited for congenital heart imaging by relatively poor temporal resolution (particularly for assessment of the coronary arteries) and often complex acquisition protocols requiring a long examination that frequently necessitate general anesthesia for young children [13]. Only a few studies in the literature critically assess the use of 64-slice MDCT angiography for congenital heart disease. The current study analyzed the utility of this technique in 60 consecutive cases of congenital heart disease across a broad spectrum of pathologic anatomy.
Materials and Methods
The study enrolled 60 consecutive patients with congenital heart disease who underwent 64-slice MDCT angiography in our center during the period June 2006 through September 2007. The investigations were performed to answer specific anatomic questions raised by inconclusive echocardiographic or angiographic evaluation. All studies were performed in the presence of a qualified pediatric cardiologist and radiologist working in unison.
Data Acquisition
An anesthesiologist intravenously sedated patients who were not able to hold their breath, and imaging was done during quiet breathing. The examinations were performed with Siemens Somatom Sensation (Muenchen, Germany). The MDCT data were obtained using the following parameters: rotation time (0.37 s), pitch (0.9), slice thickness (0.75–1 mm), low voltage (120 kV), current (180–200 mA), collimation (0.6 mm), table feed (5.4 mm/rotation), and reconstruction interval (0.1–0.5 mm). Images depicting a region of interest in the aorta or pulmonary artery were obtained with an acquisition delay of 4 s and an automatic triggering threshold of 100 Hounsfield Unit (HU). A weight-based, low-dose MDCT protocol (120 kVp, 180–200 mA) was used [10].
Scanning was performed from the thoracic inlet level to the L1–L2 level or lower as needed. Nonionic contrast agent Ioversol (Optiray 350 mg/ml; Tyco Healthcare Canada Inc., Pointe Claire, QC, Canada) was injected in a peripheral vein at a dosage of 1–2 ml/kg and a flow rate of 2–3 ml/s. A leg vein was the preferred route. However, an arm vein was used for patients who had undergone a bidirectional cavopulmonary shunt or Fontan procedure. Electrocardiography and respiration-gated techniques were not used. The children were observed for 2–4 h before being sent home.
Image Processing
The acquisition volume was analyzed with software version Syngo CT 2006 A (Siemens AG, Forchheim, Germany). Various image-reformatting techniques including linear or curved planar reformatting, maximum intensity projection (MIP), minimum intensity projection, and volume rendering (VR) were used depending on target structure and purpose [15]. The plane of the reformatted image was adjusted to correspond to the long axis of the structure of interest. Curved planar reformatting was used to evaluate curved structures such as the pulmonary artery system, and MIP was used mainly for evaluation of the cardiovascular structures. Minimum intensity projection was used to evaluate the airway and lung parenchyma. For 3D reformatting, VR was used to evaluate the cardiovascular structures. Thin-section multiplanar reformatting was used for accurate measurement of the diameter or area of the structure in question [6].
The information obtained was compared with that provided by echocardiography/angiography for relevance of additional information. Perioperative anatomic descriptions, wherever available (n = 26), formed the gold standard for comparison. The information obtained was classified based on the diagnostic group.
Results
The ages of the study participants ranged from 7 months to 26 years (median, 4.7 years). The male:female ratio was 0.9:1. Patient weight ranged from 4 to 70 kg (median, 16.5 kg). There were no procedure-related complications.
The results were classified as diagnostic categories (groups 1–5; Tables 1–5), and the impact of the procedure on strategizing management was critically analyzed. The most common indication for MDCT angiography in the current series was the need to evaluate pulmonary artery anatomy (n = 36) (Table 1). A total of 14 cases were studied to assess the confluence of pulmonary arteries. Six cases with confluent pulmonary arteries proceeded to two ventricle repairs or a Fontan track based on intracardiac anatomy. Of the eight cases with nonconfluent pulmonary arteries, three proceeded to unifocalization and radical repair, whereas five had conservative management because of an anatomy too complex for surgical intervention. In 15 cases, growth of the pulmonary arteries was monitored after a surgical procedure (commonly a systemic pulmonary shunt for tetralogy of Fallot/pulmonary atresia). The outcome is shown in Table 1. In seven cases, MDCT angiography enabled decision making regarding pulmonary angioplasty/stenting, and five of these proceeded to planned intervention. Except for the seven cases that did not have further intervention, all the remaining cases in group 1 had pulmonary artery anatomy documented by surgical/angiographic evaluation, and an excellent correlation was obtained.
Tables 2–5 summarize other major groups, MDCT angiographic findings, and the subsequent course of management. Figures 1–8 show representative images.
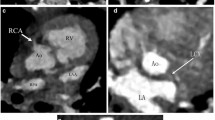
(a) Axial image showing nonconfluent pulmonary arteries in a patient with tetralogy of Fallot/pulmonary atresia. The main pulmonary artery (MPA) continues as the left pulmonary artery (LPA). The right pulmonary artery is not seen. There are only distal intrapulmonary branches. (b) Volume-rendered image of the same patient showing the anatomy better. A large ductus (PDA) is supplying the left pulmonary artery. PDA, patent ductus arteriosus
(a) Axial image showing midline liver, right-sided stomach (Sto), and juxtaposed aorta (Ao) and inferior vena cava (IVC) on the left side. (b) Multiplanar reconstructed view showing eparterial bronchi. (c) Coronal image of bilateral superior vena cava (RSVC, LSVC) and confluent pulmonary arteries. LSVC, left superior vena cava; RSVC, right superior vena cava
(a) Coronal image of left isomerism showing interrupted inferior vena cava (IVC) with hemiazygos continuation. Note the hepatic veins draining directly to the atrium. The superior mesenteric artery distribution suggests malrotation of the gut. (b) Coronal image showing multiple splenic masses (S). (c) Multiplanar reconstructed image of hyparterial bronchi, which have only two branches on both sides
Discussion
The new generation of 64-slice MDCT has changed the approach to noninvasive assessment of congenital heart disease. Ou et al. [13] recently reviewed the application of 3D CT scanning for congenital heart disease, concluding that it had become an invaluable diagnostic and decision-aiding tool, a complement to echocardiography, and often a substitute for diagnostic angiography.
The current study was an attempt to examine the specific benefit provided by MDCT angiography over conventional imaging for a variety of anatomic abnormalities across the spectrum of congenital heart disease. For assessment of pulmonary arteries, MDCT angiography was particularly useful in demonstrating confluence or discontinuity of pulmonary arteries and extent of pulmonary artery stenosis. It served well for choosing balloon/stent size for balloon angioplasty/endovascular stenting and for assessing the growth of pulmonary artery after systemic pulmonary shunt (Figs. 1–3). Thus this technique helped to evolve a management algorithm (Fig. 9) based on the frequently asked questions about the pulmonary arteries. For aortic anomalies, MDCT angiography precisely identified the site of coarctation, the degree of narrowing, and the extent of involvement in degenerative aortic diseases (Fig. 8).
In particular, MDCT angiography was useful for visceral heterotaxy, demonstrating the diverse range of abnormalities in multiple organs as well as cardiac abnormalities (Figs. 4 and 5). Thus, MDCT angiography helped to identify pulmonary situs, gut malrotation, asplenia/polysplenia, and visceral malposition. No other imaging method, with the exception of MRA, shows all the anatomic details of heterotaxy in one shot. The intracardiac anatomy, systemic and pulmonary venous connection, outflow tract obstruction, and collaterals all were well delineated and corroborated with surgical findings.
Chen et al. [4] showed the usefulness of 3D electron-beam CT scanning for evaluating tracheobronchial anomalies in children with congenital heart disease. We found 64-slice MDCT to be extremely useful in demonstrating the eparterial/hyparterial position of bronchi in heterotaxy syndrome (Figs. 4b and 5c). In particular, MDCT angiography was useful for evaluating William’s syndrome, in which the involvement of the aorta and the pulmonary artery could be diffuse. A range of miscellaneous conditions (aortopulmonary window, blocked Fontan circuit) also was well demonstrated by MDCT angiography (Fig. 6). This range is bound to expand with the passage of time.
Demonstration of coronary artery angiography in children by MDCT is fraught with the problem of tachycardia-related loss of resolution. However, in selected cases, this limitation did not preclude demonstration of the origin and course of coronary arteries in this series. In anomalous origin of left coronary artery from pulmonary artery, MDCT angiography demonstrated the sinus of origin and the distance for surgical translocation precisely in a 4-month-old baby. Postoperative assessment also was performed 6 months after surgery using the same technique.
The major concern in MDCT angiography is the degree of exposure to radiation. The reported average radiation exposure per study is 18–28.4 millisieverts despite the short period of scanning [3, 9]. We have tried to minimize the exposure by following the as-low-as-reasonably-achievable (ALARA) principle. Average exposure per MDCT study was calculated at 10–14.2 millisieverts. In addition, MDCT angiography was more cost effective for children than conventional angiography (US $100–175 vs $400–$450). It also avoided hospitalization.
Lee et al. [11] suggested that after initial assessment with echocardiography, MDCT could probably replace diagnostic cardiac catheterization for further anatomic clarification in neonates. The current study showed that this concept could be extended to all age groups for congenital heart disease. Since the introduction of MDCT angiography, the number of diagnostic catheterization procedures has decreased steeply at our institution. Cardiac catheterization has been needed only for cases in which physiologic data were crucial. For younger children, MDCT may be preferable to MRI due to the simplicity of the examination and the rapidity of image acquisition, generally in less time than 2 s [13]. Because MDCT takes less time and has fewer requirements for sedation than MRI, it can be performed more easily for an unstable patient who needs intensive monitoring and care. In addition, the MDCT postprocessing time is shorter than the MRI postprocessing time needed for morphologic and functional evaluation.
Conclusion
The 64-slice MDCT angiography obtained using the current technology is a major breakthrough in cardiovascular imaging for children. This study has shown the major diagnostic and decision-aiding role of this technique in the assessment of congenital heart disease, especially for answering questions not resolved by echocardiography. This would avoid conventional angiography for a large number of patients and would be a useful tool for planning interventional cardiac catheterization.
Limitations of the Study
This study was not designed as a head-to-head comparison of 64-slice MDCT angiography with conventional imaging techniques. We have not attempted to compare CT angiography with its major competitor, MRA.
References
Bean MJ, Pannu H, Fishman EK (2005) Three-dimensional computed tomographic imaging of complex congenital cardiovascular abnormalities. J Comput Assist Tomogr 29:721–724
Boxt LM (2004) Magnetic resonance and computed tomographic evaluation of congenital heart disease. J Magn Reson Imaging 19:827–847
Chandran A, Fricker FJ, Schowengerdt KO, et al. (2005) An institutional review of the value of computed tomographic angiography in the diagnosis of congenital cardiac malformations. Cardiol Young 15:47–51
Chen SJ, Lee WJ, Wang JK, et al. (2003) Usefulness of three-dimensional electron beam computed tomography for evaluating tracheobronchial anomalies in children with congenital heart disease. Am J Cardiol 92:483–486
Goo HW, Park IS, Ko JK, et al. (2005) Computed tomography for the diagnosis of congenital heart disease in pediatric and adult patients. Int J Cardiovasc Imaging 21:347–365
Goo HW, Park IS, Ko JK, et al. (2003) CT of congenital heart disease: normal anatomy and typical pathologic conditions. RadioGraphics 23:S147–S165
Haramati LB, Glickstein JS, Issenberg HJ, et al. (2002) MR imaging and CT of vascular anomalies and connections in patients with congenital heart disease: significance in surgical planning. Radiographics 22:337–347
Matsui H, Yasukochi S, Haseyama K, et al. (2007) Quantification of right and left ventricular volumes with congenital heart disease by multidetector-row computed tomography. Pediatr Cardiol 28:267–271
Hollingsworth CL, Yoshizumi TT, Frush DP, et al. (2007) Pediatric cardiac-gated CT angiography: assessment of radiation dose. AJR Am J Roentgen 189:12–18
Jelnin V, Co J, Muneer B, et al. (2006) Three-dimensional CT angiography for patients with congenital heart disease: scanning protocol for pediatric patients. Catheter Cardiovasc Interv 67:120–126
Lee T, Tsai IC, Fu YC, et al. (2006) Using multidetector-row CT in neonates with complex congenital heart disease to replace diagnostic cardiac catheterization for anatomical investigation: initial experiences in technical and clinical feasibility. Pediatric Radiol 36:1273–1282
Leschka S, Oechslin E, Husmann L, et al. (2007) Pre- and post-operative evaluation of congenital heart disease in children and adults with 64-section CT. Radiographics 27:829–846
Ou P, Celermajer DS, Calcagni G, et al. (2007) Three-dimensional CT scanning: a new diagnostic modality in congenital heart disease. Heart 93:908–913
Samyn MM (2004) A review of the complementary information available with cardiac magnetic resonance imaging and multislice computed tomography (CT) during the study of congenital heart disease. Int J Cardiovasc Imaging 20:569–567
Westra SJ, Hill JA, Alejos JC, et al. (1999) Three-dimensional helical CT of pulmonary arteries in infants and children with congenital heart disease. AJR Am J Roentgenol 173:109–115
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khatri, S., Varma, S.K., Khatri, P. et al. 64-Slice Multidetector-Row Computed Tomographic Angiography for Evaluating Congenital Heart Disease. Pediatr Cardiol 29, 755–762 (2008). https://doi.org/10.1007/s00246-008-9196-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-008-9196-1