Abstract
Metal concentrations were measured in sediments of the mine-affected Molonglo River to determine current metal concentrations and distribution along the river. Compared with an uncontaminated site at 6.5 km upstream of the Captains Flat mine, sediments collected from the river at ≤12.5 km distance below the mine had a significantly higher percentage of finely divided silt and clay with higher concentrations of cadmium (Cd), copper (Cu), lead (Pb), and zinc (Zn). The measured metal concentrations in the mine affected sites of the river were in the following order: Zn = 697–6818 > Pb = 23–1796 > Cu = 10–628 > Cd = 0.13–8.7 µg/g dry mass. The highest recorded metal concentrations were Cd at 48, Cu at 45, Pb at 240, and Zn at 81 times higher than the background concentrations of these metals in the river sediments. A clear sediment metal-contamination gradient from the mine site to 63 km downstream was established for Cd, Cu, Pb, and Zn in the river sediments. Compared with sediment metal concentrations before a major flood in 2010, only Zn concentrations increased. For all of the mine-affected sites studied, Cd and Zn concentrations exceeded the (ANZECC/ARMCANZ, Australian and New Zealand guidelines for fresh and marine water quality. Australian and New Zealand Environment and Conservation Council/Agriculture and Resource Management Council of Australia and New Zealand, 2000) interim sediment-quality guidelines low values for Cd (1.5 µg/g dry mass) and the high value for Zn (410 µg/g dry mass). Existing metal loads in the riverbed sediments may still be adversely affecting the river infauna.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Historic mining activities at Captains Flat, NSW, have left a significant legacy of metal contamination in the Molonglo River, NSW (Brooks 1980; Nicholas and Thomas 1978). The former Captains Flat ore body consisted predominantly of metal-bearing sulphide such as pyrite (FeS2), sphalerite ((Zn,Fe)S), galena (PbS), chalcopyrite (CuFeS2), arsenopyrite (FeAsS), and gold, which occurred in a north–south trending belt of Silurian shale and volcanics (Jacobson and Sparksman 1988). Ore deposits were mined through underground excavation to produce mainly Cu, Pb, Zn (Jacobson and Sparksman 1988), and ore processing, i.e., milling, smelting, and storage of waste materials was performed near the Molonglo River. At present, no active mine operates at Captains Flats. During the active period of the mine, waste contaminated with metals was directly and indirectly released into the Molonglo River below the Captains Flat mine (Dames and Moore 1993). Among various mine-waste contamination sources to the river, failure of mine waste tailings dumps, which were located at the southern and northern ends of the mine (Fig. 1), have been identified as a major metal contamination source to the river (Hogg 1990). To minimise the release of mine waste into the river and improve the river condition, mine site rehabilitation work was undertaken in 1976 (Dames and Moore 1993). None of the remedial works involved a clean-up of tailings associated with river bed sediments and surrounding floodplains (Nicholas and Thomas 1978), which have been identified as major contaminant sources to the river during the mines operation, after mine closure, and even after rehabilitation works were completed (Nicholas and Thomas 1978).
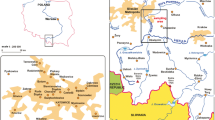
Map of the former Captains Flat mine and potential path ways of mine contaminants entering into the Molonglo River (Dames and Moore 1993)
Metal contaminants incorporated into surrounding floodplains enter the river system by seepage, surface runoff, and erosion. Sediment deposited in upstream river areas can be remobilised and flushed downstream during floods, which may result in widespread metal contamination in mine-affected aquatic environments (Brooks 1980). Metals that are discharged in soluble and particulate forms tend to be associated with bottom sediments. Sediments in aquatic environments, therefore, have been identified as a major source for metal contaminants (Walling et al. 2003). A recent study performed in 1996 (Sloane and Norris 2003) reported high concentrations of Cd 11.8 µg/g, Cu 748 µg/g, Pb 2568 µg/g, and Zn 3534 µg/g dry mass in Molonglo River sediments. No studies have been performed to investigate metal contamination in river sediments since 1996.
The Molonglo River formerly was a good habitat for river fauna. The effects of metal contamination on Molonglo River aquatic life have been investigated on several occasions since the 1960s. Before the remedial work was undertaken at Captains Flat, fish survived only a few days in tanks in situ (Weatherley et al. 1967). The same response was also observed by Graham et al. (1986) after the remedial work had been completed. Nicholas and Thomas (1978) found that metal contamination had almost eliminated animals and plants from the Molonglo River many kilometres downstream from Captains Flat, and the river bed immediately below the Captains Flat was virtually lifeless. Norris (1986) reported that Zn contamination had greatly reduced the abundance and diversity of invertebrate fauna in the river. Increasing macroinvertebrate abundance and species richness were observed with increasing distance downstream of the Captains Flat mine site (Weatherley et al. 1980), probably as a result of dilution of metal contamination in the river by water and sediments from nearby tributaries. Lintermans unpublished data (1992–1993) showed that even 18 years after the remedial work was completed, the Molonglo River between Queanbeyan and Captains Flat had only minor recovery of fish species compared with the status of the river before the mining activities commenced (Trueman 2012).
Identification of the presence and measurement of metal concentrations in sediments is important to assess the health status of rivers affected by historic mining activities and to identify the effectiveness of the rehabilitation efforts and current contamination sources to the river and river biota. Among various point and diffuse metal contamination sources to the Molonglo River, previous studies have shown that the oxidation of tailings in the river bed that are contaminated with pyrite, galena, chalcopyrite, and sphalerite is the major diffuse metal contamination source to the river (Brooks 1980; Nicholas and Thomas 1978; Weatherley et al. 1967).
This study reports the current level of metal contamination in Molonglo River sediments. Metal concentrations of river sediments were compared with those of reference sites, which are located upstream of the Captains Flat mine and in nearby tributaries of the river to represent regional background concentrations of metals in the sediments and also with previous studies carried out in the river.
Materials and Methods
Sampling Site
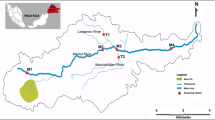
The Molonglo River rises on the western side of the Great Dividing Range and flows through the township of Captains Flat, NSW, where the abandoned mine is located, before the river is joined by the major tributary (the Queanbeyan River). The river then flows toward Canberra, where the river has been dammed to form Lake Burley Griffin, and eventually empties into the Murrumbidgee River near Uriarra Crossing. Captains Flat mine is located close to the Molonglo River in NSW upstream approximately 50 km southeast of Canberra, ACT. Eleven sites (10 test and 1 reference) in the Molonglo River and three reference sites (sites no. 12–14) in nearby tributaries were selected (Fig. 2). Selection of test sites/mine-affected sites was based on the study of Sloane and Norris (2003). Site no. 1, upstream of the Captains Flat mine, and sites no. 12–14 on tributaries were selected to represent the regional background metal concentrations in sediments.
Sediment Collection and Sample Preparation
Three replicate sediment samples were collected in acid-washed 50 mL polypropylene tubes from a depth of 0–5 cm from each site in December 2012 during river low-flow conditions. Samples were transported to the laboratory on ice and stored at 4 °C until analysis. Sediment samples were oven dried at 70 °C for 72 h, and foreign objects such as dried leaves and rocks were discarded. Each sediment sample was then homogenized manually using a stainless steel spatula before analysis. Physicochemical parameters in water were also measured in the field using Horiba U-52 Multiparameter (Table S1).
Sediment Particle Size Distribution
Sediments grain sizes were determined by dry sieving using stainless steel sieves into sizes 1.4 mm and 500, 250, 125, and 63 μm, and they were categorised as coarse sand (>1.4 mm), medium sand (>500 µm), fine sand (>250 µm), very fine sand (>125 µm), and silt and clay (<63 µm). Sediment particles retained in each sieve were weighed and the percentage composition of each fraction contributed to the sample was calculated.
Sediment Metal Analysis
Sediment metal analysis was performed the <63 µm, silt, and clay fraction (Batley and Simpson 2014).Sediments were digested using a microwave digestion procedure described previously by Telford et al. (2008). Approximately 0.2 g of oven-dried <63 µm fractions were weighted into 55 mL Teflon polytetrafluroacetate closed digestion vessels (CEM, USA). HNO3 (Suprapur Nitric Acid, Merck, Germany), 2 mL, and 1 mL of 30 % HCl (Suprapur Hydrochloric Acid, Merck, Germany) were then added to each digestion vessel. Vessels were kept covered for 2 h and were then tightly capped, and digestion was performed using a MARS microwave oven (CEM MDS 2000) using a two-step time-and-temperature program: 5 min at 800 W and 120 °C and 15 min at 1600 W and 150 °C.
After digestion, vessels were allowed to cool to room temperature (∼25 °C) for approximately 20 min, and then extracts were transferred to 50 mL polyethylene vials and diluted up to 30 mL with high-grade deionised water. Extracts were centrifuged at 3500 rpm for 10 min using an Eppendorf 5804R centrifuge. Further dilution of samples were performed on-line (Gilson GX 271, Middleton, USA) with inductively coupled plasma–mass spectrometry (ICP-MS) mixed seven element internal standards (EM science) to obtain a final 1 % (v/v) acidification for ICP-MS analysis. Cd, Cu, Pb, and Zn concentrations were measured using an ELAN DRC-e ICP-MS (PerkinElmer, SCIEX, USA) (Maher et al. 2001). For the purpose of quality assurance, the certified reference material (CRM) Buffalo River sediments (NIST- RM 8704) and acid blanks were also digested, diluted, and analysed with test samples. Concurrently, measured concentrations in the CRM were Cd 2.85 ± 0.08, Pb 110 ± 7, and Zn 413 ± 18 µg/g dry mass (mean ± SD; n = 4) and they were in agreement with the certified values Cd 2.94 ± 0.29, Pb 150 ± 17, and Zn 408 ± 15 µg/g dry mass.
Statistical Analysis
All statistical tests were performed using SPSS 21 and visually represented by Microsoft Excel 2010. Tests of normality of data were verified using Shapiro–Wilk test, and homogeneity of variance of the data were checked by Levene’s test before a comparison of means. Significance differences between sites for different grain sizes were compared by a factorial analysis of variance (ANOVA). If data showed significant difference, post hoc comparisons between sites for grain size, particularly <63 µm fraction, was made by Tukey–Kramer’s test. One-way ANOVA was used to compare the significant differences of individual metal concentrations and total metal concentrations between sites. If data showed significant differences (significant at p ≤ 0.05), post hoc test for pair-site comparisons were made by Tukey–Kramer’s test. Regression analysis was used to find the metal contamination gradient in the Molonglo River for individual and total metals. Significance levels of tests are indicated by asterisks according to the following probability ranges: *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001.
Results
Sediment Particle Size Distribution
The percentage distributions of particle sizes between sites were significantly different (F = 49; p < 0.00). Of the mine-affected sites no. 2–11, nos. 2–5, which were located closest to the mine (Fig. 2), had similar sediment particle size distributions (Table 1) with the highest silt and clay percentages being measured at sites no. 2, 4, and 5 (Fig. 3). Site no. 3, below the confluence of Copper Creek, had a lower percentage of silt and clay than the other three sites. Reference sites no. 13 and 14 in Yandyguinula and Primrose Valley Creeks, respectively, had similar silt and clay percentages to those of the sites no. 2, 4, and 5 in the Molonglo River (Fig. 3). Sediment particle size distribution at site no. 6, below the confluence with Ballalaba Creek, was similar to that of site no. 3 and that of the Ballalaba Creek, site no. 12. The percentages of silt and clay fractions in these three sites were not significantly different to that of site no. 1 upstream of the mine (Fig. 3). Silt and clay percentages of site no. 7, below the Primrose Valley Creek confluence, were significantly higher than those at site no. 6 and similar to that of the two sites located in nearby reference sites no. 13 and 14 in Yandyguinula and Primrose Valley Creeks, respectively (Figs. 2 and 3). Sites no. 8–11, furthest downstream from the mine, had similar patterns of particle size distribution to those of the sediment particle distribution at site no. 1, which is upstream of the Captains Flat mine.
Sediment Metal Concentrations
The mean individual and total metal concentrations (when the four major contaminant metals Cd, Cu, Pb, and Zn were combined) in sediments were significantly different between sites (Tables S2 and S3; p < 0.000). The background metal concentrations measured in the Molonglo River sediments at site no. 1 were not significantly different from the regional background metal concentrations measured at sites no. 12 through 14 in nearby tributaries (Figs. 4 and 5). Metal concentrations measured at the reference sites were below the ANZECC/ARMCANZ (2000) ISQG low values for freshwater sediment; Cd 1.5, Cu 65, Pb 50, and Zn 200 µg/g.
Mean total metal concentrations (combined molar concentrations of Cu, Pb, and Zn) in contaminated Molonglo River sediments (sites no. 2–11 [black bars]) and reference sites (sites no. 1 [white bar] and 12–14 [gray bars]). Mean ± SE, n = 12. Different letters denote significant differences between sites (Tukey–Kramer; p ≤ 0.05)
Mean metal concentrations (Cd, Cu, Pb, and Zn µg/g dry mass) in contaminated Molonglo River sediments (no. 2–11 [black bars]) and uncontaminated reference sites (no. 1 [white bar] and 12–14 [gray bars]). Mean ± SE, n = 3. Different letters denote significant differences between sites (Tukey–Kramer; p ≤ 0.05). Dotted lines denote ANZECC/ARMCANZ (2000) ISQG low values for freshwater sediment: Cd 1.5, Cu 65, Pb 50, and Zn 200 µg/g
Except for site no. 11, all other sites downstream of the mine had significantly higher total metal concentrations than reference sites. A clear increase of total metal concentration occurs from sites no. 1–5 and then there was a significant decrease at site no. 6 followed by slight but no significant increase at site no. 7. Sites no. 6–10 had similar total metal concentrations (Fig. 4).
Site no. 5 had significantly higher Cd concentrations than all other sites which was sixfold higher number than that of the ANZECC/ARMCANZ (2000) ISQG low value for Cd. Except for site no. 11, all of the other sites downstream of the mine had higher Cd concentrations than the reference sites (Fig. 5), which were above the ANZECC/ARMCANZ (2000) ISQG low value but below the high value. Cd concentrations at site no. 11, which is 63 km downstream of the mine were similar to Cd concentrations at the reference sites.
The highest Cu concentrations (628 ± 30 µg/g dry mass) was measured at site no. 4, which was twofold higher than the ANZECC/ARMCANZ (2000) ISQG high values for Cu. The sites closest to the mine (sites no. 2–5) had significantly higher Cu concentrations than all other downstream and the reference sites, which were not significantly different from each other (Fig. 5).
The Pb concentration at site no. 4 (1796 ± 71 µg/g dry mass) was significantly higher than all other sites, which was eightfold higher than that of the ANZECC/ARMCANZ (2000) ISQG high value. Sites no. 2, 3, and 5 also had significantly higher Pb concentrations than all other sites but significantly lower than Pb concentrations at site no. 4. Pb concentrations at sites no. 6–11 were not significantly different to Pb concentrations measured at reference sites (Fig. 5).
Zn (6818 ± 388 µg/g dry mass) was the highest measured metal contaminant in river sediments. All of the sites located downstream of the mine had significantly higher Zn concentrations than reference sites, and these values exceeded the ANZECC/ARMCANZ (2000) ISQG high values for Zn (Fig. 5). Among these sites, site no. 5 had significantly higher Zn concentrations than all other sites being 17-fold higher than the ANZECC/ARMCANZ (2000) ISQG high values for Zn.
Regression analysis of metal concentrations with distance along the Molonglo River showed a clear contamination gradient from below the mine site to 63 km downstream for total metals, Cd, Cu, Pb, and Zn in Molonglo River sediments (Table 2).
Regression analysis between metal concentrations in Molonglo River sediments with percentage of clay and silt fractions showed that total metal concentrations and concentrations of the individual metals increased with increased silt and clay fraction in sediments (Table 3).
Discussion
Sediment Particle Size Distribution
Sediment particle size analysis is important because the surface area of sediments is grain size-dependent and controls the adsorption and desorption of metals from sediments, which may modify sediment metal chemistry and bioavailability of metals in aquatic ecosystems (Simpson et al. 2005). Depending on the particle size, the availability of binding sites for metals in aquatic sediments can vary (Simpson et al. 2005). Direct and indirect release of tailings from metal-mining activities increases the metal-contaminated sediment load input to freshwater aquatic ecosystems (Brooks 1980). As a consequence, mine-generated materials can be traced for many kilometres downstream of mine workings (Hudson-Edwards et al. 1999; Taylor and Kesterton 2002).
According to the present study, even 67 years after the second tailings dump failure at the Captains Flat mine, a high percentage of finely divided tailing materials are still present in the river sediments close to the mine. Compared with the uncontaminated site upstream of the mine (Fig. 1) sites immediately downstream of the mine (sites no. 2, 4, and 5) had a high percentage of finely divided silt and clay with significantly higher concentrations of metals (Figs. 3, 4 and 5; Table 1). Depending on the textural properties of the sediments (e.g., particle size) and the flow regime of the water body, sediments contaminated with tailings are transported, sorted, and deposited on river sediments. The composition of sediment particle size, therefore, varies with location and time. Coarser materials are heavy and are generally deposited close to the discharge source, and metals bound to finer particles are carried downstream and deposited in pool areas in freshwater bodies. Barriers such as dams may alter the movements of sediments. According to a study by Brooks (1980), all types of sediments released from the mine activities, particularly those from the collapse of the southern tailings dump, were effectively trapped by the town reservoir at Captains Flat; however, clay materials containing metals passed over the dam and were flocculated and incorporated within river bed sediments due to the addition of soluble salts from mining activities. As reported in a study by Hogg (1990), almost all of the sediments in the river near Captains Flat originated from the mine. These findings are consistent with the present study because high percentages of finely divided sediments with high concentrations of metals were present at sites closest to the mine.
Sites no. 2, 4, and 5 (located below the town reservoir at Captains Flat) are pools, which are deep and slow flowing, and there is no input of clean sediment from tributaries at these points (Fig. S1). The river at site no. 3, however, is at the confluence with Copper Creek, therefore, the lower percentage of silt and clay fraction in this site may be due to the relatively shallow river bed and/or flushing off of finer sediment particles with increased volume of water input at this point (Fig. S1). In addition to the upstream supply, particle size composition at site no. 6 and sites located further downstream may also depend on the particle size of sediment entering from Ballalaba Creek and inputs from river banks (Fig. 2). The river before site no. 7 confluences with two other creeks, Yandyguinula and Primrose Valley Creek, where reference sites no. 13 and 14 were located, and all of these sites had similar sediment particle size distribution (Table 1) and similar percentage of silt and clay in sediments (Fig. 3). Moreover, site no. 7, located after Carwoola and Fox Flow floodplains, is deep and the water flow rate relatively low (Fig. S1), therefore, sediment particle composition at this site and sites further downstream may depend on several factors such as upstream supply, physical properties of the river, water flow rates, and inputs of sediments from tributaries, floodplains, and the river bank. The river sediments furthest from the mine (sites no. 8–11) had similar particle size distributions with lower percentages of finely divided silt and clay (Fig. 2; Table 1). Except for site no. 11, total metal concentrations at these sites were significantly higher than those at reference sites (Fig. 4), therefore, sediments in these sites contain mine-contaminated sediments due to downstream movement of finely divided tailings and/or adsorption or coprecipitation of dissolved metals due to changes of physicochemical parameters with increasing distance from the mine (Table S1) (Hudson-Edwards et al. 1999; Taylor and Kesterton 2002).
Sediment Metal Concentrations
In the present study, metal analysis in sediments was performed using the <63 µm sediment fraction. As recommended (Simpson and Graeme 2016), metal analysis in this fraction should be used to characterise metal contamination in aquatic environments. In the present study, even 50 years after mining ceased and 36 years after the last major remedial works were completed at the Captains Flat mine, high Cd, Cu, Pb, and Zn concentrations in sediments were found in most of the mine-affected sites in the Molonglo River compared with the background concentrations of these metals in river sediments (Fig. 5). The concentrations of metals in the sediments of contaminated sites in the Molonglo River were in the order Zn > Pb > Cu > Cd. River bed sediments have extremely high Zn (697–6818 µg/g dry mass) compared with background concentrations of Zn in the Molonglo River upstream of the mine (84 µg/g dry mass) and other reference sites (38 to 105 µg/g dry mass).
According to the study by Weatherley et al. (1967), mine tailings discharged from the Captains Flat were mainly composed of pyrite, galena, chalcopyrite, and sphalerite. The river bed sediment is likely to still contain these materials based on the significantly higher concentrations of these metals in the contaminated sites compared with the reference sites.
Comparisons of metal concentrations in the river bed sediments in the present study with previous studies to understand the recovery of the river from metal contamination was difficult due to the use of different sediment collection periods, analysis techniques, and the use of different fractions of sediments for metal analysis. In this respect, in the present study, to understand the present magnitude of metal contamination in the river sediments, comparison with river background metal concentrations and ANZECC/ARMCANZ (2000) ISQG low and high values for Cd, Cu, Pb, and Zn were made.
Metal concentrations at the most contaminated river sites (site no. 4 for Cu and Pb and site no. 5 for Cd and Zn) sediments are enriched with Cd (48x), Cu (45x), Pb (240x), and Zn (81x) compared with background concentrations of these metals in the river. Extreme enrichment of river sediments with Cd, Pb, and Zn were also reported in other mine-affected freshwater environments (e.g., Crooked Creek, Missouri, USA) (Gale and Wixson 1979). Cd (except for site no. 11), and Zn concentrations in all of the mine-affected sites exceeded the ANZECC/ARMCANZ (2000) the ISQG low value for Cd (1.5 µg/g dry mass) and the high value for Zn (200 µg/g dry mass) and were measured up to 63 km downstream of the mine (Fig. 5). Likely sources include inputs of contaminated sediments from upstream of the river, particularly high-flow conditions; adsorption and coprecipitation of dissolved metal with increasing distance from the mine; reduced dilution of contaminated sediments from uncontaminated sediments released from nearby tributaries; high mobility of these two metals compared with Cu and Pb; and reintroduction from floodplains.
Pb concentrations at sites no. 2–5 exceeded the ANZECC/ARMCANZ (2000) ISQG high value of 220 µg/g dry mass, and those at sites no. 6–8 exceeded the ISQG low value for Pb of 50 µg/g dry mass. The river bed at site no. 4 was brass yellow to deep red in colour and had significantly higher Cu concentrations than the other mine-affected sites. Out of 10 mine-affected sites studied, a significant increase in Cu was only detected at five sites: concentrations at sites no. 2, 4, and 5 exceeded the ANZECC/ARMCANZ (2000) ISQG high value (270 µg/g dry mass), and those at sites no. 3 and 7 exceeded the ISQG low value for Cu (65 µg/g dry mass).
Depending on the changes in physical, chemical, and biological process, metals can be incorporated into bottom sediments or released from sediments to the water column (Macklin et al. 1996). Among these processes, in mine-affected rivers, weathering of minerals is one of the major processes that causes metal mobility in sediments (Lacal et al. 2003). In the present study, for example, elevated Zn concentrations were observed in sediments at all sites below the mine (Fig. 5). Compared with Pb, Zn has a higher mobility in sediments due to weathering of minerals which Zn is associated (Lacal et al. 2003), therefore, remobilisation of sediment-bound Zn would be more likely to occur than for the other metals (Brooks 1980). As shown in Eq. 1, Zn-containing sulphides (e.g., sphalerite) convert into soluble Zn by oxidative weathering of pyrite due to the formation of ferric sulphate. In addition to downstream movements of sediment-bound Zn, Zn in solution can also be transported over a greater distance and incorporated into sediments by way of precipitation and coprecipitation of dissolved Zn (Brooks 1980). This might be contributing to high Zn concentrations in the river even far downstream of the mine. The exchange of Zn between water and sediment of the Molonglo River system was previously been investigated under laboratory conditions by Nicholas and Thomas (1978). Their results showed that under acidic conditions (pH 3–4), Zn was steadily released from sediments to the overlying water, and the opposite was recorded at higher pH.
Unlike Zn, Pb concentrations were only significantly elevated at the sites close to the mine (Fig. 5). This may be attributed to the formation of insoluble Pb sulphate by oxidation of sulphide minerals deposited close to the mine (Eq. 2) (Hogg 1990). As concluded by Brooks (1980), Pb in downstream sediments is therefore most likely derived from the downstream movement of contaminated sediments during high river flow.
River sediments with the highest total and individual (Cd, Cu, Pb, and Zn) metal concentrations were measured at sites closest to the mine (sites no. 2–5) (Figs. 2, 3, and 5). These sites may still contain previously deposited tailings because they had higher percentages of <63 µm particles compared with the site above the mine and sites located further downstream of the mine (Fig. 3). These factors, together with metals still being released from point and diffuse contamination sources from the mine, explain the significantly higher concentrations of Cd, Cu, Pb, and Zn at these sites.
As shown in Figs. 4 and 5, metal concentrations at site no. 6, approximately 17 km downstream from the mine, had significantly lower metal concentrations than those at site no. 5. This is attributed to dilution of river bed sediments from the inputs of uncontaminated sediments from the Ballalaba Creek (Fig. 2), the burial of previously deposited contaminated sediments with newly deposited sediments, and/or chemical processes such as chemical weathering and pH buffering occurring with increasing distance from the mine. The percentage of finely divided sediment particles at this site was significantly lower than those at the sites above this site (Fig. 3). Below the Carwoola and Foxlow floodplains, at site no. 7, approximately 32 km downstream from the mine, Cd, Cu, and Zn concentrations increased (Fig. 5). This is likely due to inputs of sediments from the erosion of river bank and/or surface runoff of metals that were previously held in floodplain sediments immediately above this site and/or the flushing of contaminated sediments from upstream of the river during floods. A similar trend was also reported by Hogg (1990) for Zn concentrations in sediments at these two sites. Hence, the floodplain may still be a potential source of metals to the river; however, further investigations are required to confirm this hypothesis.
In mine-affected rivers, downstream attenuation of metals in sediments can occur due to influxes of clean sediments from tributaries, hydrogeochemical reactions, and abrasion (Hudson-Edwards et al. 1996, 2001), chemical weathering, pH buffering, acid neutralisation (Byrne et al. 2011; Hudson-Edwards, Macklin et al. 1996) and uptake by benthic biota if present. In the present study, gradual downstream attenuation of all metal concentrations in sediments was evident (Figs. 4 and 5; Table 2) because metal concentrations decreased with increased distance from the mine.
With different uncertainties associated with previous studies, as described earlier, the following comparisons were made. A previous study undertaken in 1996 by Sloane and Norris (2003) also reported Cd, Cu, Pb, and Zn as the major metal contaminants in Molonglo river bed sediments. In addition to the present study, other studies have shown Zn to be a major metal contaminant of river sediments (Nicholas and Thomas 1978; Sparksman 1982; Weatherley et al. 1967). Brooks (1980) study, however, showed that sediments from the river close to the mine had very similar concentrations of Pb and Zn, and in some cases the Pb concentrations reported in their study were higher than Zn concentrations.
To identify the effects of the 2010 flood on Molonglo River sediment metal concentrations, the present results were compared with the study performed by Sloane and Norris (2003) before this flood. This type of comparison, however, has limited accuracy due to different uncertainties associated with sediment metal analysis. The highest recorded metal concentrations in river sediments (<100 µm fraction) by Sloane and Norris (2003) were Cd 11.8, Cu 748, Pb 2568, and Zn 3534 µg/g dry mass. Except for Zn (6818 µg/g), the highest recorded Cd (8.7 µg/g), Cu (628 µg/g), and Pb (1766 µg/g) concentrations in this study were all lower than those reported by Sloane and Norris (2003). The exceptionally high Zn concentration in sediments in the present study might possibly be due to newly deposited sediments after the input of dissolved and particulate Zn from point and diffusive mine sources during the 2010 flood. The highest recorded Pb concentrations in sediments before the flood were 2568 µg/g; this decreased to 1766 µg/g in the present study. A similar drop of Pb concentrations in sediments was also observed before (1300 µg/g) and after (350 µg/g) a severe flood that occurred in mid-October 1976 (Hogg 1990). Compared with the Sloane and Norris (2003) study, Cu concentrations recorded in the present study were also lower. This trend was also reported for Cu concentrations in the Molonglo River before and after the major flood in 1976.
Conclusions
Molonglo River sediments closest to mine had a higher percentage of finely divided silt and clay particles representing previously deposited mine tailings in the river bed. The highest concentrations of Cd, Cu, Pb, and Zn were also found at sites with the highest percentage of silt and clay in sediments. Compared with background metal concentrations, the most contaminated sites in the present study showed a strong enrichment of metals in sediments. Sediments collected from all of the sites downstream of the mine had significantly higher Cd and Zn concentrations than those at reference sites. Cu and Pb concentrations in sediments at sites closest to the mine were significantly higher than those at reference sites. Downstream attenuation of metal concentrations in sediments was evident with increasing distance from the mine. Comparisons made between the present (after the 2010 flood) and a previous study by Sloane and Norris (2003) (before flooding) showed that except for Zn, the flood may not have increased the other metal contaminants in the river. Despite remedial works completed to minimise metal contamination to the river, sediments in the river are still contaminated with metals.
References
ANZECC/ARMCANZ (2000) Australian and New Zealand guidelines for fresh and marine water quality. Australian and New Zealand Environment and Conservation Council/Agriculture and resource Management council of Australia and New Zealand
Batley GE, Simpson SL (2014) Chapter 2: Sediment sampling, sample preparation and general analysis. In: Simpson SL, Batley GE (eds) Sediment quality assessment: a practical handbook. CSIRO, Clayton South
Brooks KA (1980) Mine waste pollution of the Molonglo River: an investigation into the effect of remedial measures carried out in 1976 at Captains Flat. Chemical Laboratory, N.S.W. Dept of Mineral Resources
Byrne P, Wood PJ, Reid I (2011) The Impairment of river systems by metal mine contamination: a review including remediation options. Crit Rev Environ Sci Technol 42(19):2017–2077
Dames, Moore (1993) Final report: captains flat mine site assessment of options for further remediation. Report to N.S.W. Environment Protection Authority
Gale NL, Wixson BG (1979) Cadmium in forest ecosystems around lead smelters in Missouri. Environ Health Perspect 28:23
Graham G, Byron G, Norris RH (1986) Survival of Salmo gairdneri (rainbow trout) in the zinc polluted Molonglo River near Captains Flat, New South Wales, Australia. Bull Environ Contam Toxicol 36(1):186–191
Hogg D (1990) Evaluation of the remedial works at Captains Flat Mine. Report to the ACT Government by David Hogg Pty. Ltd. Environmental Consultants
Hudson-Edwards KA, Macklin MG, Curtis CD, Vaughan DJ (1996) Processes of formation and distribution of Pb-, Zn-, Cd-, and Cu-bearing minerals in the Tyne Basin, Northeast England: implications for metal-contaminated river systems. Environ Sci Technol 30(1):72–80
Hudson-Edwards KA, Macklin MG, Taylor MP (1999) 2000 years of sediment-borne heavy metal storage in the Yorkshire Ouse basin, NE England, UK. Hydrol Process 13(7):1087–1102
Hudson-Edwards KA, Macklin MG, Miller JR, Lechler PJ (2001) Sources, distribution and storage of heavy metals in the Rıo Pilcomayo, Bolivia. J Geochem Explor 72(3):229–250
Jacobson G, Sparksman G (1988) Acid mine drainage at Captains Flat, New South Wales. BMR J Aust Geol Geophys BJAGDT 10(4):391–393
Lacal J, da Silva MP, García R, Sevilla MT, Procopio JR, Hernández L (2003) Study of fractionation and potential mobility of metal in sludge from pyrite mining and affected river sediments: changes in mobility over time and use of artificial ageing as a tool in environmental impact assessment. Environ Pollut 124(2):291–305
Macklin M, Payne I, Preston D, Sedgwick C (1996) Review of the Porco mine tailings dam burst and associated mining waste problems, Pilcomayo basin, Bolivia. Report to the UK Overseas Development Agency, 33
Maher W, Forster S, Krikowa F, Snitch P, Chapple G, Craig P (2001) Measurement of trace elements and phosphorus in marine animal and plant tissues by low-volume microwave digestion and ICP-MS. Atomic Spectrosc 22(5):361–370
Nicholas WL, Thomas M (1978) Biological release and recycling of toxic metals from lake and river sediments. Australian Water Resource Council Technical Paper No. 33
Norris R (1986) Mine waste pollution of the Molonglo River, New South Wales and the Australian Capital Territory: effectiveness of remedial works at Captains Flat mining area. Mar Freshw Res 37(2):147–157
Simpson SL, Batley GE, Chariton AA, Stauber JL, King CK, Chapman JC, et al. (2005) Handbook for sediment quality assessment. Centre for Environmental Contaminants Research
Simpson SL, Graeme EB (2016) Sediment quality assessment: a practical guide, 2nd edn. CSRIO, NSW, Australia
Sloane P, Norris R (2003) Relationship of AUSRIVAS-based macroinvertebrate predictive model outputs to a metal pollution gradient. J North Am Benthol Soc 22(3):457–471
Sparksman G (1982) Heavy metal pollution in the Molonglo River. Bureau of Mineral, Resources, Geology and Geophysics. Record 1983/16
Taylor MP, Kesterton RG (2002) Heavy metal contamination of an arid river environment: Gruben River, Namibia. Geomorphology 42(3):311–327
Telford K, Maher W, Krikowa F, Foster S (2008) Measurement of total antimony and antimony species in mine contaminated soils by ICPMS and HPLC-ICPMS. J Environ Monit 10(1):136–140
Trueman WT (2012) True tales of the trout cod: river histories of the Murray-Darling Basin (Murrumbidgee River catchment booklet). Canberra, Murray-Darling Basin Authority
Walling D, Owens P, Carter J, Leeks G, Lewis S, Meharg A et al (2003) Storage of sediment-associated nutrients and contaminants in river channel and floodplain systems. Appl Geochem 18(2):195–220
Weatherley AH, Beevers JR, Lake PS (1967) The ecology of a zinc polluted river. In: Australian inland waters and their fauna: Eleven studies. ANU Press, Canberra
Weatherley AH, Lake PS, Rogers SC (1980) Zinc pollution and the ecology of the freshwater environment. In: Niagru OJ (ed) Zinc in the environment. Wiley, New York, pp 337–418
Acknowledgments
Richard Norris Scholarship. Funding University of Canberra Ecochemistry laboratory. We thank Chitral Siriwardana for assistance with field work and Chamoda Siriwardana and Bhagya Siriwardana for valuable assistance with labelling. We also thanks Alica Tschierschke for support to create the geographic information system map.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Marasinghe Wadige, C.P.M., Taylor, A.M., Krikowa, F. et al. Sediment Metal Concentration Survey Along the Mine-Affected Molonglo River, NSW, Australia. Arch Environ Contam Toxicol 70, 572–582 (2016). https://doi.org/10.1007/s00244-015-0259-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-015-0259-z