Abstract
An efficient biosorbent, sugarcane bagasse was used in native, HCl-treated, and Na-alginate immobilized form for the removal of Direct Violet 51 dye from aqueous solutions. Batch study was performed to optimize important process parameters, such as pH, contact time, biosorbent dose, initial dye concentration, and temperature. Removal of Direct Violet 51 was found to be favorable at pH 2 with the biosorbent dose of 0.05 g. Biosorption process was found to be exothermic in nature. Maximum dye biosorption (39.6 mg/g) was achieved by using HCl-treated biomass. The pseudo-second-order kinetic and Langmuir adsorption isotherm models showed best fitness to the experimental data. Thermodynamic study was also performed to determine the feasibility of biosorption process. Continuous mode study was performed to optimize the important process parameters, such as bed height, flow rate, and initial dye concentration for maximum removal of Direct Violet 51 dye. The higher bed height, low flow rate, and high initial dye concentration were found to be the better conditions for maximum dye biosorption (17.28 mg/g). The linearized form of the Thomas model equation fitted well to the experimental data. The bed depth service time model was used to express the effect of bed height on breakthrough curves. Characterization of biosorbent was performed by scanning electron microscopy and Fourier transform infrared (FT-IR) analysis. The FT-IR spectral analyses showed the involvement of hydroxyl, carbonyl, and carboxyl groups in biosorption process. These results indicated that sugarcane bagasse biomass could be used as a novel biosorbent for the removal of Direct Violet 51 dye from real textile and related industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Rapid industrialization has increased the concerns about ongoing deterioration of the global environment. The textile industry is playing a key role in the economy of many countries. During dyeing operation in textile industries, huge amounts of colored wastewater is produced due to low level of dye fiber fixation (El-Khaiary 2007). This colored wastewater is directly released to the water bodies, and it shows its negative effects on aquatic life by obstructing sunlight penetration and hence retards the photosynthetic activity of aquatic plants. These synthetic dyes are recalcitrant molecules that are difficult to biodegrade. People of different areas consume this wastewater for washing, bathing, and drinking (Sharma and Sobti 2000). The carcinogenic and mutagenic nature of synthetic dyes imparts many harmful effects to human beings, such as kidney dysfunction and damage to the reproductive system, central nervous system, liver, and brain (Shen et al. 2009). Therefore, it is important to remove these dyes from the wastewater before its discharge into the environment.
Scientists are performing extensive research work in search of efficient wastewater remediation technologies (Asgher and Bhatti 2012). A wide variety of physical and chemical methods for the treatment of colored wastewater include the following: photocatalytic degradation (Mahmoodi et al. 2005), coagulation (Bozdogan and Goknil 1987) membrane seperation (Wu et al. 1998), microbiological decomposition (Pearce et al. 2003) and adsorption techniques (Abdelwahab et al. 2006; Ho et al. 2005). All of these methods have different color-removal abilities, capital costs, and operating rates (Amin 2009). The adsorption process is preferred over other processes due its low cost, easy operation, flexibility, and simplicity. For the efficient application of adsorption process, the importance should not only be given to its low cost, but prime importance should be given to the selection of adsorbents with high adsorption capacity, stability, and easy availability (Crini 2006).
Agricultural waste materials are excellent substances to be used for the treatment of wastewater because these wastes are easily available and are produced worldwide (Pehlivan et al. 2013). The inadequate disposal of agricultural wastes to the environment causes aesthetic problems, so it is better to exploit these materials for the remediation of different pollutants from the environment (Raymundo et al. 2010). Among the different low-cost materials, sugarcane bagasse is proven to be an efficient biosorbent for the removal of pollutants from aqueous solutions. The growth rate of the sugar cane plant is remarkably efficient. The high annual production and nature of sugarcane bagasse has increased its attractiveness for the remedy of environmental pollution in different ways (Almazan et al. 1998; Ritter 2007; Arnaud 2008). The high biosorption capacity of sugarcane bagasse is due to the presence of macromolecules in its structure along with humic and fulvic substances, cellulose, hemicelluloses, lignin, and proteins that have carbonyl, carboxylic, amine, and hydroxyl functional groups, which show the capacity to adsorb dye molecules by the ion-exchange phenomena or by complexation (Dávila-Jiménez et al. 2005).
The biosorption capacity of agricultural byproducts can be enhanced by various physical and chemical treatments. Low mechanical strength of biosorbents, due to their low density and low rigidity, may create some difficulties in solid–liquid separation, inability to reuse of biosorbent, and development of high-pressure decrease in the column mode (Vegliò and Beolchini 1997; Vijayaraghavan and Yun 2007). Several well-known techniques are available to make biosorbents suitable for process applications. Among these, immobilization techniques, such as entrapment and cross-linking, have been found to be practical for biosorption (Vegliò and Beolchini 1997). The biosorbents can be immobilized using different immobilization matrices, such as sodium alginate (Xiangliang et al. 2005), polyacrylamide (Bai and Abraham 2003), polysulfone (Beolchini et al. 2003; Vijayaraghavan et al. 2007), and polyurethane (Hu and Reeves 1997). The results of the present study, in which the biosorbent was used in native, HCl-treated, and Na-alginate immobilized form during batch study and continuous mode experiments were performed with native form of biosorbent, show the usefulness of sugarcane bagasse for the removal of Direct Violet 51 dye from aqueous solution.
Materials and Methods
Chemicals
Analytical grade PEI ((H(NHCH2CH2)nNH2); molecular weight = 25,000 g/L) and sodium alginate used in this study were purchased from Sigma–Aldrich (St. Louis, MO, USA). All other chemicals used in this study were of analytical grade and were also procured from Sigma-Aldrich.
Preparation of Biomass
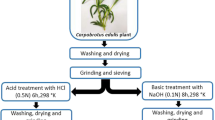
Sugarcane bagasse was collected from the local market of Faisalabad, Pakistan, to use as biosorbents in this study. The biomass was cut into small pieces and rinsed several times with distilled water to remove dust and foreign particles. The cleaned biomass was dried in sunlight and oven dried overnight at 60 °C. The dried biomasses were ground with a food processor (Moulinex, France) and sieved using Octagon sieve (OCT-DIGITAL 4527-01) to a 300-μm mesh size and stored in air tight bottle.
Preparation of Aqueous Dye Solutions
Direct Violet 51 dye was obtained from Sandal Dyestuff Industries, Faisalabad, Pakistan, and was used without further purification. Stock solution of dye was prepared by dissolving 1 g of dye in 1,000 mL of double-distilled water. The experimental solutions of different concentrations, ranging from 10 to 200 mg/L, were made by further dilutions. Standard curve was developed through the measurement of the dye solution absorbance by UV/Visible Spectrophotometer (Schimadzu, Japan). Direct Violet 51 dye was anionic in nature, and its λmax was 549 nm.
Immobilization of Biomass
Sodium-alginate (2.0 g) was dissolved in 100 mL of water by heating, and then the solution was cooled. Sugarcane bagasse biomass (1 g/100 mL) was added to each of previously described mixtures and mixed until to form one homogeneous mixture. Then the mixture was dropped into a solution of 0.1 M CaCl2 to form uniform beads of immobilized biomass. The beads were washed with distilled water and stored at 4 °C in 0.05 M CaCl2 solution (Zhang et al. 2007).
Pretreatment of Sugarcane Bagasse Biomass
Sugarcane bagasse biomass was chemically treated with HCl. For this purpose, 1 g of the biosorbent was treated with 5 % solution of HCl. The modified biomass was washed with double-distilled water and filtered. The modified biosorbent was dried in oven at 60 °C for 24 h and ground (Bhatti et al. 2009). The dried ground treated biomass was stored in an air-tight bottle.
Characterization of Sugarcane Bagasse Biomass
The chemical characteristics of sugarcane bagasse biomass were analyzed and interpreted by Bruker Tensor 27 Fourier transform infrared spectrometer with the samples prepared as KBr discs. The surface structure of sugarcane bagasse biomass was analyzed using a JEOL JMT 300 scanning electron microscope (SEM).
The point-of-zero charge (pHpzc) was determined by solid addition method (Mall et al. 2006). A series of 0.1 M KNO3 solutions (50 mL each) were prepared, and their pH was adjusted in the range of 1.0 to 12.0 by addition of 0.1 N HCl and NaOH. To each solution, 0.1 g of sugarcane bagasse was added, the suspensions were shaken manually, and the solution was kept for a period of 48 h with intermittent manual shaking. The final pH of the solution was recorded and difference between initial and final pH (∆pH) (Y-axis) was plotted against initial pH (X-axis). The point of intersection of this curve yielded point of zero charge.
Batch Experimental Program
Batch experiments were performed with native, HCl-treated, and immobilized sugarcane bagasse biomass in 250-mL Erlenmeyer conical flasks containing 50 mL of dye solution of known dye concentration in an orbital shaking incubator (PA250/25H) at 120 rpm. Optimization of important process parameters such as pH (2–9), contact time (0 to 180 min), biosorbent dose (0.05–0.3 g), initial dye concentration (10–200 mg/L), and temperature (303–333 K)—for the removal of Direct Violet 51 was performed by using classical approach. The blank solutions were also run under same conditions except the addition of biosorbent. Effect of presence of different salts (NaCl, KNO3, CaCl2, MgSO4 and AlCl3) on the biosorption of Direct Violet 51 was also investigated at different concentrations (0.1–0.5 M) of these salts in 50 mg/L of dye solution. Effect of presence of heavy-metals ions (cadmium, lead, chromium, cobalt, and copper) at different concentrations (50–250 mg/L) was also studied for the adsorptive removal of Direct Violet 51 by sugarcane bagasse biomass. Presence of surfactants was also investigated by using 1 % of different surfactants Triton X-100, CTAB, SD, and two commercial surfactants, Arial and Excel. The pH of the solution was adjusted using 0.1 M HCl and NaOH solutions. All of the experiments were performed in triplicate, and reported values are mean ± SD. After a certain time, the samples were removed, centrifugation was performed at 5,000 rpm for 20 min, and concentration of remaining dye solution was determined by using a UV–Vis spectrophotometer (Schimadzu, Japan).
The equilibrium dye uptake, qe (mg/g), was calculated using the following relationship (Eq. 1):
where C o is the initial dye concentration (mg/L), C e is the equilibrium dye concentration (mg/L), V is the volume of the solution (L), and w is the mass of the biosorbent (g).
Column Studies
Continuous biosorption experiments in a fixed-bed column were performed in a glass column (20-mm ID and 43-cm height), packed with a known quantity of sugarcane bagasse biomass. At the bottom of the column, a stainless sieve was attached followed by a layer of glass wool. A known quantity of the sugarcane bagasse biomass was packed in the column to yield the desired bed height of the adsorbent (2, 3, and 4 cm). Direct Violet 51 dye solution of known concentrations (25, 50, and 75 mg/L) at pH 2 was pumped upward through the column at a desired flow rate (1.8, 3.6, and 5.4 mL/min) controlled by a peristaltic pump (Prominent, Heidelberg, Germany). The dye solutions at the outlet of the column were collected at regular time intervals, and the concentration was measured using a double-beam ultraviolet (UV)-visible spectrophotometer (Shimadzu, Japan) at 549 nm. All of the experiments were performed at room temperature (28 ± 1 °C).
Results and Discussion
Characterization of Biosorbent
The FT-IR spectra of sugarcane in its native, HCl-treated, and immobilized dye-loaded form is presented in Fig. 1. The spectra were studied in the range of 400–4,000 cm−1. In all three forms of biosorbent, a broad band at approximately 3,340 cm−1 indicates the presence of O–H group (carboxylic acids, phenols, and alcohols) on the surface of biosorbent as in cellulose, pectin, and lignin. The presence of peak at approximately 2,900 cm−1 is due to the C–H stretching and indicates the presence of –CH and CH groups in the structure of sugarcane bagasse. The peak at approximately 1,700 cm−1 allocates the C=O stretching vibrations in native and pretreated forms. This peak was absent in immobilized from of biosorbent. This shows that immobilization of biosorbent has masked this group on the surface of sugarcane bagasse. The presence of peaks in the region of 2,370 cm−1 might be due to the presence of C≡C bonds. A new peak was observed in case of HCl-treated sugarcane bagasse in the region of 3,757 cm−1, which indicates the presence of N–H group. This shows that the treatment of biomass with acid resulted in the exposure of buried amino groups on the surface of biosorbent, which leads to the higher biosorption capacity of HCl treated biomass. Due to specific interaction between biosorbent and dye molecules, a change in the spectra was observed due to vanishing and broadening of some peaks (Fig. 1). The –OH stretching peaks in dye-loaded biosorbent absorbed at lower frequency, which confirmed the involvement of hydroxyl groups in the biosorption mechanism. The FT-IR spectra indicate the exchanging sites and functional groups on which biosorption takes place (Akar et al. 2009).
The surface features and morphological characteristics of the biosorbent were studied using an SEM. This was used to determine the particle shape and porous structure of biomass. The greater the number of pores, the greater was the biosorption of dye onto the biosorbent surface. Typical SEM photographs of free sugarcane bagasse biomass and Direct Violet 51 loaded biomass are shown in Fig. 2a, b, respectively. These photographs indicated the porous and fibrous texture of the biosorbent with high heterogeneity that could contribute to the biosorption of the dyes.
Point-of-zero charge was determined to understand the mechanism of biosorption process. Adsorption of cations is favored at pH pHpzc, whereas adsorption of anions is favored at pH pHpzc. Point-of-zero charge of sugarcane bagasse biomass was determined by solid addition method (Mall et al. 2006), and it was found to be 4.8 (Fig. 2c). This indicates that below this value, pH sugarcane bagasse acquires positive charge due to protonation of functional groups, which results in electrostatic attraction between dye anions, whereas above this pH value a negative charge exists on the surface of sugarcane bagasse biomass. Hence, the adsorption of Direct Violet 51 dye should be favorable at pH pHpzc because of the anionic nature of Direct Violet 51 dye.
Effect of pH
Medium pH is an important controlling factor in the biosorption process. It seems to affect the solution chemistry of dyes and functional groups of biosorbents (Vijayaraghavan and Yun 2008). The effect of pH on the biosorption of Direct Violet 51 by native, HCl-treated, and immobilized bagasse biomass is given in Fig. 3a. Maximum biosorption of dye with all forms of biosorbent was observed at pH 2. With an increase in pH, dye removal decreased. Pretreated biomass showed maximum biosorption potential (24.7 mg/g) among all three forms of sugarcane bagasse. Direct Violet 51 produces molecular anions in aqueous solutions. At lower pH, protonation of functional groups on the surface of biosorbent takes place due to excess H+ ions in the solution and positively charged surface of biosorbent facilitates in the attachment of dye anions to the biosorbent. At greater pH levels, the concentration of OH− ions increase in the solution, which compete to the negatively charged dye anions for the attachment to the biosorbent surface (Sadaf and Bhatti 2011).
Effect of Contact Time
The biosorption potential of sugarcane bagasse was investigated as a function of time, and the results are presented in Fig. 3b. The results indicated that biosorption of Direct Violet 51 onto bagasse is a fast process. The equilibrium was attained in 30 min in the case of native and pretreated biomass, whereas equilibrium with immobilized biomass was found to be slow. In the case of immobilized biomass, the biomass retains inside the matrix and the dye molecules suffer a mass-transfer resistance problem due to which there was a delay in the attainment of equilibrium (Guo et al. 2003). Vijayaraghavan et al. (2007) used the immobilized Corynebacterium glutamicum biomass for the removal of dye and found delay in the attainment of equilibrium.
Effect of Biosorbent Dose
The results of effect of biosorbent dose on the removal of Direct Violet 51 dye are presented in Fig. 3c. The results indicated that by increasing the biosorbent dose, the biosorption capacity (mg/g) of the biosorbent decreased. Maximum dye removal was seen by using biosorbent dose of 0.05 g. Thus, 0.05-g biosorbent dose was selected for further study. It can be attributed to the overlapping or aggregation of active sites resulting in a decrease of the total biosorbent surface area available for the attachment of dye molecules and an increase in diffusion path length (Senturk et al. 2010). Another important factor is that at high biosorbent dosage, the available dye molecules are insufficient to completely cover the available binding sites on the biosorbent, which usually results in low solute uptake (Tangaromsuk et al. 2002).
Effect of Initial Dye Concentration
The initial dye concentration provides an important driving force to overcome mass-transfer resistance between solid and aqueous phase, and equilibrium is established when the dynamic balance between dye concentration and biosorbent surface takes place (Bouberka et al. 2006). This experiment was performed to investigate the biosorption capacity of sugarcane bagasse for the removal of Direct Violet 51 dye from aqueous solution over a dye concentration range of 10–200 mg/L. It was observed that maximum biosorption capacity (39.6 mg/g) was achieved with pretreated biomass (Fig. 3d). The biosorption capacity of immobilized biomass was lower compared with the free and treated biomass. Greater biosorption capacity at greater initial dye concentrations is attributed due to the fact that at greater dye concentrations, the active sites available for biosorption become fewer compared with the moles of solute present, and hence the removal of solute is strongly dependent on the initial solute concentration (Ho and Mckay 2000).
Effect of Temperature
Temperature shows a significant effect on the biosorption process. The effect of temperature was observed over a temperature range of 303–333 K, and results are presented in Fig. 3e. The results indicated that with the increase in temperature from 303 K, the biosorption capacity decreased. Maximum biosorption capacity was obtained at 303 K with pretreated and immobilized biomass, whereas native biomass showed maximum biosorption potential at 308 K. Further increase in temperature results in decrease in dye removal. This shows that biosorption of Direct Violet 51 dye onto sugarcane bagasse is an exothermic process. The decrease in biosorption of dyes at great temperatures is due to the weakening of adsorptive forces responsible for the adsorption of dye molecules on the surface of biosorbents. This can also be due to the fact that deactivation of biosorbent active sites takes place, which leads to the decreased biosorption at greater temperatures (Asgher and Bhatti 2012).
Effect of Electrolytes
During the dyeing process in textile industries, large amounts of salts are consumed (Aksu and Balibek 2010). Thus, the concentration of salts in textile effluents is one of the important factors that control both electrostatic and nonelectrostatic interactions between the biosorbent surface and dye molecules and therefore affects biosorption capacity (Dogan et al. 2008). The effect of presence of electrolytes on the biosorption ability of the sugarcane bagasse for Direct Violet 51 was investigated using NaCl, KNO3, CaCl2, MgSO4, and AlCl3 solutions of concentrations ranging from 0.1 to 0.5 M (Fig. 4a). The results indicate that the presence of salts increases biosorption potential due because increased ionic strength increases the positive charge of the surface of biosorbent, which increases the electrostatic interaction between dye anions and biosorbent; this ultimately results in increased biosorption potential of biosorbent. This indicates that sugarcane bagasse can be used for the removal of Direct Violet 51 dye in the presence of salts.
Effect of Heavy-Metal Ions
The presence of heavy-metal ions in the dye solution also affects the biosorption capacity of the biosorbent. Different heavy metals, i.e., Cr, Cu, Co, Pb, and Cd, were used in different concentrations (50–250 mg/L). The results indicated that in the presence of Cd, Pb, and Co, the biosorption potential of bagasse increased, whereas in presence of Cr and Cu, a decrease in biosorption of dye was observed (Fig. 4b). The increase in biosorption capacity in the presence of heavy-metal ions is due to the fact that interaction between heavy metals and dye molecules result in the precipitation or aggregation of dye molecules, which decreases the solubility in the solution and enhances biosorption of dye onto the biosorbent (Haq et al. 2011). Zhou and Banks (1993) also reported the similar results. O’Mahony et al. (2002) reported that the presence of high levels of heavy-metal ions decreases the biosorption capacity of the biomass due to competition between metal ions and dye molecules.
Effect of Surfactants
Surfactants are also used in the textile industries during different operations, and hence their presence in the textile effluents also affects the biosorption potential of biosorbent. Different surfactants (1 %) were used (1 %) to determine their effect on dye removal from the solution. The results indicated that the presence of surfactants in dye solution significantly decreases biosorption capacity (Fig. 4c). This might be due to the competition between dye molecules and surfactants for attachment to the biosorbent surface (Haq et al. 2011). Brahimi-Horn et al. (1992) also observed that the presence of detergent in dye solution decrease binding capacity of the biosorbents.
Biosorption Kinetics
The kinetics of Direct Violet 51 onto sugarcane bagasse biomass was analyzed using pseudo-first-order, pseudo-second-order, and intraparticle diffusion kinetic models. The applicability of these kinetic models was determined by measuring the correlation coefficients (R 2). When the value of R 2 is high, the model is best applicable to the data.
Pseudo-first-order kinetic model is based on the fact that the change in dye concentration with respect to time is proportional to power one (Lagergren 1898). The integral form of the pseudo-first-order model generally expressed as Eq. 2:
where q e and q t are the biosorption capacity (mg/g) at equilibrium and time t, respectively, K 1 is the rate constant (L/min), and t is the contact time (min). The values of rate constant K 1, q e calculated, q e experimental, and R 2 for the biosorption of Direct Violet 51 using native, pretreated, and immobilized sugarcane bagasse biomass are listed in Table 1. By Lagergren pseudo-first-order model, a plot of log(qe–qt) versus t gives a straight line with a poor R 2. Pseudo-first-order kinetic model predicted much lower values of the equilibrium biosorption capacity (q e) compared with the experimental values. Thus, results indicate incompatibility of pseudo-first-order kinetic model with the kinetic data of Direct Violet 51. Mostly, the first-order kinetic model is not fitted well for whole data range of contact time and can be applied for the preliminary stage of adsorption mechanism (McKay and Ho 1999).
The biosorption mechanism over a complete range of the contact time is explained by the pseudo-second-order kinetic model (Ho et al. 2000). The pseudo-second-order kinetic model can be presented as Eq. 3:
where K 2 (g/mg min) is the second-order rate constant of the biosorption process. A plot between t/q t versus t gives the value of the constants K 2 (g/mg h), and q e (mg/g) can also be calculated. The second-order parameters K 2, q e calculated, q e experimental, and R 2 for biosorption of Direct Violet 51 are listed in Table 1. The results indicate that the values calculated and experimental q e values are closer to each other. The values of R 2 are much greater for native, pretreated, and immobilized biomasses. Thus, the pseudo-second-order kinetic model show the best fitness to the kinetic data, and it is more appropriate and effective than the pseudo-first-order kinetic model.
The intraparticle diffusion equation is written as follows (Eq. 4 [Weber and Morris 1963]):
where C i is the intercept that describes the boundary layer thickness, and Kpi (mg/g min1/2) is the rate constant of intraparticle diffusion. The values of K pi and C i for Direct Violet 51 are listed in Table 1. The poor value of correlation coefficient (R 2) indicates that the biosorption of Direct Violet 51 onto the sugarcane bagasse is not depended on intraparticle diffusion. It may be concluded that surface adsorption and intraparticle diffusion were concurrently operating during the biosorption of Direct Violet 51 dye onto sugarcane bagasse biomass.
Biosorption Isotherm
The biosorption isotherms give an idea of the biosorption capacity of the biosorbent (Salleh et al. 2011). To simulate the biosorption isotherm, different models, such as Freundlich, Langmuir, Temkin, and Doubinin-Radushkevich, were selected to explicate dye biosorbent interaction. The Freundlich isotherm model is valid for multilayer biosorption and is derived by assuming a heterogeneous surface with interaction between adsorbed molecules with a nonuniform distribution of heat of sorption over the surface (Freundlich 1906). Mathematically it can be expressed as Eq. 5:
where q e is the amount of dye adsorbed per unit of adsorbent at equilibrium time (mg/g), and C e is equilibrium concentration of dye in solution (mg/L). K F and n are isotherm constants where K F indicate the biosorption capacity, and n is a measure of deviation from linearity of the biosorption and used to verify types of biosorption. It is suggested that if n is equal to unity, the biosorption is linear; n less than unity indicates that biosorption is a chemical process, whereas n greater than unity is associated with a favorable biosorption (Salleh et al. 2011). The values of R 2, K F, and n are listed in Table 2. Greater values R 2 for native, pretreated, and immobilized biomass confirms the applicability of model to the equilibrium data of Direct Violet 51 dye.
The Langmuir isotherm model is valid for biosorption of a solute from a liquid solution as monolayer adsorption on a surface containing a finite number of binding sites (Langmuir 1918). The linear form of Langmuir can be written as Eq. 6:
The Langmuir constants, q m (maximum biosorption capacity) (mg/g) and b (values for Langmuir constant related to the energy of biosorption (L/mg)), are predicted from the plot between C e/q e versus C e. The results are listed in Table 2. Greater values of R 2 for native, pretreated, and immobilized biomass (0.995, 0.999, and 0.98) and close agreement between experimental and calculated biosorption capacities indicate good fitness of this model to the equilibrium data of Direct Violet 51.
The essential characteristics of the Langmuir isotherm model can be expressed in terms of dimensionless constant separation factor for equilibrium parameter, R L (Hall et al. 1966), which can be calculated as Eq. 7:
where Co is the initial dye concentration, and b is the Langmuir constant. The values of RL indicate the type of isotherm to be favorable (0 < RL < 1), unfavorable (RL > 1), irreversible (RL = 0), or linear (RL = 1). Value of RL in the present study was in the range of 0 to 1, which shows that biosorption of Direct Violet 51 onto sugarcane bagasse was a favorable process.
The Temkin isotherm model (Temkin and Pyzhev 1940) suggests an equal distribution of binding energies over the number of the exchanging sites on the surface. The distribution of these energies depends on the number of functional groups on the dye molecule and the biosorbent surface. The linear form of the Temkin isotherm model can be written a Eq. 8:
where B = RT/b; T is the absolute temperature in Kelvin; b is Temkin constant; and R is the universal gas constant (8.314 J mol−1 K−1). A is the equilibrium binding constant, and B is corresponding to the heat of sorption. These constants and R 2 values can be calculated by plotting a graph between qe and lnCe. The value of R 2 and other constants are listed in Table 2. R 2 values for the data of biosorption of Direct Violet 51 onto native, pretreated, and immobilized biomass suggest that the experimental data are better fitted to the Temkin isotherm model.
The Doubinin-Radushkevich (D-R) isotherm model is based on the fact that there is no homogeneous surface or constant biosorption potential (Doubinin and Radushkevich 1947). It is used for estimation of the porosity apparent free energy. The linear form of the D-R isotherm model can be expressed as Eq. 9:
where β is a constant corresponding to the adsorption energy; q m is the theoretical saturation capacity; and ε is the Polanyi potential calculated from Eq. 10:
where R (8.314 Jmol−1K−1) is the gas constant and T (K) is the absolute temperature.
The mean free energy of biosorption E can be defined as the free energy change when one mole of ion is transferred from infinity in solution to the biosorbent. E can be calculated from the β value by the following relation (Eq. 11 [Kundu and Gupta 2006] ):
The value of this parameter can give information about biosorption mechanism. When one mole of ions is transferred, the value range 1–8 kJ/mol indicates physical adsorption (Onyango et al. 2004). A value of E between 8 and 16 kJ/mol indicates the adsorption process followed by ion-exchange mechanism (Helfferich 1962), whereas its value in the range of 20–40 kJ/mol is indicative of chemical adsorption (Tahir and Rauf 2006). Thus, here it seems that chemical adsorption mechanism is involved in the case of native and pretreated biomass, whereas the physical-adsorption mechanism is involved in the case of biosorption of dye molecules onto immobilized biomass (Table 2). Values of R 2 correlation coefficient for native, pretreated, and immobilized biomass indicate low fitness of the D-R model to the experimental data.
Thermodynamic Studies
Thermodynamic parameters, such as Gibbs free energy change (ΔG), enthalpy change (ΔH), and entropy change (ΔS), were calculated from the thermal data obtained from the biosorption of Direct Violet 51 onto sugarcane bagasse biomass. The thermodynamic parameters can be calculated using equations Eqs. 12 and 13:
Where K d = qe/Ce; R is the gas constant (8.314 J/mol K); and T is the absolute temperature. Thus. it can also be written as Eq. 14:
The values of ΔH and ΔS were determined from the slope and intercept of the Van’t Hoff graph and are listed in Table 3. The biosorption of Direct Violet 51 dye onto native, pretreated, and immobilized biomass is an exothermic reaction, which is also confirmed by negative values of ΔH. The negative values of ΔS suggest the decrease in randomness at the solid/solution interface during the biosorption of Direct Violet 51 dye onto sugarcane bagasse biomass.
Column Study
The biosorption of Direct Violet 51 dye onto sugarcane bagasse in fixed-bed systems was investigated as a function of bed height, flow rate, and initial dye concentration, and the results are presented in the form of breakthrough curves. Effluent volume (Veff) can be calculated as Eq. 15:
where ttotal and F are the total flow time (min) and volumetric flow rate (mL/min), respectively. Breakthrough capacity Q0.5 (at 50 % or Ct/Co = 0.5) expressed in mg of dye adsorbed per gram of biosorbent was calculated by the following equation (Eq. 16):
Effect of Bed Height
Bed height is an important process parameter for the removal of dye in continuous mode. The breakthrough curves at different bed heights was checked by varying the bed height from 2 cm to 4 cm keeping the flow rate and initial dye concentration constant (Fig. 5a). The results indicated that an increase in bed height significantly enhanced dye removal from 13.2 to 16.2 mg/g, which is due to the availability of more binding sites for the attachment of dye molecules (Lezehari et al. 2012). The breakthrough and exhaustion times also increased with increased bed height. The shorter breakthrough time at lower bed height is due to axial dispersion, which is the governing mechanism for mass transfer, which indicates that dye molecules don’t have enough time to diffuse into the whole biosorbent (Uddin et al. 2009). These trends indicate that beds of an increased height may be required for better column performance.
Effect of Flow Rate
The effect of flow rate was investigated by varying the flow rate from 1.8 to 5.4 mL/min, and the results are shown in Fig. 5b. The results clearly demonstrate a decrease in biosorption potential of sugarcane bagasse by increasing the flow rate at constant bed height and initial dye concentration. The biosorption of Direct Violet 51 dye decreased from 16.2 to 10.8 mg/g by increasing the flow rate from 1.8 to 5.4 mL/min. This might be due to shorter residence time of the dye solution in the column at higher flow rates and dye solution leaving the column before attainment of the equilibrium point (Ghorai and Pant 2005).
Effect of Initial Dye Concentration
Initial dye concentration is an important parameter to determine the capacity of biosorbent for the removal of dye. Experiments were performed to determine the effect of dye concentration on the biosorption potential of bagasse by changing the concentration of Direct Violet 51 from 25 to 75 mg/L, and results are presented in Fig. 5c. The results showed an increase in dye removal at higher initial dye concentrations. The maximum dye biosorption was 17.28 mg/g at an initial dye concentration of 75 mg/L. The major driving force for biosorption is the concentration difference between the dye on the biosorbent and the dye in the solution (Aksu and Gonen 2003; Vijayaraghavan and Prabu 2006). The maximum biosorption of Direct Violet 51 with native biomass in continuous mode is lower (17.28 mg/g) then in the batch mode study (36.2 mg/g) because of the decrease in effective surface area of sugarcane bagasse in packed columns than that in stirred-batch vessels (Sadaf et al. 2013).
Application of Thomas Model
The Thomas model (Thomas 1944), is derived from the assumption that the rate driving force obeys second-order reversible reaction kinetics. This model also assumes a constant separation factor, but it is applicable to either favorable or unfavorable isotherms. The linearized form of the Thomas model can be expressed as Eq. 17:
where k Th (mL/min mg) is the Thomas rate constant; q o (mg/g) is the equilibrium dye uptake per g of the biosorbent; C o (mg/L) is the inlet dye concentration; C t (mg/L) is the outlet concentration at time t; W (g) is the mass of biosorbent; Q (mL/min) is the flow rate; and ttotal (min) stands for flow time.
The column data were fitted to the Thomas model to determine the Thomas rate constant (K Th) and maximum solid-phase concentration (q o). From Table 4, it is seen that values of determined R 2 range from 0.959 to 0.997. Results indicate that q o increased significantly with increase in bed height and initial dye concentration, but K Th decreased, and an opposite trend was seen in the case of flow rate. With the increase in flow rate, the value of q o decreased, but the value of K Th increased.
Application of Bed Depth Service Time Model
The bed depth service time (BDST) approach is based on the equation of Bohart and Adam, equation, and it is a widely used model (Mukhopadhyay et al. 2008). The BDST model is based on surface reaction rate theory. It gives an idea of the efficiency of the column under constant operating conditions for achieving a desired breakthrough level. In fixed-bed systems, the main design criterion is to predict how long the biosorbent will be able to sustain removing a specific amount of impurity from solution before regeneration is needed. This period of time is called the “service time of the bed.” BDST is a simple model for predicting the relationship between bed height (Z) and service time (t) in terms of process concentrations and biosorption parameters. Hutchins proposed a linear relationship between bed height and service time given in Eq. 18:
where C o is the initial dye concentration (mg/L); C b is the breakthrough dye concentration (mg/L); U is the linear velocity (cm/min); No is the biosorption capacity of bed (mg/L); ka is the rate constant in the BDST model (L/mg/min); t is the time (min); and Z is the bed height (cm) of the column. Eq. (18) can be re written in the form of a straight line (Eqs. 19 through 21).
where
and
From the slope and intercept of these fitted BDST equations, the BDST parameters, namely, the biosorption rate constant (ka), sorption capacity (No), and critical bed depth (Zo) were calculated and are listed in Table 5. The good values of the R 2 showed that the variation of the service time with bed depth is highly linear for all of the systems, thus indicating the validity of the BDST model when applied to the continuous-column studies.
Conclusion
This study highlighted the feasibility of sugarcane bagasse biomass for the removal of Direct Violet 51 dye from aqueous solution. Sugarcane bagasse was found to be a promising biosorbent with a noticeable biosorption capacity. Biosorption of Direct Violet 51 dye onto sugarcane bagasse was rapid and exothermic in nature. Maximum dye removal with native, HCl-treated, and immobilized biomasses was 36.17, 39.6, and 21.9 mg/g, respectively. Experimental data showed better agreement with pseudo-second-order kinetic model and Langmuir adsorption isotherm model. Continuous-mode experiments were also performed, and maximum bed height and initial dye concentration were better conditions for maximum dye removal in the continuous-mode study. The results showed that sugarcane bagasse biomass could be used as a novel biosorbent for the removal of Direct Violet 51 dye from real textile and related industries.
References
Abdelwahab O, El Nemr A, El-Sikaily A, Khaled A (2006) Biosorption of Direct Yellow 12 from aqueous solution by marine green algae Ulva lactuca. Chem Ecol 22:253–266
Akar T, Anilan B, Gorgulu A, Akar ST (2009) Assessment of cationic dye biosorption characteristics of untreated and non-conventional biomass: pyracantha coccinea berries. J Hazard Mater 168:1302–1309
Aksu Z, Balibek E (2010) Effect of salinity on metal-complex dye biosorption by Rhizopus arrhizus. J Environ Manag 91:1546–1555
Aksu Z, Gonen F (2003) Biosorption of phenol by immobilized activated sludge in a continuous packed bed: prediction of breakthrough curves. Proc Biochem 39:599–613
Almazan O, Gonzalez L, Galvez L (1998) Maurice Paturau Memorial Lecture, Keynote Address, The sugar cane, its by-products and co-products. In: Proceedings of the Third Annual Meeting of Agricultural Sciences, University of Mauritus, Reduit, Mauritus
Amin NK (2009) Removal of direct blue-106 dye from aqueous solution using new activated carbons developed from pomegranate peel: adsorption equilibrium and kinetics. J Hazard Mater 165:52–62
Arnaud CH (2008) Sweet success. Chem Eng News 86(18):44–45
Asgher M, Bhatti HN (2012) Evaluation of thermodynamics and effect of chemical treatments on sorption potential of citrus waste biomass for removal of anionic dyes from aqueous solutions. Ecol Eng 38:79–85
Bai RS, Abraham T (2003) Studies on chromium (VI) adsorption desorption using immobilized fungal biomass. Bioresour Technol 87:17–26
Beolchini F, Pagnanelli F, Toro L, Veglio F (2003) Biosorption of copper by Sphaerotilus natans immobilized in polysulfone matrix: equilibrium and kinetics analysis. Hydrometallurgy 70:101–112
Bhatti HN, Khalid R, Hanif MA (2009) Dynamic biosorption of Zn(II) and Cu(II) using pretreated Rosa gruss an teplitz (red rose) distillation sludge. Chem Eng J 148:434–443
Bouberka Z, Khenifi A, Benderdouche N, Derriche Z (2006) Removal of Supranol Yellow 4GL by adsorption onto Cr-intercalated montmorillonite. J Hazard Mater 133:154–161
Bozdogan A, Goknil H (1987) The removal of the color of textile dyes in wastewater by the use of recycled coagulant, MU Fen. Billimeri Dergisi Sayi 4:83–90
Brahimi-Horn MC, Lim KK, Liany SL, Mou DG (1992) Binding of textile azo dyes by Myrothecium verrucaria Orange II 10B (blue) and RS (red) azo dye uptake for textile wastewater decolourization. J Ind Microbiol 10:245–261
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97:1061–1085
Dávila-Jiménez MM, Elizalde-González MP, Peláez-Cid AA (2005) Adsorption interaction between natural adsorbents and textile dyes in aqueous solution. Colloid Surf A 254:107–114
Dogan M, Abak H, Alkan M (2008) Biosorption of methylene blue from aqueous solutions by hazelnut shells: equilibrium, parameters and isotherms. Water Air Soil Pollut 192:141–153
Doubinin MM, Radushkevich LV (1947) Proceedings of the Academy of Sciences of the USSR. Phys Chem 55:327–329
El-Khaiary MI (2007) Kinetics and mechanism of adsorption of methylene blue from aqueous solution by nitric acid treated water-hyacinth. J Hazard Mater 147:28–36
Freundlich HMF (1906) Ober dies adsorption in losungen. J Phys Chem 57:385–470
Ghorai S, Pant KK (2005) Equilibrium, kinetics and breakthrough studies for adsorption of fluoride on activated alumina. Sep Purif Technol 42:265–271
Guo B, Hong L, Jiang HX (2003) Macroporous poly (calcium acrylatedivinyle/benzene) Bead—A selective orthophosphate sorbent. Ind Eng Chem Res 42:5559–5567
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore and solid diffusion kinetics in fixed bed adsorption under constant pattern conditions. IEC Fundam 5:212–223
Haq I, Bhatti HN, Asgher M (2011) Removal of Solar Red BA textile dye from aqueous solution by low cost barley husk: equilibrium, kinetic and thermodynamic study. Can J Chem Eng 89:593–600
Helfferich F (1962) Ion exchange. McGraw-Hill, New York
Ho YS, McKay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res 34:735–742
Ho YS, Mckay G, Wase DAJ, Foster CF (2000) Study on the sorption of divalent metal ions onto peat. Adsorpt Sci Technol 18:639–650
Ho YS, Chiang TH, Hsueh YM (2005) Removal of basic dye from aqueous solution using tree fern as a biosorbent. Process Biochem 40:119–124
Hu MZC, Reeves M (1997) Biosorption of uranium by Pseudomonas aeruginosa strain CSU immobilized in a novel matrix. Biotechnol Prog 13:60–70
Kundu S, Gupta AK (2006) Adsorptive removal of As (III) from aqueous solution using iron oxide coated cement (IOCC): evaluation of kinetic, equilibrium and thermodynamic models. Sep Purif Technol 51:165–172
Lagergren S (1898) Zur theorie der sogenannten adsorption gelster stoffe. Kungliga Svenska Vetenskapsakademiens Handlingar 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lezehari M, Baudu M, Bouras O, Basly JP (2012) Fixed-bed column studies of pentachlorophenol removal by use of alginate-encapsulated pillared clay microbeads. J Colloid Interface Sci 379(1):101–106
Mahmoodi NM, Arami M, Limaee NY, Tabrizi NS (2005) Decolorization and aromatic ring degradation kinetics of Direct Red 80 by UV oxidation in the presence of hydrogen peroxide utilizing TiO2 as a photocatalyst. Chem Eng 112:191–196
Mall ID, Shrivastava VC, Kumar GVA, Mishra IM (2006) Characterization and utilization of mesoporous fertilizer plant waste carbon for adsorptive removal of dyes from aqueous solution. Colloids Surf A Physicochem Eng Asp 278(1–3):175–187
McKay G, Ho YS (1999) The sorption of lead(II) on peat. Water Res 33:578–584
Mukhopadhyay M, Noronha SB, Suraishkumar GK (2008) Copper biosorption in a column of pretreated Aspergillus niger biomass. Chem Eng J 144:386–390
O’Mahony T, Guibal E, Tobin JM (2002) Reactive dye biosorption by Rhizopus arrhizus biomass. Enzyme Microb Technol 31:456–463
Onyango MS, Kojima Y, Aoyi O, Bernardo EC, Matsuda H (2004) Adsorption equilibrium modeling and solution chemistry dependence of fluoride removal from water by trivalent-cation-exchanged zeolite F-9. J Colloid Interface Sci 279:341–350
Pearce CL, Lloyd JR, Guthrie JT (2003) The removal of colour from textile wastewater using whole bacterial cells: a review. Dyes Pigments 58:179–196
Pehlivan E, Tran HT, Ouédraogo WKI, Schmidt C, Zachmann D, Bahadir M (2013) Sugarcane bagasse treated with hydrous ferric oxide as a potential adsorbent for the removal of As(V) from aqueous solutions. Food Chem 138:133–138
Raymundo AS, Zanarotto R, Belisário M, Pereira MG, Ribeiro JN, Ribeiro AVFN (2010) Evaluation of sugar-cane bagasse as bioadsorbent in the textile wastewater treatment contaminated with carcinogenic Congo Red dye. Braz Arch Biol Technol 53(4):931–938
Ritter SK (2007) Biofuel bonanza. Chem Eng News 85(26):15–24
Sadaf S, Bhatti HN (2011) Biosorption of Foron turquoise SBLN using mixed biomass of white rot fungi from synthetic effluents. Afr J Biotechnol 10(62):13548–13554
Sadaf S, Bhatti HN, Ali S, Rehman K (2013) Removal of Indosol Turquoise FBL dye from aqueous solution by bagasse, a low cost agricultural waste: batch and column study. Desalin Water Treat. doi:10.1080/19443994.2013.780985
Salleh MAM, Mahmoud DK, Karim WA, Idris A (2011) Cationic and anionic dye adsorption by agricultural solid wastes: a comprehensive review. Desalination 280(1–3):1–13
Senturk HB, Ozdes D, Duran C (2010) Biosorption of Rhodamine 6G from aqueous solutions onto almond shell (Prunus dulcis) as a low cost biosorbent. Desalination 252:81–87
Sharma MK, Sobti RC (2000) Rec effect of certain textile dyes in Bacillus subtilis. Mutat Res, Genet Toxicol Environ Mutagen 465:27–38
Shen D, Fan J, Zhou W, Gao B, Yue Q, Kang Q (2009) Adsorption kinetics and isotherm of anionic dyes onto organo-bentonite from single and multisolute systems. J Hazard Mater 172:99–107
Tahir SS, Rauf N (2006) Removal of cationic dye from aqueous solutions by adsorption onto bentonite clay. Chemosphere 63:1842–1848
Tangaromsuk J, Pokethitiyook P, Kruatrachue M, Upatham ES (2002) Cadmium biosorption by Sphingomonas paucimobilis biomass. Bioreour Technol 85:103–105
Temkin MJ, Pyzhev V (1940) Recent modifiications to Langmuir isotherms. Acta Physiochim USSR 12:217–222
Thomas HC (1944) Heterogeneous ion exchange in a flowing system. J Am Chem Soc 66:1466–1664
Uddin MT, Rukanuzzaman M, Khan MMR, Islam MA (2009) Adsorption of methylene blue from aqueous solution by jackfruit (Artocarpus heteropyllus) leaf powder: a fixed-bed column study. J Environ Manag 90:3443–3450
Vegliò F, Beolchini F (1997) Removal of metals by biosorption: a review. Hydrometallurgy 44:301–316
Vijayaraghavan K, Prabu D (2006) Potential of Sargassum wightii biomass for copper(II) removal from aqueous solutions: application of different mathematical models to batch and continuous biosorption data. J Hazard Mater 137:558–564
Vijayaraghavan K, Yun YS (2007) Chemical modification and immobilization of Corynebacterium glutamicum for biosorption of Reactive black 5 from aqueous solution. Ind Eng Chem Res 46:608–617
Vijayaraghavan K, Yun YS (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291
Vijayaraghavan K, Han MH, Choi SC, Yun YS (2007) Biosorption of Reactive Black 5 by Corynebacterium glutamicum biomass immobilized in alginate and polysulfone matrices. Chemosphere 68:1838–1845
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanity Eng Div Am Soc Civ Eng 89:31–59
Wu J, Eiteman MA, Law SE (1998) Evaluation of membrane filtration and ozonation processes for treatment of reactive dye wastewater. J Environ Eng 124:272–277
Xiangliang P, Jianlong W, Daoyong Z (2005) Biosorption of Pb(II) by Pleurotus ostreatus immobilized in calcium alginate gel. Process Biochem 40:2799–2803
Zhang L-S, Wu W-Z, Wang J-J (2007) Immobilization of activated sludge using improved polyvinyl alcohol (PVA) gel. J Environ Sci 19:1293–1297
Zhou LL, Banks CJ (1993) Mechanism of humic acid color removal from natural waters by fungal biomass biosorption. Chemosphere 27:607–620
Acknowledgments
The authors are thankful to Greater Education Commission of Pakistan for financial assistance under Project No. 20-159/R7D/09/1841 and the Indigenous Ph.D. Fellowship Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadaf, S., Bhatti, H.N., Nausheen, S. et al. Potential Use of Low-Cost Lignocellulosic Waste for the Removal of Direct Violet 51 from Aqueous Solution: Equilibrium and Breakthrough Studies. Arch Environ Contam Toxicol 66, 557–571 (2014). https://doi.org/10.1007/s00244-013-9992-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-013-9992-3