Abstract
Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) are two kinds of emerging contaminants most studied in recent years. However, there is limited information about their combined toxicity to aquatic organisms. In the present study, the single and combined toxicity of PFOA and PFOS to zebrafish (Danio rerio) embryos were investigated. PFOS was more toxic than PFOA for the single toxicity. In four mixtures, PFOS and PFOA showed complex interactive effects that changed from additive to synergistic effect, then to antagonistic effect, and at last turnover to synergic effect again, with increased molar ratios of PFOS. Neither the concentration-addition model nor the independent-action model could predict the combined effects when strong interactive effects existed. Although the interactive effects of PFOS and PFOA affected their combined toxicity, the trend of mixture toxicity still showed an increase with increasing molar ratios of PFOS in the mixture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Perfluorinated compounds (PFCs) are a large class of man-made compounds and used widely in commercial and industrial applications as surfactants, paper and textile coatings, and food packaging (Kissa 2001). Because of their worldwide application, PFCs have been released to the environment all over the world by various ways during manufacture, distribution, use, and disposal processes (Paul et al. 2009). Recently, numerous studies have showed that PFCs exist in nearly all environmental matrices, wildlife, and humans around the world (Giesy and Kannan 2002; Kannan et al. 2005; Houde et al. 2006; Fromme et al. 2009; Butt et al. 2010). Among them, perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) are two typical and predominant PFCs, and they are of greatest concern due to their persistence, potential bioaccumulation, and toxicity to humans and animals.
To assess health impacts of PFOS and PFOA, mammalian toxicity has been assessed on rodents, nonhuman primates, and human cell lines (Lau et al. 2007; Andersen et al. 2008). In addition, toxicological studies have been performed on aquatic organisms, such as algae, water flea, frog, and fish, to evaluate their ecological risk (Ankley et al. 2004; Liu et al. 2008; Li 2009; Huang et al. 2010; Jeon et al. 2010; Ding et al. 2012a, b). However, most of the toxicological studies available are focused on assessing the single toxicity of PFOS and PFOA, and there are few reports on their mixture toxicity with each other or with other pollutants (Jernbro et al. 2007; Hu and Hu 2009; Watanabe et al. 2009; Wei et al. 2009; Rodea-Palomares et al. 2012). In the real environment and organisms, PFOS and PFOA have often been shown to commonly coexist (Houde et al. 2006; Suja et al. 2009). Therefore, it is necessary to test their combined toxicity to better assess their ecological risk and human health risk.
To determine mixture toxicity, Hu and Hu (2009) investigated combined effects of PFOS and PFOA on hepatoma Hep G2 cells. The study showed that the combined effect of PFOA and PFOS was a summation effect that was neither synergistic nor antagonistic. Wei et al. (2009) tested the combined effects of six polyfluorinated and perfluorinated compounds, including PFOS and PFOA, on primary cultivated hepatocytes from rare minnows using a custom cDNA microarray. The study showed that mixtures, as well as individual compounds, consistently regulated a particular gene set, which suggests that these conserved genes may play a central role in the toxicity mediated by PFCs. Furthermore, certain genes were regulated by the mixture, whereas they were not affected by the individual substances. Rodea-Palomares et al. (2012) examined toxicological interactions of PFOA and PFOS using a combination-index method. PFOA and PFOS showed an antagonistic interaction at the whole range of effect levels. Therefore, PFOS and PFOA may have complicated toxicological interactions and thus have different effects on different organisms.
Due to the temporal and spatial variability of the mixture composition, a direct effects measurement is, although desirable, not feasible in most cases. When the mixture components are fixed, prediction of mixture toxicity from the toxicity of the individual compounds is a promising alternative. Several approaches for the prediction of mixture toxicity have been reported (Rider and LeBlanc 2005). Among them, concentration addition (CA) and independent action (IA) are mostly used for the predictive assessment of combination effects.
Because the CA and IA models assume that no interactions are present in the analyzed mixtures, they might overestimate or underestimate the combined toxic effects when interactions exist. To describe and quantify such deviations, many methods have been introduced, such as toxic unit summation (TU), additivity index, and mixture toxicity index (MTI) (Altenburger et al. 2003; Koutsaftis and Aoyama 2007). Among them, MTI is a popular method for mixture toxicity assessment. Therefore, in the present study, the single and mixture toxicity of PFOA and PFOS were first tested on zebrafish (Danio rerio) embryos, and then mixture toxicity was predicted by CA and IA models and assessed by MTI.
Materials and Methods
Test Chemicals
Salts of PFOS and PFOA were used in the present study to test the toxicity of PFOS and PFOA. PFOS potassium salt (PFOSK; CAS no. 2795-39-3, purity 98 %) and ammonium perfluorooctanoate (CAS no. 3825-26-1, purity 98 %) were purchased from Sigma-Aldrich. The chemicals were dissolved in reconstituted water for the tested concentrations, and no solvents were used. The stock solutions were kept at 26 °C for use.
Zebrafish Maintenance and Embryo Collection
Adult zebrafish (D. rerio) maintenance and embryo collection were performed according to the guide in the zebrafish book (Westerfield 2000). Briefly, adult zebrafish were kept in aerated and biologically filtered reconstituted freshwater at 26 ± 1 °C with a photoperiod of 14 h of light to 10 h of dark. Water was totally renewed, and aquaria were cleaned each week. The fish were fed twice daily with either the zebrafish diet (Zeigler, Aquatic Habitats, Apopka, FL) or live Artemia (Jiahong Feed Co., Tianjin, China).
The day before a test, male and female zebrafish, at a ratio of 1:1, were placed in spawning tanks before the onset of darkness. Mating, spawning, and fertilization take place within 30 minutes after light onset in the morning. Eggs were collected from spawn traps and washed with clean water (Organisation for Economic Co-operation and Development 1992). Unfertilized or abnormal eggs were removed under a stereomicroscope.
Toxicity Bioassays
For a toxicity test, six exposure concentrations were performed with three replicates. Twenty normally fertilized eggs/exposure concentration were divided into a 24-well plate with 1 embryo/well containing 2 mL test solution. The remaining four wells were filled with control water and fertilized eggs used as the control. An embryo was considered dead when 1 of 4 end points (i.e., coagulation of the embryo, nondetachment of the tail, nonformation of somites, and nondetection of the heart beat) was observed. The survival rates were monitored and documented at 72 and 96 h postfertilization (hpf). The test solutions were half renewed every 24 h.
The mixture toxicity of PFOS and PFOA was tested with fixed mixture ratios (1:1, 1:3, 1:6, and 1:10) of individual chemicals. Although the mixture ratio was kept constant, the total concentration of the mixture was varied so that a complete concentration–response relationship of the mixture could be determined experimentally.
Data Analysis
The concentration–response relationships of the individual chemicals and their mixtures were fitted using a general best-fit method (Scholze et al. 2001). A set of ten different two or three-parametric nonlinear regression models (including probit, logit, and Weibull models) were chosen. For each individual set of data, the best-fitting model was chosen based on the residual sum of squares and adjusted R-square. The observation-based 95 % confidence limits of dose–response curves were calculated according to the study of Zhu et al. (2009). The calculations were performed using Origin 8.0 and Matlab 7.1.0 software.
Prediction and Assessment of Mixture Toxicity
The CA and IA models were used to predict mixture toxicity. For calculation of the effect concentrations by CA, Eq. 1 was used as follows (Eq. 1):
where EC x,i and EC x,mix are the individual concentration and the total concentration of the mixture provoking x effect, respectively, and p i denotes the fraction of component i in the mixture.
Equation 2 was used as a starting point to calculate the mixture effects according to IA. The concentration–response relationships F i of the individual components (i = 1, . . ., n) were used to calculate their effects E(c i ):
The MTI method was chosen to assess the interactions in the mixture toxicity. First, TU of component i, TU i , was calculated as \( TU_{i} = \frac{{c_{i} }}{{EC_{50,i} }} \), where c i and EC 50,i are concentration and EC 50 of component i, respectively. Then M and M 0 were calculated as \( M = \sum {TU_{i} } \), and \( M_{0} = \frac{M}{{\hbox{max} (TU_{i} )}} \), where max(TU i ) is the maximum value of TU i . Finally, MTI was determined as \( MTI = 1 - \frac{\log M}{{\log M_{0} }} \).
When the value of MTI is <0, mixture potency is defined as antagonism; when it equals 0, there is no addition (IA); when it is >0 but <1, mixture potency is regarded as partial addition; when it equals 1, mixture potency is defined as addition; and when it is >1, mixture potency is defined as synergism.
Results and Discussion
Single Toxicity of PFOS and PFOA
According to the 10 nonlinear regression models, concentration–response relationships for single toxicity of PFOS and PFOA on zebrafish embryos were determined. On the basis of the residual sum of squares and adjusted R-squares of the models, best-fit models were chosen for PFOS and PFOA. The best-fit models for the concentration–response relationships of PFOA and PFOS were the Generalized Logit I model and the Aranda–Ordaz model, respectively. Model parameters and the fitted LC50 values are listed in Table 1. For the single toxicity of PFOA, the 72- and 96-h LC50 values were determined to be 1.448 and 0.896 mM, respectively. The obtained 72- and 96-h LC50 values for PFOS were 0.102 (54.9 mg/L) and 0.101 mM (54.4 mg/L), respectively.
For the single toxicity of PFOS, Shi et al. (2008) exposed 4-hpf zebrafish embryos to 0.1, 0.5, 1, 3, and 5 mg/L PFOS until 132 hpf. Before 84 hpf, there was no significant difference in the percentage of survivorship in any of the exposed groups compared with the control group. At PFOS concentrations of 0.1 and 0.5 mg/L, no significant increase in mortality was observed over the whole exposure time, whereas a significant increase in mortality was observed at PFOS concentrations of 1, 3, and 5 mg/L at 132 hpf. Huang et al. (2010) also tested the single toxicity of PFOS to zebrafish embryos from 6 to 120 hpf. It was reported that PFOS ≥1.0 mg/L was lethal to embryos, whereas embryos exposed to 0.25 and 0.5 mg/L PFOS showed no increased mortality over the exposure time. The calculated LC50 value of PFOS at 120 hpf was 2.20 mg/L. These two previous studies showed more lethal effects of PFOS to zebrafish embryos. Differences between these studies could be related to different PFOS chemicals and solvent used. The previous studies both used PFOS for exposure and dimethylsulfoxide as a solvent, whereas this study used PFOSK for exposure without the use of solvents.
Mixture Toxicity of PFOS and PFOA
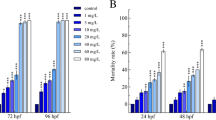
For mixture M1 with a c PFOS:c PFOA (mg/L:mg/L) ratio of 1:10, the combined toxicity was best characterized by the Generalized Logit I model (Fig. 1). The model parameters and the fitted LC50 values are also listed in Table 1. From the model, the 72- and 96-h LC50,mix values were calculated to be 0.733 and 0.628 mM, respectively.
The CA and IA models were both used to predict the mixture toxicity of PFOS and PFOA based on the effects of the individual compounds. The observed and predicted mixture toxicity is presented in Fig. 1. It could be seen that the predictions of the CA and IA models all fell in the range of 95 % confidence limit of the dose–response estimation, although there were some deviations. For 72-h mortality, the predictions of the CA model agreed well with the observed effects. The predictions of the IA model also agreed with the observed effects at lower concentrations, whereas it underestimated the combined effects at greater concentrations. In general, the CA and IA models were used to predict the mixture toxicity of compounds with similar or different modes of actions, respectively. The results of M1 might suggest that PFOS and PFOA act on similar modes of action at 72 h of exposure. For 96-h mortality, both models slightly overestimated the observed mixture toxicity for most of the concentration range. At greater concentrations, the IA model corresponded with the observed experimental data, which might suggest that PFOS and PFOA have different modes of actions at greater concentrations after 96 h of exposure.
Table 1 also lists MTI values for M1. Because the MTI for the 72-h toxicity tends toward 1.00, PFOS and PFOA show an additive effect after 72 h of exposure. For the 96-h toxicity, MTI is 0.81, suggesting that PFOS and PFOA have a partial additive effect. Therefore, the CA model could also predict the combined toxicity at 96 h for M1 with some deviations.
For mixture M2 with a c PFOS:c PFOA (mg/L:mg/L) ratio of 1:6, the joint toxicity was also best fitted by the Generalized Logit I model (Fig. 2). Based on the fitted model, 72- and 96-h LC50,mix values were determined to be 0.556 and 0.409 mM, respectively.
For the mixture toxicity of M2, the predictions of the CA and IA models all fell in the range of the 95 % confidence limit of the dose–response estimation, but the predictions of the IA model tended to be closer to the lower confidence bounds at greater concentrations. It could be seen that the IA model underestimated the observed mixture toxicity of M2 at greater concentrations after 72 and 96 h of exposure, whereas the CA model performed better than the IA model for the effects at 72 and 96 h. This might suggest that PFOS and PFOA showed more or less similar modes of actions when the c PFOS:c PFOA ratio equaled 1:6.
As the MTI value for the 72-h toxicity of M2 is 1.05, PFOS and PFOA mainly show an additive effect after 72 h of exposure. For the 96-h toxicity, MTI increased to 1.21, indicating that PFOS and PFOA tend to have a weak synergistic effect. Therefore, the CA model slightly underestimates the combined toxicity at 96 h.
When Hu and Hu (2009) investigated the effects of PFOS and PFOA on hepatoma Hep G2 cells, they found that cells exposed to a mixture of PFOA and PFOS and individual compounds did not show a significant difference on the apoptotic rate and then suggested that the combined effect of the two compounds was an additive effect. These findings coincide with part of the results of M1 and M2 that PFOS and PFOA acted independently and the combined effect was the sum of the effects of the individual compounds.
For mixture M3 with a c PFOS:c PFOA (mg/L:mg/L) ratio of 1:3, the joint toxicity was best fitted by the Aranda–Ordaz model (Fig. 3), which gave 72- and 96-h LC50,mix values of 0.568 and 0.561 mM, respectively. For prediction of mixture toxicity, the CA and IA models both overestimated the combined effects at 72 and 96 h of exposure with the predictions above the upper bounds of the 95 % confidence limit of the dose–response estimation. Because the experimental data did not correspond with the prediction of the CA and IA models, PFOS and PFOA might have complex interactions in the mixture.
For M3, the MTI values for 72- and 96-h toxicity were −0.67 and −0.42, respectively. As the values were both <0, PFOS and PFOA show an antagonistic interaction. Therefore, the CA and IA models overestimated the combined effects of M3. This result agreed with the results of Rodea-Palomares et al. (2012), which indicated that PFOA and PFOS had an antagonistic interaction at the whole range of effect levels on bioluminescent cyanobacterium Anabaena CPB4337.
For mixture M4 with a c PFOS:c PFOA (mg/L:mg/L) ratio of 1:1, the joint toxicity was again best fitted by the Generalized Logit I model (Fig. 4). From the fitted model, 72- and 96-h LC50,mix values of M4 were 0.164 and 0.081 mM, respectively. For prediction of 72-h mixture toxicity, the CA and IA models both underestimated the combined effects at greater concentrations. However, they greatly underestimated 96-h mixture toxicity for all of the concentration range.
The MTI values for 72- and 96-h toxicity of M4 were 3.99 and 7.80, respectively. The values were both >1, which suggests that PFOS and PFOA have a synergistic effect for M4. Therefore, the CA and IA models underestimated the combined effects at 72 and 96 h of exposure. Wei et al. (2009) assessed the combined effects of PFOS and PFOA on primary cultured hepatocytes from rare minnow (Gobiocypris rarus) using a custom cDNA microarray. Their results showed that the mixture of PFOS and PFOA with equal ratios regulated 52 genes (28 upregulated and 24 downregulated), which were not affected by either of the two individual compounds. Therefore, PFOS and PFOA could induce synergistic effects that individual compounds did not cause.
In addition, the results of Wei et al. (2009) also showed that a total of 334 genes regulated individually by PFOA or PFOS were not affected by their mixture, and 21 genes displayed consistent increases or decreases in the expression responses among the single and mixture exposure. Therefore, PFOS and PFOA could have complex toxic effects when they are coexposed to organisms. In the present study, PFOS and PFOA showed a variation of combined toxic effects with different mixture ratios over different time frames, which agreed with the results of Wei et al. (2009).
Relationship Between Mixture Toxicity and Molar Ratios of PFOS in the Mixture
Figure 5 gives the linear regression between logLC50,mix and c PFOS/(c PFOS + c PFOA) values. From the figure, it can be seen that the mixture toxicity increases with increasing molar ratios of PFOS in the mixture. However, PFOS and PFOA have complex interactive effects with variable molar ratios of PFOS in the mixtures. The interactive effects changes from addition to synergistic effect, then to antagonistic effect, and at last turnover to synergic effect again, with increased molar ratios of PFOS in the mixture.
The toxicity of PFOS and PFOA has been extensively investigated, and modes of action have been analyzed (Andersen et al. 2008; Hu et al. 2002, 2003; Kleszczyński and Składanowski 2009; Kleszczyński et al. 2009; Lau et al. 2007; Liu et al. 2008, 2009; Shi et al. 2008; Wei et al. 2008, 2009). These previous studies showed that the toxicity of PFOS and PFOA was involved in multiple biological processes, including lipid metabolism and transport, membrane integrity, oxidative stress, hormone action, immune responses, and mitochondrial functions. There are both similarities and differences in toxicological effects and modes of action between PFOS and PFOA, and for the same toxicity effect, the potential is different for these two chemicals (Hu et al. 2003; Lau et al. 2007; Liu et al. 2008, 2009; Kleszczyński and Składanowski 2009; Shi et al. 2008; Wei et al. 2008, 2009). Therefore, when PFOS and PFOA are mixed at a certain concentration ratio, they might interact and show an antagonistic effect based on the same mode of action of individual chemicals. Otherwise, they will represent an additive or synergistic effect.
In the present study, the interactive effects of PFOS and PFOA in the mixture affected their combined effects, which could deviate the mixture toxicity from the relationship between logLC50,mix and c PFOS/(c PFOS + c PFOA) values. Although the mixture toxicity was affected by the interactive effects, the trend of the mixture toxicity still showed an increase with increasing molar ratios of PFOS in the mixture.
Conclusion
In the present study, single and mixture toxicity of PFOS and PFOA on zebrafish embryos were investigated. PFOS showed stronger single toxicity than PFOA. In four different mixtures, PFOS and PFOA showed complex interactive effects that changed from addition to synergistic effect, then to antagonistic effect, and at last turnover to synergic effect again, with increased molar ratios of PFOS. Neither the CA nor the IA model could predict the combined effect when strong interactions existed. Although the interactive effects of PFOS and PFOA affected their mixture toxicity, the trend of the mixture toxicity still showed an increase with increasing molar ratios of PFOS in the mixture.
References
Altenburger R, Nendza M, Schüürmann G (2003) Mixture toxicity and its modeling by quantitative structure-activity relationships. Environ Toxicol Chem 22:1900–1915
Andersen ME, Butenhoff JL, Shang SC, Farrar DG, Kennedy GL, Lau C (2008) Perfluoroalkyl acids and related chemistries—toxicokinetics and modes of action. Toxicol Sci 102:3–14
Ankley GT, Kuehl DW, Kahl MD, Jensen KM, Butterworth BC, Nichols JW (2004) Partial life-cycle toxicity and bioconcentration modeling of perfluorooctanesulfonate in the northern leopard frog (rana pipiens). Environ Toxicol Chem 23:2745–2755
Butt CM, Berger U, Bossi R, Tomy GT (2010) Levels and trends of poly- and perfluorinated compounds in the arctic environment. Sci Total Environ 408:2936–2965
Ding GH, van den Brandhof EJ, Baerselman R, Peijnenburg WJ (2012a) Acute toxicity of poly- and perfluorinated compounds to two cladocerans, Daphnia magna and Chydorus sphaericus. Environ Toxicol Chem 31:605–610
Ding GH, Wouterse M, Baerselman R, Peijnenburg WJ (2012b) Toxicity of poly- and perfluorinated compounds to lettuce (Lactuca sativa) and green algae (Pseudokirchneriella subcapitata). Arch Environ Contam Toxicol 62:49–55
Fromme H, Tittlemier SA, Völkel W, Wilhelm M, Twardella D (2009) Perfluorinated compounds—exposure assessment for the general population in Western countries. Int J Hyg Environ Health 212:239–270
Giesy JP, Kannan K (2002) Perfluorochemical surfactants in the environment. Environ Sci Technol 36:146A–152A
Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DCG (2006) Biological monitoring of polyfluoroalkyl substances: a review. Environ Sci Technol 40:3463–3473
Hu XZ, Hu DC (2009) Effects of perfluorooctanoate and perfluorooctane sulfonate exposure on hepatoma Hep G2 cells. Arch Toxicol 83:851–861
Hu WY, Jones PD, Upham BL, Trosko JE, Lau C, Giesy JP (2002) Inhibition of gap junctional intercellular communication by perfluorinated compounds in rat liver and dolphin kidney epithelial cell lines in vitro and Sprague-Dawley rats in vivo. Toxicol Sci 68:429–436
Hu WY, Jones PD, DeCoen W, King L, Fraker P, Newsted J et al (2003) Alterations in cell membrane properties caused by perfluorinated compounds. Comp Biochem Physiol C Toxicol Pharmacol 135:77–88
Huang H, Huang C, Wang L, Ye X, Bai C, Simonich MT et al (2010) Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS). Aquat Toxicol 98:139–147
Jeon J, Lim HK, Kannan K, Kim SD (2010) Effect of perfluorooctanesulfonate on osmoregulation in marine fish, Sebastes schlegeli, under different salinities. Chemosphere 81:228–234
Jernbro S, Rocha PS, Keiter S, Skutlarek D, Färber H, Jones PD et al (2007) Perfluorooctane sulfonate increases the genotoxicity of cyclophosphamide in the micronucleus assay with V79 cells-further proof of alterations in cell membrane properties caused by PFOS. Environ Sci Pollut Res Int 14:85–87
Kannan K, Tao L, Sinclair E, Pastva SD, Jude DJ, Giesy JP (2005) Perfluorinated compounds in aquatic organisms at various trophic levels in a Great Lakes food chain. Arch Environ Contam Toxicol 48:559–566
Kissa E (2001) Fluorinated surfactants and repellants, 2nd edn. Marcel Decker, New York
Kleszczyński K, Składanowski AC (2009) Mechanism of cytotoxic action of perfluorinated acids. I. Alteration in plasma membrane potential and intracellular pH level. Toxicol Appl Pharmacol 234:300–305
Kleszczyński K, Stepnowski P, Składanowski AC (2009) Mechanism of cytotoxic action of perfluorinated acids. II. Disruption of mitochondrial bioenergetics. Toxicol Appl Pharmacol 235:182–190
Koutsaftis A, Aoyama I (2007) Toxicity of four antifouling biocides and their mixtures on the brine shrimp Artemia salina. Sci Total Environ 387:166–174
Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J (2007) Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99:366–394
Li MH (2009) Toxicity of perfluorooctane sulfonate and perfluorooctanoic acid to plants and aquatic invertebrates. Environ Toxicol 24:95–101
Liu W, Chen S, Quan X, Jin YH (2008) Toxic effect of serial perfluorosulfonic and perfluorocarboxylic acids on the membrane system of a freshwater alga measured by flow cytometry. Environ Toxicol Chem 27:1597–1604
Liu W, Zhang YB, Quan X, Jin YH, Chen S (2009) Effect of perfluorooctane sulfonate on toxicity and cell uptake of other compounds with different hydrophobicity in green alga. Chemosphere 75:405–409
Organisation for Economic Co-operation and Development (1992) Test guideline 203. OECD Guideline for Testing of Chemicals. Fish, Acute Toxicity Test
Paul AG, Jones KC, Sweetman AJ (2009) A first global production, emission, and environmental inventory for perfluorooctane sulfonate. Environ Sci Technol 43:386–392
Rider CV, LeBlanc GA (2005) An integrated addition and interaction model for assessing toxicity of chemical mixtures. Toxicol Sci 87:520–528
Rodea-Palomares I, Leganés F, Rosal R, Fernández-Piñas F (2012) Toxicological interactions of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) with selected pollutants. J Hazard Mater 201–202:209–218
Scholze M, Boedeker W, Faust M, Backhaus T, Altenburger R, Grimme LH (2001) A general best-fit method for concentration-response curves and the estimation of low-effect concentrations. Environ Toxicol Chem 20:448–457
Shi X, Du Y, Lam PK, Wu RS, Zhou B (2008) Developmental toxicity and alteration of gene expression in zebrafish embryos exposed to PFOS. Toxicol Appl Pharmacol 230:23–32
Suja F, Pramanik BK, Zain SM (2009) Contamination, bioaccumulation and toxic effects of perfluorinated chemicals (PFCs) in the water environment: a review paper. Water Sci Technol 60:1533–1544
Watanabe MX, Jones SP, Iwata H, Kim EY, Kennedy SW (2009) Effects of coexposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin and perfluorooctane sulfonate or perfluorooctanoic acid on expression of cytochrome P450 isoforms in chicken (Gallus gallus) embryo hepatocyte cultures. Comp Biochem Physiol C Toxicol Pharmacol 149:605–612
Wei YH, Liu Y, Wang JS, Tao Y, Dai JY (2008) Toxicogenomic analysis of the hepatic effects of perfluorooctanoic acid on rare minnows (Gobiocypris rarus). Toxicol Appl Pharmacol 226:285–297
Wei YH, Shi XJ, Zhang HX, Wang JS, Zhou BS, Dai JY (2009) Combined effects of polyfluorinated and perfluorinated compounds on primary cultured hepatocytes from rare minnow (Gobiocypris rarus) using toxicogenomic analysis. Aquat Toxicol 95:27–36
Westerfield M (2000) The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio), 4th edn. University of Oregon Press, Eugene
Zhu XW, Liu SS, Ge HL, Liu Y (2009) Comparison between two confidence intervals of dose–response relationships [in Chinese]. China Environ Sci 29(2):955–960
Acknowledgments
This study was supported by the Fundamental Research Funds for the Central Universities (Grant No. DLMU 2012TD027), the Liaoning Biodiversity Protection Action Plan (Grant No. 2012-Inhyhbc-0004), and the National Nature Science Foundation of China (Grant No. 40976064). The authors thank Willie Peijnenburg for reviewing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, G., Zhang, J., Chen, Y. et al. Combined Effects of PFOS and PFOA on Zebrafish (Danio rerio) Embryos. Arch Environ Contam Toxicol 64, 668–675 (2013). https://doi.org/10.1007/s00244-012-9864-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-012-9864-2