Abstract
In the light of the alarming decline of the European eel (Anguilla anguilla L.) population, there is an urgent need to define ecological indicators for eel habitat quality. Due to an increasing shortage of glass eels available for local stock enhancement, the decision of whether restocking is a valuable management tool to increase high-quality silver eel escapement to the sea needs to be evaluated. Organic contaminants, such as polycyclic aromatic hydrocarbons (PAHs), are among the major threats to fish in their habitat. Therefore, the aim of the investigation presented here was to examine metabolites of PAHs in eel bile as one possible marker for habitat quality. In total, 170 yellow eels were collected in the rivers Rhine, Ems, Weser, Elbe, Havel, Schlei, Eider, Trave, Warnow, Peene, Uecker, and Oder in 2009. PAH metabolites in eel bile were analyzed using high-performance liquid chromatography with fluorescence detection. Metabolites of pyrene and phenanthrene were investigated. Concentrations of PAH metabolites in eel bile varied significantly between several rivers, with the highest mean concentrations of 1-hydroxypyrene and 1-hydroxyphenanthrene in eel bile from the river Trave (2421 and 632 ng/ml). Moreover, huge differences in the ratio of 1-hydroxypyrene to 1-hydroxyphenanthrene, with the highest mean value in eel bile from the river Ems (7.43) and the lowest mean value in eel bile from the river Uecker (0.70), indicate different sources of PAH contamination. A comparative analysis of PAH-metabolite contamination of eels in different river systems is seen as a first step toward a classification of freshwater habitats for restocking purposes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The abundance of the European eel (Anguilla anguilla L.) population is at a historical minimum and continues to decrease (ICES 2009). Therefore, the European Commission decided on a management framework (European Union [EU] 1100/2007) with the objective being to rebuild the stock. This council regulation requires national eel-management plans (EMPs) to guarantee for each European river basin a minimum escapement to the sea of 40% of the silver eel biomass relative to the best estimate of escapement that would have existed if no anthropogenic influences had impacted the stock. The management tools used to reach this target are up to the EU Member States and include decreased fishing efforts (such as catch limitations, closed seasons, or increased minimum landing-size limits), habitat restoration, and restocking of juvenile eel.
In some EU member states, restocking represents the most important measure in EMPs. However, in the light of the dramatically decreased recruitment, the scientific opinion toward common stocking practices of eels have been discussed controversially regarding the question of local versus global surplus to spawning stock to the risk of spreading diseases and parasites (Svedäng and Wickström 1997; Limburg et al. 2003; Westin 2003). Because glass eels and eel elvers are caught in the wild and transported during long distances to their release sites, it is further debatable whether these fish are still able to return to their spawning grounds in the Sargasso Sea after reaching maturation. In addition, due to the fact that the European eel consists of one single panmictic population, restocking should only be conducted if stocked eels face greater habitat quality and/or lower anthropogenic mortalities at their final destination compared with their waters of origin. Recently, increasing attention has been paid to such qualitative aspects, in particular, diseases and organic contaminants, such as polycyclic aromatic hydrocarbons (PAHs; ICES 2010a). However, eel habitat-quality characterization based on organic contaminates is completely lacking in Germany.
PAHs are a group of hydrophobic organic compounds that are ubiquitous pollutants derived from pyrogenic and petrogenic sources (Vuorinen et al. 2006). The load of these contaminants in aquatic ecosystems is often correlated with degree of urbanization (Meador et al. 1995; Van der Oost et al. 2003), shipping traffic, and combustion of fossil fuels, oil, and coal (Varanasi et al. 1989; O’Malley et al. 1996). Atmospheric input can be an important source of aquatic PAH pollution as well (Ruddock et al. 2003). In rivers, PAHs tend to accumulate in sediments (Notar et al. 2001), where they can be present in thousand-fold greater concentrations than those in the overlying water (James and Kleinow 1993). Studies applying sediment bioassays, as well as bioassay-directed fractionation on German river sediments, showed PAHs as being major lipophilic toxicants (Brack et al. 1999). Fish absorb PAHs mainly by way of the gills but also through the body surface and by ingestion of contaminated sediment or food (Varanasi et al. 1989). The assimilated PAHs can be rapidly metabolized in two main phases. In the phase I reaction, the catalytic activity of oxidases causes the addition of hydroxyl groups to the parent molecule. In the phase II reaction, various polar moieties are conjugated to the phase I products to facilitate excretion (Di Giulio et al. 1995).
PAHs and related metabolic compounds, such as epoxides and diolepoxides, have repeatedly been shown to cause deleterious effects in fish (Stein et al. 1990; Aas et al. 2000b). Although Myers et al. (2003) linked PAH exposure to increased levels of neoplastic and neoplasia-related aberrations or tumors in fish liver, epoxides and dioepoxides are known to cause DNA adducts, which may induce cancer (van Schooten et al. 1995). Thus, PAH contamination in aquatic ecosystems is a cause for serious concern.
The major site of PAH metabolism is the liver, and metabolites produced here are secreted into bile and stored in the gall bladder before being excreted into the intestine until the next feeding (Varanasi and Stein 1991; Au et al. 1999). The extensive PAH metabolism in fish (Meador et al. 1995) does not render an adequate assessment of exposure by measuring PAHs directly (Fenet et al. 2006). Thus, an alternative but indirect indicator of exposure is the concentration of PAH metabolites in the bile fluid of fish. Assessment of bile PAH metabolites by high-performance liquid chromatography (HPLC) with fluorescence detection has proven useful as a method for PAH monitoring (Kammann 2007). A review on analytical methods to determine PAH metabolites has been published recently (Beyer et al. 2010). The major metabolite in fish bile is 1-hydroxypyrene, which contributes ≤76% of the sum of PAH metabolites (Ruddock et al. 2003). Other metabolites detected in considerably lower levels are 1-hydroxyphenanthrene, 1-hydroxychrysene, and three metabolites of benzo(a)pyrene (Ruddock et al. 2003). Determination of PAH metabolites has previously been performed in bile of several marine fish species, such as dab Limanda limanda (van Schanke et al. 2001; Luthe et al. 2002; Kammann 2007), hornyhead turbot Pleuronichthys verticalis (Roy et al. 2003), eelpout Zoarces viviparous (Vuorinen et al. 2006), and flounder Platichthys flesus (Reynolds et al. 2003) either after (experimental) exposure of fish to PAHs in vivo or in fish caught in the wild. Consequently, PAH monitoring has been established as a part of several international environmental programs (Organization for the Protection of the Marine Environment of the North-East Atlantic 1998).
As benthic and largely benthivorous fish, fat-rich species of eels are known to show high affinity for organic contaminants compared with other freshwater and diadromous fishes. Some studies concerning the assessment of PAH contamination in European eel are restricted to PAH parent compounds (Pointet and Milliet 2000; Buet et al. 2006), which are rapidly metabolized in fish. Due to this rapid metabolisation, the concentration of PAH in fish tissue and fluids are generally low and do not reflect the pollution level. Some studies have been published on PAH metabolites in eel bile (Ruddock et al. 2002; Gorbi et al. 2005; Ribeiro et al. 2005). In these publications, fish from estuaries and lagoons have been used. Little information is available on PAH-metabolite levels in freshwater eels (van der Oost et al. 1996; Fenet et al. 2006). Most of the studies mentioned previously did not consider important parameters, such as sex or feeding status of eels, which have been reported to influence the biliary PAH-metabolite concentration (Aas et al. 2000a; Vuorinen et al. 2006). In the light of the German EMPs, there is a strong need for information of PAH contamination of eels living in European freshwater areas to identify suitable eel habitats. A comparative analysis of organic contamination of eels from different river systems is seen as a first step toward classification of freshwater habitats for restocking purposes. In the light of still-declining glass eel recruitment along the European coast, science based criteria on how to best use this limited resource is urgently needed.
Thus, the aim of the present study was to perform the first comprehensive investigation of biliary PAH-metabolite concentrations in eels from 12 rivers to elucidate spatial differences in PAH contamination and to describe PAH pollution as one factor of habitat quality of European eels.
Materials and Methods
Sampling of Fish
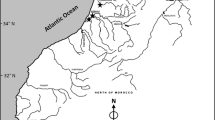
European eels were caught under natural conditions at one location per river site (mostly downstream) where commercial eel fishery was available. The German rivers under investigation were the Rhine, Ems, Weser, Elbe, Havel, Schlei, Eider, Trave, Warnow, Peene, Uecker, and Oder. Fifteen yellow eels per river were collected between May and June in 2009 (Fig. 1). After transferring eels into the laboratory, they were killed by exposure to an overdose of clove oil (Carl Roth GmbH, Karlsruhe, Germany) or by cervical vertebrate dislocation. Eels were weighed and measured to the nearest 0.5 g and 0.5 cm, respectively, to calculate condition factor (CF) as follows: CF (%) = total body weight (g)/total length (cm)3 * 100. Proportion of liver to whole-body weight was calculated as the hepatosomatic index (HSI) as follows: HSI (%) = liver weight (g)/total body weight (g) * 100.
According to Durif et al. (2005), female eels were classified into five maturation stages (silvering index [SI]). The stages corresponded to growth phase (stage I and II, yellow eel), a premigrant phase (stage III), and two migrating phases (stage IV and V, silver eel). Eels with an SI of IV and V were excluded (stage IV n = 5 and stage V n = 2; Table 1). These eels differ in feeding behavior compared with yellow eels (stage I and II) and in dietary status, which may influence the concentration of PAH metabolites in bile (Collier and Varanasi 1991; Aas et al. 2000a). Bile fluid was removed from the gallbladder using a syringe (Henke Sass Wolf, Tuttlingen, Germany) with a disposable hypodermic needle (0.6 × 30 mm; B. Braun, Emmenbrücke, Germany), and samples were stored at −20°C until further examination.
Chemicals
Standard solutions of 1-hydroxypyrene (1-OH-Pyr) and 1-hydroxyphenanthrene (1-OH-Phen) were supplied by Dr. Ehrenstorfer GmbH (Augsburg, Germany) with certified concentrations. Ascorbic acid was purchased from Sigma-Aldrich (Munich, Germany). Acetonitrile (hypergrade for HPLC), methanol (gradient grade for HPLC), ethanol (absolute), trifluoroacetic acid (spectroscopic quality) and ß-glucuronidase–arylsulfatase mixture were purchased from VWR International GmbH (Darmstadt, Germany). HPLC water (gradient grade) came from Mallinckrodt Baker (Deventer, the Netherlands).
Treatment of Bile Samples, HPLC, and Ultraviolet Analysis
PAH metabolites in bile samples were determined by a modified version of the method described by Kammann (2007) based on Krahn et al. (1984). A volume of 25 μl bile was mixed with 95 μl water to which 5 μl ß-glucuronidase/arylsulfatase solution (30/60 U/ml) were added. The resultant solution was incubated for 2 h at 37°C on a heated shaker. The reaction was stopped by addition of 125 μl ethanol containing 5 mg/ml ascorbic acid. The final solution represents a 10-fold dilution of the bile sample and was centrifuged (5 min, 700× g). The clear supernatant was immediately used for HPLC analysis. Concentrations of 1-OH-Pyr and 1-OH-Phen were determined in a 50-μl aliquot using a LaChrom HPLC system (Merck Hitachi) comprising a quaternary pump (L-7100), an auto sampler (L-7200), and a fluorescence detector (L-7480). Standard solutions were diluted in acetonitrile containing 5 mg/ml ascorbic acid and stored in the dark. According to Kammann (2007), samples were chromatographed with a flow of 0.55 ml/min on a Nucleosil 100-3 C18 (3 × 125 mm) reverse-phase column equipped with a guard column. The initial mobile phase was acetonitrile/0.1% trifluoroacetic acid 50/50 (v/v). After 10 min, the solvent composition progressively changed to 60% acetonitrile during 4 min and changed afterward to 100% acetonitrile during 2 min. The excitation/emission wavelength pairs for 1-OH-Pyr and 1-OH-Phen were 346/384 and 256/380 nm, respectively. Ultraviolet (UV)-absorption measurement was performed in 1:20 diluted bile fluids by means of a microplate reader (FLUOstar OPTIMA; BMG Labtech, Offenburg, Germany) at 380 and 660 nm, respectively. Biliverdin was calculated from the absorption at 660 nm using the molar extinction coefficient according to Grossbard et al. (1987).
Quality Assurance
Every sample was hydrolysed and subjected to HPLC analysis twice. The limit of detection (LD) and the limit of quantification (LQ) were calculated from a standard curve according to Deutsches Institut für Normung 32645 (1994) with a confidence level of 99%. Considering the dilution of the sample during sample preparation, an LD of 3.4 and an LQ of 22.5 ng/ml bile were determined for 1-OH-Pyr. For 1-OH-Phen, an LD of 0.5 and an LQ of 1.7 ng/ml were calculated. A fish-bile sample as laboratory reference material was included in every sample batch to monitor the stability of the method by measuring 1-OH-Pyr. Calibrations consisting of five standard concentrations were repeated daily with every sample batch.
Statistics
KYPLOT (version 2.0 Beta 15, 1997–2001; Tokyo, Japan) was used to perform statistical analysis of length, weight, CF, HIS, SI, biliverdin, and A380. Data are expressed as means ± SDs. Intergroup differences were considered significant at p < 0.05 according to one-way analysis of variance followed by Tukey–Kramer pairwise comparison. Fischer’s least significance difference (LSD) test was used to identify significant differences in means of 1-OH-Pyr and 1-OP-Phen concentrations between rivers (p < 0.05, p < 0.01, and p < 0.001). Regression-tree analysis (De’Ath and Fabricius 2000) was used to determine the most likely inducer of variability among the numeric response variables (metabolite concentrations). We included eel length, weight, CF, HSI, and SI as predictor variables. Multiple comparisons of partitions were conducted based on chi-square statistics according to Pearson with Bonferroni adjustment (p < 0.05).
Results
Mean values of the investigated variables total length, wet weight, CF, HSI, SI, as well as bile pigments biliverdin and A380 (sum parameter of pigments), varied significantly between rivers (Table 1). Variation in mean total length and mean weight of eels may indicate size selection by fishing gear (stow net, eel pot, fyke net, electrofishing) and/or selection by different mesh sizes. These variations were reflected in mean CF, which ranged from 0.14 in the river Eider to 0.19 in the rivers Ems and Warnow (Tukey–Kramer test, p < 0.001). HSI differed significantly between rivers, with highest mean value in the river Schlei (2.20 ± 0.43%) and the lowest mean value in the river Rhine (1.33 ± 0.23%; Tukey–Kramer test, p < 0.001). Regarding maturation stage of eels, significant differences in SI were observed in the rivers Uecker (2.85 ± 0.38) and Warnow (2.77 ± 0.44) compared to the rivers Eider (2.0 ± 0.63), Schlei (1.69 ± 0.75), Weser (2.0 ± 0.56), and Ems (1.86 ± 0.54) (Table 1; Tukey–Kramer test, p < 0.001).
For all rivers, the dominant PAH metabolite was 1-OH-Pyr. The individual results of 1-OH-Pyr covered a broad range, from <22.5 ng/ml in the river Uecker to 3724.5 ng/ml in the river Trave (see Table 2). The highest mean value of 1-OH-Pyr was found in eel bile from the river Trave (2420.8 ng/ml; range 1315.5–3724.5; see Table 2), which was significantly greater than 1-OH-Pyr mean values in eels from of all of the other rivers (Fischer’s LSD test, p < 0.001). The lowest mean 1-OH-Pyr value was detected in the river Uecker, with a concentration of 109.7 ng/ml. The mean 1-OH-Pyr concentrations were arranged in the following rank order: Trave > Elbe > Oder > Warnow > Eider > Weser > Peene > Ems > Havel > Rhine > Schlei > Uecker. The individual results of 1-OH-Phen were considerably lower than the values for 1-OH-Pyr but with a similarly range in their concentration pattern (Table 2; Fig. 2). The minimum concentration of 1-OH-Phen was measured in eel bile from the river Rhine (12.8 ng/ml), and the overall maximum was detected in eel bile from the river Warnow (1633.7 ng/ml; Fig. 2; Table 2). In line with the results for 1-OH-Pyr, the highest mean value of 1-OH-Phen was found in eel bile of the river Trave (632.4 ng/ml; Fischer’s LSD test, p < 0.001). The lowest mean value of 1-OH-Phen was detected in the river Ems (52.7 ng/ml; Table 2). The rank order of the mean 1-OH-Phen concentrations was as follows: Trave > Warnow > Oder > Peene > Elbe > Uecker > Rhine > Eider > Weser > Schlei > Havel > Ems.
Regression-tree analysis identified the location of commercial eel catches (rivers) as the major inducer of variability on the concentrations of 1-OH-Pyr (chi-square test, F = 116.1, p < 0.05) and 1-OH-Phen (chi-square test, F = 43.6, p < 0.05). In general, the highest PAH-metabolite concentrations were detected in the river Trave (see previous text). Beyond the location of catch, none of the biological parameters influenced variability of biliary PAH-metabolite concentrations in eel.
Evaluation of the ratio of 1-OH-Pyr to 1-OH-Phen provides the significantly highest mean ratio (7.4) in eel bile of the river Ems compared with eel bile from all of the other rivers (Fischer’s LSD test, p < 0.001). The lowest ratio of 1-OH-Pyr to 1-OH-Phen was observed in eel bile from the river Uecker (0.7). Among the eel bile samples from 12 rivers, the ratio of 1-OH-Pyr to 1-OH-Phen of the river Uecker was < 1, suggesting a greater proportion of phenanthrene in the PAH contamination of the river Uecker compared with other rivers. The rank order of the mean ratio of 1-OH-Pyr to 1-OH-Phen was as follows: Ems > Eider > Havel > Elbe > Weser > Trave > Rhine > Oder > Schlei > Warnow > Peene > Uecker (Fig. 3). Because of the weak correlation between 1-OH-Pyr and bile pigments (biliverdin [r 2 = 0.07]; A380 [r 2 = 0.009]) the PAH-metabolite results are presented as nanograms per milliliter without previous normalization to bile pigments.
Discussion
1-OH-Pyr was found to be the major PAH metabolite in eel bile, with individual concentrations varying from <22.5 to 3725.5 ng/ml, supporting its role as an indicator metabolite of PAH contamination in fish (Lin et al. 1994; Eggens et al. 1996). This is in line with reports for the marine species flounder (Pleuronectes platessa), plaice (Platichthys flesus), and dab (Limanda limanda) (Ariese et al. 1993; Kammann 2007).
Using this PAH metabolite as indicator for PAH exposure, eel from the river Trave is exposed to the highest PAH contamination. Trave eels were caught next to the city of Luebeck (200,000 inhabitants). Cities and port activities are known PAH sources for the aquatic environment (Notar et al. 2001; Fenet et al. 2006; Kammann and Gercken 2010). Surprisingly, eels from the rivers Elbe and Oder, which were caught in the immediate vicinity of shipping routes or bigger cities, are only medium contaminated. In contrast, samples from the other rivers were taken in agriculture-dominated areas. Eels from the river Uecker exhibit the lowest contamination and at the same time a relatively high amount of 1-OH-Phen compared with 1-OH-Pyr, indicating a different contamination source. The small river Uecker has no importance for commercial shipping. However, one single sampling point may not be representative for a longer river, which may have different possible point sources of PAHs. More sampling along one larger river in Germany is planned for future studies to shed additional light on sources of PAH contamination in eel.
1-OH-Phen was generally detected in lower concentrations than 1-OH-Pyr. This may be related to the fact that phenanthrenes strongly bind to sediment particles, resulting in decreased bioavailability (Yuan et al. 2001). However, in our study, a huge variation in the ratio of 1-OH-Pyr to 1-OH-Pheno was observed, indicating different PAH contamination sources mainly to the river Uecker on one hand and the river Ems on the other hand. Comparing absolute 1-OH-Pyr values to published results for other species, considerably lower concentrations have been reported for marine fish caught offshore, with maximum levels approximately 120 ng/ml (Kammann 2007). Fish caught in estuarine or coastal waters have been found to exhibit significantly greater concentrations ≤3000 ng/ml (Kammann and Gercken 2010; Vuorinen et al. 2006; Pikkarainen 2006; Escartin and Porte 1999). Investigations of PAH metabolites in European eels caught in the United Kingdom estuaries showed 1-OH-Pyr concentrations in bile ranging from 120 to 7600 ng/ml (Ruddock et al. 2003), which is in accordance with the concentrations found in the present study.
PAH-metabolite concentrations in fish bile are known to vary significantly between fish species (Escartin and Porte 1999). In addition, metabolite levels seem to be affected by sex (Vuorinen et al. 2006) as well as environmental factors, such as temperature (Buhler and Williams 1989; Vuorinen et al. 2006) and dietary status (Collier and Varanasi 1991; Aas et al. 2000a). Taking this into account, only female yellow eels with a mean SI between 1.69 and 2.85 (SI calculated according to Durif et al. 2005) were analyzed in this study. Previous studies dealing with PAH pollution in European eels either did not determine sex at all (Pointet and Milliet 2000; Roche et al. 2002; Ruddock et al. 2002, 2003) or analyzed immature eels (Ribeiro et al. 2005). In contrast, only female eels of comparable size and weight were analyzed by van Schooten et al. (1995) to assess PAH exposure in eels from Dutch freshwaters. The dietary status of fish affects the concentration of PAH metabolites in bile (Collier and Varanasi 1991; Aas et al. 2000a) because food in the gut causes the release of bile into the intestine (Brumley et al. 1998). In fish that have not been recently fed, metabolites tend to become concentrated in bile due to resorption of water across the gallbladder (Ariese et al. 1993). One approach to compensate for this concentration effect is to relate metabolite levels to biliverdin absorbance. The green bile pigment, biliverdin, has been used before as a relating parameter (Ariese et al. 1993; Aas et al. 2000b; Ruddock et al. 2003; Richardson et al. 2004). UV absorption of the bile at 380 nm (A380) describes the bile pigments as a sum and can also been used for normalization (Ariese et al. 1997). Both parameters are linked to the color of bile. Richardson et al. (2004) compared the coefficients of variation (CVs) before and after normalization and detected no or little improvement by using biliverdin as a relating parameter. Due to fairly poor correlations, the CV of 1-OH pyrene within one group (eels from one river) typically did not improve (decrease) much with normalization: The CV for 1-OH-pyrene decreased in only 6 of 12 groups after normalization to A380 compared with nonnormalized results (data not shown). The statistical spread within a group does therefore not improve much with normalization. Furthermore, normalization itself adds another source of variability. Regarding normalization, our data are consistent with the results of Aas et al. (2000a) and Richardson et al. (2004). Both investigators presented their data as nonnormalized in ng/ml but recommended that bile be normalized to biliverdin, especially if marked differences in feeding status of fish might occur.
We conclude that neither biliverdin nor A380 absorbance are candidate parameters for PAH-metabolite normalization in bile of female yellow eels with a similar maturation stage and dietary status. Whether eels show any biological response or even suffer from PAH contamination is still unknown. Derived from toxicological studies with fish exposed to crude oil, ICES (2010b) recently published environmental-assessment criteria for PAH metabolites that toggle this problem for some marine fish species, with threshold values set in the range of 483–909 and 262–1832 ng/ml for 1-OH-Pyr and 1-OH-Phen, respectively. Because some of the values found here exceed these threshold levels, negative biological responses cannot be excluded.
Conclusion
As a benthivorous species, the European eel (A. anguilla) may suffer from harmful contaminants, such as PAHs, in freshwater habitats where they accumulate in the sediment.
It is suggested that the PAH metabolites in eel bile could be used as one parameter among others to describe the quality of freshwater habitats. The dominant PAH metabolite was 1-OH-Pyr, and thus it is therefore the best indicator in eel bile for PAH pollution. The patterns of single PAHs may indicate different derivation of PAH pollution in the rivers. Therefore, the relation of 1-OH-Pyr to 1-OH-Phen may reflect different sources of PAH contamination. This first comprehensive investigation of biliary PAH metabolites in eels from 12 rivers in Germany can be helpful in the evaluation of PAH contamination of the aquatic environment and could support the effort to identify suitable eel habitats.
References
Aas E, Baussant T, Balk L, Liewenborg B, Andersen OK (2000a) PAH metabolites in bile, cytochrome P4501A and DNA adducts as environmental risk parameters for chronic oil exposure: a laboratory experiment with Atlantic cod. Aquat Toxicol 51:241–258
Aas E, Beyer J, Goksoyr A (2000b) Fixed wavelength fluorescence (FF) of bile as a monitoring tool for polyaromatic hydrocarbon exposure in fish: an evaluation of compound specificity, inner filter effect and signal interpretation. Biomarkers 5:9–23
Ariese F, Kok SJ, Verkaik M, Gooijer C, Velthorst NH, Hofstraat JW (1993) Synchronous fluorescence spectrometry of fish bile: a rapid screening method for the biomonitoring of PAH exposure. Aquat Toxicol 26:273–286
Ariese F, Burgers I, Oudhoff K, Rutten T, Stroomberg G, Vethaak D (1997) Comparison of analytical approaches for PAH metabolites in fish bile samples for marine and estuarine monitoring. Institute for Environmental Studies, Vrije Universiteit, Amsterdam, R-97/9, p 29
Au DWT, Wu RSS, Zhou BS, Lam PKS (1999) Relationship between ultrastructural changes and EROD activities in liver of fish exposed to benzo[a]pyrene. Environ Pollut 104:235–247
Beyer J, Jonsson G, Porte C, Krahn MM, Ariese F (2010) Analytical methods for determining metabolites of polycyclic aromatic hydrocarbon (PAH) pollutants in fish bile: a review. Environ Toxicol Pharmacol 30:224–244
Brack W, Altenburger R, Ensenbach U, Möder M, Segner H, Schuurmann G (1999) Bioassay-directed identification of organic toxicants in river sediment in the industrial region of Bitterfeld (Germany) – a contribution to hazard assessment. Arch Environ Contam Toxicol 37:164–174
Brumley CM, Haritos VS, Ahokas JT, Holdway DA (1998) The effects of exposure, duration and feeding status on fish bile metabolites: implications for biomonitoring. Ecotoxicol Environ Saf 39:147–153
Buet A, Banas D, Vollaire Y, Coulet E, Roche H (2006) Biomarker responses in European eel (Anguilla anguilla) exposed to persist organic pollutants. A field study in the Vaccarès lagoon (Camargue, France). Chemosphere 65:1846–1858
Buhler DR, Williams DE (1989) Enzymes involved in metabolism of PAH by fishes and other aquatic animals: oxidative enzymes (or phase I enzymes). In: Varanasi U (ed) Metabolism of polycyclic aromatic hydrocarbons in the aquatic environment. CRC Press, Boca Raton, pp 151–184
Collier TK, Varanasi U (1991) Hepatic activities of xenobiotic metabolizing enzymes and biliary levels of xenobiotics in English sole (Parophrys vetulus) exposed to environmental contaminants. Arch Environ Contam Toxicol 20:462–473
De’Ath G, Fabricius KE (2000) Classification and regression trees: a powerful yet simple technique for ecological data analysis. Ecology 81:3178–3192
Deutsches Institut für Normung e.V. (1994) 32645, Nachweis-, Erfassungs- und Bestimmungsgrenze. Beuth Verlag, Berlin
Di Giulio RT, Benson WH, Sanders BM, van Veld PA (1995) Biochemical mechanisms: metabolism, adaptation, and toxicity. In: Rand GM (ed) Fundamentals of aquatic toxicology. Taylor & Francis, Washington, DC, pp 523–561
Durif C, Dufour S, Elie P (2005) The silvering process of Anguilla anguilla: a new classification from yellow resident to silver migrating stage. J Fish Biol 66:1025–1043
Eggens ML, Opperhuizen A, Boon JP (1996) Temporal variation of CYP1A indices, PCB and 1-OH pyrene concentration in flounder, Platichthys flesus, from the Dutch Wadden Sea. Chemosphere 33:1579–1596
Escartin E, Porte C (1999) Assessment of PAH pollution in coastal areas from the NW Mediterranean through the analysis of fish bile. Mar Pollut Bull 38:1200–1206
European Union Council Regulation (EC) No. 1100 (2007) Establishing measures for the recovery of the stock of European eel. Official Journal of the European Union, L 248/17
Fenet H, Gomez E, Rosain D, Casellas C (2006) Polycyclic aromatic hydrocarbon metabolites and 7-ethoxyresorufin O-deethylase activity in caged European eels. Arch Environ Contam Toxicol 51:232–236
Gorbi S, Baldini C, Regoli F (2005) Seasonal variability of metallothioneins, cytochrome P450, bile metabolites and oxyradical metabolism in the European eel Anguilla anguilla L. (Anguillidae) and striped mullet Mugil cephalus L. (Mugilidae). Arch Environ Contam Toxicol 49:62–70
Grossbard ML, Boyer JL, Gorden ER (1987) The excretion pattern of biliverdin and bilirubin in bile of the small skate (Raja erinacea). J Comp Physiol B 157:61–66
International Council for the Exploration of the Sea (2009) Report of the ICES Advisory Committee 2009. ICES Advice 2009, Book 9. http://www.ices.dk/products/icesadvice.asp. Accessed 21 June 2011
International Council for the Exploration of the Sea (2010a) Report of the ICES Advisory Committee 2010, ICES Advice 2010. Book 9, pp 115–123. http://www.ices.dk/products/icesadvice.asp. Accessed 21 June 2011
International Council for the Exploration of the Sea (2010b) Advice 1.5.5.1. Further development of guidance on integrated monitoring and assessment of chemicals and biological effects, p 57. http://www.ices.dk/committe/acom/comwork/report/2010/Special%20Requests/OSPAR%20Guidance%20on%20integrated%20monitoring%20and%20assessment.pdf. Accessed 21 June 2011
James MO, Kleinow KM (1993) Trophic transfer of chemicals in the aquatic environment. In: Malins DC, Ostrander GK (eds) Aquatic toxicology: molecular, biochemical and cellular perspectives. Lewis, London, pp 69–92
Kammann U (2007) PAH metabolites in bile fluids of dab (Limanda limanda) and flounder (Platichthys flesus)―spatial distribution and seasonal changes. Environ Sci Pollut Res 14:102–108
Kammann U, Gercken J (2010) PAK-Metaboliten in Aalmuttern (Zoarces viviparus) aus der Wismar-Bucht. Umweltwiss Schadst Forsch 22:541–546
Krahn MM, Myers MS, Burrows DG, Malins DC (1984) Determination of metabolites of xenobiotics in the bile of fish from polluted waterways. Xenobiotica 14:633–646
Limburg KE, Wickström H, Svedäng H, Elfman M, Kristiansson P (2003) Do stocked freshwater eels migrate? Evidence from the Baltic suggests “Yes”. Am Fish Soc Symp 33:275–284
Lin ELC, Cormier SM, Racine RN (1994) Synchronous fluorometric measurement of metabolites of polycyclic aromatic hydrocarbons in the bile of brown bullhead. Environ Toxicol Chem 13:707–715
Luthe G, Stroomberg GJ, Ariese F, Brinkman UAT, van Straalen NM (2002) Metabolism of 1-fluoropyrene and pyrene in marine flatfish and terrestrial isopods. Environ Toxicol Pharmacol 12:221–229
Meador J, Stein J, Reichert W, Varanasi U (1995) Bioaccumulation of polycyclic aromatic hydrocarbons by marine organisms. Rev Environ Contam Toxicol 143:79–165
Myers MS, Johnson LL, Collier TK (2003) Establishing the causal relationship between polycyclic aromatic hydrocarbon (PAH) exposure and hepatic neoplasms and neoplasia-related liver lesions in English sole (Pleuronectes vetulus). Hum Ecol Risk Assess 9:67–94
Notar M, Leskosek H, Faganeli J (2001) Composition, distribution and sources of polycyclic aromatic hydrocarbons in sediments of the Gulf of Trieste, Northern Adriatic Sea. Mar Pollut Bull 42:36–44
O’Malley VP, Abrajano TA, Hellou J (1996) Stable carbon isotopic apportionment of individual polycyclic aromatic hydrocarbons in St. John’s Harbour, Newfoundland. Environ Sci Technol 30:634–639
Organization for the Protection of the Marine Environment of the North-East Atlantic (1998) JAMP guidelines for contaminant-specific biological effects monitoring. Oslo and Paris Commission, London
Pikkarainen AL (2006) Ethoxyresorufin-O-deethylase (EROD) activity and bile metabolites as contamination indicators in Baltic Sea perch: determination by HPLC. Chemosphere 65:1888–1897
Pointet K, Milliet A (2000) PAHs analysis of fish whole gall bladders and livers from the Natural Reserve of Camargue by GC/MS. Chemosphere 40:293–299
Reynolds WJ, Feist SW, Jones GJ, Lyons BP, Sheahan DA, Stentiford GD (2003) Comparison of biomarker and pathological responses in flounder (Platichthys flesus L.) induced by ingested polycyclic aromatic hydrocarbons (PAH) contamination. Chemosphere 52:1135–1145
Ribeiro CAO, Vollaire Y, Sanchez-Chardi A, Roche H (2005) Bioaccumulation and the effects of organochlorine pesticides, PAH and heavy metals in the eel (Anguilla anguilla) at the Camargue Natural Reserve, France. Aquat Toxicol 74:53–69
Richardson DM, Gubbins MJ, Davies IM, Moffat CF, Pollard PM (2004) Effects of feeding status on biliary PAH metabolite and biliverdin concentrations in plaice (Pleuronectes platessa). Environ Toxicol Pharmacol 17:79–85
Roche H, Buet A, Ramade F (2002) Accumulation of lipophilic microcontaminants and biochemical responses in eels from the Biosphere Reserve of Carmargue. Ecotoxicology 11:9–18
Roy LA, Steinert S, Bay SM, Greenstein D, Sapozhnikova Y, Bawardi O et al (2003) Biochemical effects of petroleum exposure in hornyhead turbot (Pleuronichthys verticalis) exposed to a gradient of sediments collected from a natural petroleum seep in CA, USA. Aquat Toxicol 65:159–169
Ruddock PJ, Bird DJ, McCalley DV (2002) Bile metabolites of polycyclic aromatic hydrocarbons in three species of fish from the Severn estuary. Ecotoxicol Environ Saf 51:97–105
Ruddock PJ, Bird DJ, McEvoy J, Peters LD (2003) Bile metabolites of polycyclic aromatic hydrocarbons (PAHs) in European eels Anguilla anguilla from United Kingdom estuaries. Sci Total Environ 301:105–117
Stein JE, Reichert WL, Nishimoto M, Varanasi U (1990) Overview of studies in liver carcinogenesis in English sole from Puget Sound: evidence for a xenobiotic chemical etiology II: biochemical studies. Sci Total Environ 94:51–69
Svedäng H, Wickström H (1997) Low fat content in female silver eels: indications of insufficient energetic stores for migration and gonadal development. J Fish Biol 50:475–486
Van der Oost R, Goksoyr A, Celander M, Heida H, Vermeulen NPE (1996) Biomonitoring of aquatic pollution with feral eel (Anguilla anguilla). 2. Biomarkers: pollution-induced biochemical responses. Aquat Toxicol 36(3–4):189–222
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Van Schanke A, Holtz F, van der Meer J, Boon JP, Ariese F, Stroomberg G et al (2001) Dose- and time dependent formation of biliary benzo[a]pyrene metabolites in the marine flatfish dab (Limanda limanda). Environ Toxicol Chem 20:1641–1647
Van Schooten FJ, Maas LM, Moonen EJC, Kleinjans JCS, van der Oost R (1995) DNA dosimetry in biological indicator species living on PAH-contaminated soils and sediments. Ecotoxicol Environ Safe 30:171–179
Varanasi U, Stein JE (1991) Deposition of xenobiotic chemicals and metabolites in marine organisms. Environ Health Perspect 90:93–100
Varanasi U, Stein JE, Nishimoto M (1989) Biotransformation and disposition of PAH in fish. In: Varanisi U (ed) Metabolism of polycyclic aromatic hydrocarbons in the aquatic environment. CRC Press, Boca Raton, pp 93–150
Vuorinen PJ, Keinänen M, Vuontisjärvi H, Barsiene J, Broeg K, Förlin L et al (2006) Use of biliary PAH metabolites as a biomarker of pollution in fish from the Baltic Sea. Mar Pollut Bull 53:479–487
Westin L (2003) Migration failure in stocked eels Anguilla anguilla. Mar Ecol Prog Ser 254:307–311
Yuan SY, Chang JS, Yen JH, Chang BV (2001) Biodegradation of phenanthrene in river sediment. Chemosphere 43:273–278
Acknowledgments
The authors are grateful to Alexander Schulz for skillful technical assistance and to Florian Sambraus for support with data evaluation. Furthermore, we thank our colleagues Klaus Wysujack, Thomas Lang, and Udo Koops, for many useful discussions. This study was partly financed by the EU-Data Collection Regulation (2008/949/EC).
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Nagel and U. Kammann contributed equally to this work.
Rights and permissions
About this article
Cite this article
Nagel, F., Kammann, U., Wagner, C. et al. Metabolites of Polycyclic Aromatic Hydrocarbons (PAHs) in Bile as Biomarkers of Pollution in European Eel (Anguilla anguilla) from German Rivers. Arch Environ Contam Toxicol 62, 254–263 (2012). https://doi.org/10.1007/s00244-011-9693-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-011-9693-8