Abstract
Formation water (produce water or oil field brine) from oil and gas production usually has high concentrations of soluble salts and metals. The objective of this study was to examine the effect of formation water from Urucu Reserve, Amazon, on whole-body uptake and internal distribution of newly accumulated Na+ in juvenile tamoatá, Hoplosternum litoralle. Groups of fish were submitted to nine treatments for 3 h in 400-ml chambers: control (well water), 5% formation water, and well water with respective concentrations of 5% formation water of Ca2+, Fe, Mn, Ba2+, Fe + Ca2+, Mn + Ca2+, and Ba + Ca2+ added. Specimens of tamoatá exposed to 5% formation water presented a very high Na+ influx, probably due to the high Na+ levels in this water. Waterborne Fe and Mn stimulated Na+ influx, but Fe increased Na+ efflux, causing Na+ loss. Waterborne Mn, on the other hand, decreased Na+ efflux, reducing Na+ loss by this species. Waterborne Ca2+ also affected Na+ influx but had no significant effect on net Na+ fluxes. These results demonstrated that spilling of formation water in ion-poor Amazon rivers would dramatically disrupt osmoregulatory balance of tamoatá and probably other Amazon fish species, impairing their survival and reduce biodiversity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Formation water, also known as produce water or oil field brine, is a byproduct of oil and gas production that usually has high concentrations of soluble salts and metals (Jackson and Reddy 2007; Manfra et al. 2007; Woodall et al. 2003). Formation water is the water associated with oil- or gas-bearing formations, which is separated from oil or gas on the drilling platform and reinjected into the well or discharged (Caliani et al. 2009). A few studies investigated the effects of the formation water (Caliani et al. 2009; Stephens et al. 2000; Zhu et al. 2008) or the alkylphenols (APs) and polycyclic aromatic hydrocarbons (PAHs) associated with the formation water (Holth et al. 2008; Lie et al. 2009; Sundt et al. 2009) on fish. In marine fishes, formation water affected gill histology and cortisol levels (Stephens et al. 2000) and cytochrome protein biomarkers (Zhu et al. 2008), induced endocrine disruption (Lie et al. 2009), and led to AP and PAH accumulation in the body (Sundt et al. 2009). Formation water enhanced the frequency of micronuclei (Caliani et al. 2009), affected the reproductive, nervous, respiratory, and immune systems, and lipid metabolism and altered connective tissue (Holth et al. 2008) in freshwater fish.
Oil and gas production in the Urucu reserve, Amazon, is on the order of 50,000 barrels equivalents/day and 10,360 m3/day, respectively (Petróbras 2008). The Urucu River, a tributary of the Solimões River, is an ion-poor “black water river” [see Baldisserotto et al. (2008) for ion levels in black water rivers]. Previous analysis revealed high levels of several metals in formation water, including Fe, Mn, and Ba (Maco Garcia 1997). However, the composition of formation water varies between fields (Jackson and Reddy 2007; Manfra et al. 2007; Pantaleão et al. 2006; Woodall et al. 2003).
To cope with the extremely low waterborne ion levels fishes from Amazon black water rivers presents a high-affinity Na+ transporter and high rates of Na+ uptake and loss or a low-affinity transporter and much lower rates of uptake and loss (Gonzalez et al. 2002). Species that survive indefinitely in the very acidic waters of the Amazonian flooded forest also possess a Na+ transporter insensitive to low pH (Gonzalez and Wilson 2001). No studies analyzed the effects of the metals found in the formation water on fish osmoregulation. Previous study demonstrated that brook charr (Salvelinus fontinalis) exposed to high waterborne Fe or Mn levels presented high net Na+ loss, but fish were also exposed to a very acidic pH (3.34) at the same time (Grippo and Dunson 1996), which makes it difficult to identify the effect of these metals.
Many fish species occur in aquatic environments located nearby the crude oil mining area of the Urucu River and are vulnerable to crude oil spills or formation water leakage as these fish species, such as Hoplosternum littorale, have evolved in this ion-poor water of the Amazon (Val and Almeida-Val 1995). Species of the genus Hoplosternum are facultative air-breathing fishes (Graham 1997), which means that these species can be affected by both the crude oil that remains on the surface of the water and to increased levels of metals and other elements originating from formation water and dissolved in the river water.
The objective of this study was to evaluate the effects of formation water from the Urucu Reserve on Na+ accumulation in several fish tissues. Accordingly short-term branchial uptake and internal distribution of radio-labeled waterborne Na+ uptake in juvenile tamoatá, H. litoralle, was examined. These measurements might provide important information regarding the possible short-term effects of formation water leakage into the rivers on osmoregulation of Amazon fish.
Material and Methods
Experimental Animals
Juvenile (8–40 g) tamoatás (H. litoralle) were obtained from Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA) fish culture facility in Manaus, Brazil. Fish were transported to the Laboratory of Ecophysiology and Molecular Evolution, National Institute for Research in the Amazon and maintained in aerated 500-l tanks with ion-poor well water (Table 1) for at least 14 days. Fish were fed once a day with commercial food with 28% crude protein to apparent satiety.
Acute Toxicity Test
After the acclimation period, short-term (24-h) toxicity tests were conducted to identify the maximum concentration of formation water that juvenile tamoatás could tolerate. Accordingly, fish were randomly separated into fifteen 20-l aquaria (N = 4 each aquaria) and exposed to five different concentrations of formation water (5, 6, 7, 10, and 20%; three replicates) for 24 h. The concentration of 5% formation water was the only one that did not kill any fish and, hence, was selected as the concentration used in the Na+ flux experiments.
Sodium Flux Experiments
A series of Na+ flux studies were conducted to evaluate the effects on osmoregulation associated with formation water or the major ions and Ca2+ contained therein. Fish were exposed to 5% formation water as well as well water spiked with Ca2+, major metals (Fe, Mn, Ba), and combinations of both (Fe + Ca, Mn + Ca, and Ba + Ca) at concentrations consistent with those in 5% formation water or unspiked well water (control). The concentrations of metals and major ions in formation water, diluted formation water, and well water are presented in Table 1.
Fish were fasted for a day prior to the flux measurements. Ten juvenile fish were weighed and transferred to 400-ml (one juvenile per chamber) flux chambers containing well water. After a 2-h settling period, the water in the chamber was completely removed and exchanged for the treatment water containing 1.0 μCi/l of 22Na+ (GE Healthcare). Fish were exposed to air for 2–3 s during this water exchange. After a further 10 min of mixing, water samples (5 ml) were taken then and 3 h later and acidified with 100 μl concentrated HNO3. Then the fish were anesthetized with buffered MS-222 (0.5 g/l) and blood was collected from the caudal vein with heparinized 1-mL syringes. Blood samples were centrifuged at 10,000g for 5 min to separate plasma. Juveniles were then sacrificed by a blow to the head, and gills, kidney, and liver were dissected and weighed separately. The remaining structures were considered “carcass.” Tissues were then partitioned for radioactivity analysis (for newly accumulated Na+).
For counting 22Na+, tissues (gill, carcass, plasma, liver, and kidney) were processed as described by Hogstrand et al. (1994). Briefly, 100 mg of tissue were placed in 1 ml of liquid tissue solubilizer (NCS’ GE Healthcare) and heated at 45°C for at least 48 h, then neutralized with glacial acetic acid, and diluted with 10 ml of an organic-compatible scintillation fluor (BCS; Amersham). Water samples (0.5 ml) were mixed with 1 ml of scintillation fluor (ACS; Amersham). Samples were counted on a liquid scintillation counter (LS 6500; Beckmann, Fullerton, CA). Counting efficiencies for 22Na+ were determined by internal standardization (i.e., by addition/recovery of known amounts of 22Na+).
Newly accumulated Na+ was calculated by the following equation (Grosell et al. 1997):
where M New is the newly accumulated Na+ concentration (nmol/g/tissue), a is the number of counts per minute (cpm) per gram of tissue or milliliter of plasma as appropriate, b is the number of cpm per liter of water, and c is the total Na+ concentration per liter of water. For the b and c terms, we used the average of measurements at the start and end of flux periods, which were never significantly different. Unidirectional whole-body uptake rates of waterborne Na+ (i.e., via the gills) were determined by summing the newly accumulated uptake values of all of the individual tissues (absolute, not weight-specific values) of a fish and dividing the result by fish weight and the length of the exposure period (3 h) to convert to a rate. It was also possible to detect small changes in the total Na+ concentration of the water (measured by atomic absorption spectrophotometry; see below) in the individual flux chambers over the 3-h period, from which whole-body net flux rates could be calculated. Sodium efflux rate was calculated from the difference between Na+ net flux rate and Na+ influx rate (see Wood 1992).
Water Analysis
Ion and metal levels in the water samples were analyzed using atomic absorption spectrophotometry (AAnalyst 800; Perkin-Elmer, Wellesley, MA). Standard solutions were made with analytical-grade reagents (Merck) dissolved in deionized water, and each standard curve was made with five different concentrations, with relationship above r 2 = 0.99 and with b of the equation near zero.
Statistical Analysis
Data were reported as means ± standard error of mean (SEM). Homogeneity of variances among groups was tested with the Levene test. All datasets did not present homogeneous variances; consequently, comparisons among different treatments were made using the Kruskall–Wallis analysis of variance (ANOVA) and Mann-Whitney test. Analyses were performed using the software Statistica (version 5.1), and the minimum significance level was set at p < 0.05.
Results
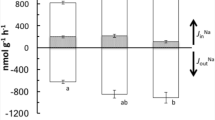
The composition of formation water of Urucu crude oil reserve contains very high ion levels, and Fe, Mn and Ba are the metals found in higher concentrations (Table 1). Juveniles maintained in well water showed a negative whole-body net flux (i.e., a Na+ loss). Whole-body net fluxes were not calculated in tamoatás exposed to 5% formation water because the high waterborne Na+ levels would have resulted in large errors without using radioactive Na+ to calculate Na+ efflux. Tamoatás exposed to 5% formation water showed a 51-fold increase in whole-body Na+ uptake compared to fish maintained in well water. Fish exposed to high waterborne Fe levels showed significantly higher whole-body Na+ uptake but significantly higher Na+ loss compared to animals maintained in well water due to the significant increase of Na+ efflux. The increase of waterborne Ca2+ levels caused an attenuation of this increase of whole-body Na+ uptake, efflux, and net Na+ loss provoked by high waterborne Fe levels. Tamoatás exposed to high waterborne Mn also showed significantly higher whole-body Na+ efflux compared to animals maintained in well water, but whole-body net Na+ loss was significantly lower because waterborne Mn increased twofold Na+ uptake. The increase of waterborne Ca2+ levels eliminated the effect of high Mn on whole-body Na+ uptake and efflux, but net Na+ fluxes were still significantly lower than those animals maintained in well water. High waterborne Ca2+ and high Ba + Ca2+ increased significantly whole-body Na+ uptake, but efflux and net flux were not affected significantly (Fig. 1).
Whole-body Na+ influxes (Jin), effluxes (Jout) and net fluxes (Jnet) of H. littorale exposed to well water (control), 5% formation water (FoW), and well water added with respective concentrations of 5% formation water of Ca2+, Fe, Mn, Ba, Fe + Ca2+, Mn + Ca2+, and Ba + Ca2+ for 3 h (N = 8–10). *Significantly different from control (p < 0.05); +significantly different from fish exposed to 5% formation water; osignificantly different from fish exposed to the same metal and low Ca2+
Newly accumulated Na+ in fish maintained in well water was slightly higher in the gills, plasma, and kidney, followed by the carcass and liver, indicating a rapid transfer from the gills to the plasma and from the plasma to the tissues. Newly accumulated Na+ was insignificant in the red blood cells. Tamoatás exposed to 5% formation water presented significantly higher newly accumulated Na+ in the gills, liver, kidney, and plasma than those maintained in well water. The same pattern on newly accumulated Na+ observed in fish maintained in well water was observed in animals exposed to 5% formation water: Three hours is enough for a fast transference of Na+ from the gills to the plasma and from the plasma to kidney and liver. However, newly accumulated Na+ increased almost 250-fold in the kidney and between 30- and 100-fold in the gills, liver, and plasma. No Na+ accumulation was detected in the carcass and red blood cells (Fig. 2). Tamoatás exposed to high waterborne Ca2+ levels significantly increased newly accumulated Na+ in the gills, plasma, liver, kidney, and red blood cells compared to those maintained in well water (Fig. 2a–e). Exposure to high waterborne Fe also significantly increased newly accumulated Na+ in the gills, liver, and kidney compared to those maintained in well water, and the increase of waterborne Ca2+ reversed the effect of high waterborne Fe on Na+ accumulation in the liver and kidney (Fig. 2).
Newly accumulated Na+ concentrations in gills (a), plasma (b), liver (c), kidney (d), red blood cells (RBC) (e), and carcass (f) of H. littorale exposed to well water (control), 5% formation water (FoW), and well water added with respective concentrations of 5% formation water of Ca2+, Fe, Mn, Ba, Fe + Ca2+, Mn + Ca2+, and Ba + Ca2+ for 3 h (N = 8–10). Different letters indicate significant difference between treatments by Kruskall–Wallis ANOVA and Mann-Whitney test (p < 0.05)
Specimens of tamoatás exposed to high waterborne Mn showed significantly higher newly accumulated Na+ in the gills, plasma, liver, and kidney compared to those maintained in well water, and the increase of waterborne Ca2+ reversed the effect of high waterborne Mn on Na+ accumulation. Exposure to high waterborne Ba significantly decreased newly accumulated Na+ in the carcass compared to those maintained in well water. On the other hand, the combination high waterborne Ba + Ca2+ significantly increased newly accumulated Na+ in the gills, plasma, and kidney compared to fish maintained in well water (Fig. 2).
When organ-specific newly accumulated Na+ was expressed relative to total-body load, in all groups except that exposed to 5% formation water, carcass represented the greatest percentage, followed by the gills, liver, and kidney (Fig. 3). In tamoatás exposed to 5% formation water, the highest percentage of newly accumulated Na+ was in the gills, followed by the kidney and liver, with an insignificant accumulation in the carcass.
Partitioning of whole-body new Na+ of H. littorale exposed to well water (control), 5% formation water (FoW), and well water added with respective concentrations of 5% formation water of Ca2+, Fe, Mn, Ba, Fe + Ca2+, Mn + Ca2+, and Ba + Ca2+, expressed as the relative contribution (i.e., mass-weighted contribution of each organ)
Discussion
Mortality below 50% is observed for the marine fish species Menidia beryllina, Cyprinodon variegates, and Gambusia affinis exposed to 22–50% formation water for 5–8 days (Caliani et al. 2009; Casini et al. 2006; Holdway 2002). Lower resistance was observed for the freshwater fish tamoatá, suggesting that the difference in the composition of marine and freshwater might play a significant role, as some ion and metal levels of the formation water used in the experiments conducted by those authors were lower than the background levels present in the marine water and much higher than those present in the freshwater. Fe, Mn, and Ba levels in the formation water from the Urucu Reserve were 4080-, 2400-, and 8-fold higher than maximum allowed limit stated by CONAMA (2005) for water designated for preservation of natural environments (class 1 waters in Brazil).
The values of whole-body net Na+ fluxes observed in tamoatás in well water indicated a light efflux and values were close to whole-body net Na+ fluxes values determined for this species in ion-poor black water by Baldisserotto et al. (2008) and for the Amazon fishes Colossoma macropomum, Serrasalmus rhombeus, and Leporinus fasciatus by Gonzalez et al. (1998). The transference of tamoatás from well water to 5% formation water exposed the fish to an 800-fold increase of waterborne Na+ levels. The Na+ levels in the 5% formation water are similar to Na+ levels in salinity around 5.0%. The exposure of killifish (Fundulus heteroclitus) and rainbow trout (Oncorhynchus mykiss) to a progressive salinity increase also provoked a large increase of Na+ influx (Prodocimo et al. 2007). Probably the very high increase of Na+ influx observed in tamoatás exposed to 5% formation water compared to euryhaline killifish and rainbow trout was due to the fact that the tamoatás were adapted to the ion-poor well water, which has 12-fold lower Na+ levels than the freshwater used in the experiment of Prodocimo et al. (2007). There is no information regarding salinity resistance by tamoatás, despite their occurrence in extreme environmental conditions, from the ion-poor water of Rio Negro to the changed environments of the small streams crossing many towns and villages of the Amazon.
The data of new Na+ accumulation in the tissues demonstrated that the 3-h period of exposure to 5% formation water was enough to transfer Na+ from the gills to the plasma and later to liver and kidney, but no Na+ accumulated in the carcass and red blood cells. As no Na+ accumulated in the carcass and in the present experiment the digestive tract was included in the carcass, probably no Na+ uptake occurred by the digestive tract through water ingestion. The very high new Na+ accumulation in the kidney indicates that this organ filtered the excess Na+ uptake from the formation water and avoided its accumulation in the carcass and red blood cells.
The presence of Fe and Mn and the increase of waterborne Ca2+ levels in the water increased whole-body Na+ influx in tamoatás. Brown trout, Salmo trutta, exposed for 5 days to a 50-fold lower waterborne Mn than the present experiment (4.55 μmol/l) also presented higher Na+ influx than unexposed fish (Reader and Morris 1988). Ferrous iron (Fe2+) enters the gill cells via a Fe2+/H+ symporter (Bury and Grosell 2003; Cooper et al. 2007), which can also transport Mn and Ca2+, and due to its promiscuous transport property, it is also know as divalent metal transporter 1 (DMT1, NRAMP2 or SLC11 A2) (Nevo and Nelson 2006). Therefore, the increase of waterborne Mn or Ca2+ could activate the DMT1, and as Na+ is likely to compete with H+ on the proton-binding site of the transporter and to generate a Na+ slippage through the proton transport pathway (Nevo and Nelson 2006), this might explain the increase of Na+ influx promoted by these metals. In addition, Fe can induce the apical epithelial Na+ channel expression and amiloride sensitive Na+ transport in fetal lung epithelial cells (Rafii et al. 2000). However, at least in the neon tetra, Paracheirodon innesi, a native fish from the Negro River, Na+ influx was mildly sensitive to amiloride (Gonzalez and Preest 1999), which indicates that the expression of this kind of channel might not be usual in Amazon fishes. The combinations Fe + Ca2+ and Mn + Ca2+ in the water did not change Na+ influx compared to the metals alone in the water because the DMT1 transporter would be already activated.
Iron and Mn increased Na+ efflux in tamoatás. Brown trout exposed for 5 days to waterborne Mn (4.55 μmol/l) also presented higher Na+ efflux than unexposed fish (Reader and Morris 1988). Loss of Na+ is usually considered to occur primarily by branchial permeability (McDonald et al. 1989). To our knowledge, there are no studies regarding epithelial Na+ permeability and Fe exposure in fish, but exposure of rainbow trout to 1–6 mg/l Fe for 1–2 days provoked gill epithelial lifting from the underlying membrane as well as hypertrophy (swelling), degeneration, and sloughing of lamellar epithelium (Fish 2009). Exposure to 1.02 mg/l Fe for 3 h might be enough to damage gill epithelia in tamoatás and increase the Na+ efflux. This hypothesis is in accordance with the fact that high waterborne Ca2+ reduced the Na+ efflux provoked by Fe, because the control of the diffusive ion efflux requires a low permeability of the paracellular tight junctions of the gills, which is dependent on Ca2+ binding (Hunn 1985). However, in several fish from the Negro River, waterborne Ca2+ does not seem to regulate branchial ion permeability (Gonzalez et al. 1998), and tamoatás exposed to high waterborne Ca2+ did not show lower Na+ influx than control fish. Barium blocks K+ channels and has no direct effect on Na+ channels (Hoffmann et al. 2002), and consequently, no effect on Na+ fluxes were expected, as observed in the present experiment.
In conclusion, the present study demonstrated that tamoatás exposed to 5% formation water presented a very high whole-body Na+ influx, probably due to the high Na+ levels in this water. Waterborne Fe and Mn stimulated whole-body Na+ influx, but Fe increased Na+ efflux, causing Na+ loss. Waterborne Mn, on the other hand, decreased whole-body Na+ efflux, reducing Na+ loss by this species. Waterborne Ca2+ also affected whole-body Na+ influx but had no significant effect on net Na+ fluxes. These results demonstrated that spilling of formation water in ion-poor Amazon rivers would dramatically disrupt osmoregulatory balance of tamoatás and probably other Amazon fish species, impairing their survival and reduce biodiversity. Waterborne Fe and Mn also affect tamoatá ion regulation, indicating that these metals must be evaluated in industrial or mining effluents in the Amazon Basin.
References
Baldisserotto B, Copatti CE, Gomes LC, Chagas EC, Brinn RP, Roubach R (2008) Net ion fluxes in the facultative air-breather Hoplosternum littorale (tamoata) and the obligate air-breather Arapaima gigas (pirarucu) exposed to different Amazonian waters. Fish Physiol Biochem 34:405–412
Bury NR, Grosell M (2003) Waterborne iron acquisition by a freshwater teleost fish, zebrafish Danio rerio. J Exp Biol 206:3529–3535
Caliani I, Porcelloni S, Mori G, Frenzilli G, Ferraro M, Marsili L, Casini S, Fossi MC (2009) Genotoxic effects of produced waters in mosquito fish (Gambusia affinis). Ecotoxicology 18:75–80
Casini S, Marsili L, Fossi MC, Mori G, Bucalossi D, Porcelloni S, Caliani I, Stefanini G, Ferraro M, di Catenaja CA (2006) Use of biomarkers to investigate toxicological effects of produced water treated with conventional and innovative methods. Mar Environ Res 62:S347–S351
CONAMA (Conselho Nacional do Meio Ambiente) (2005) Resolução CONAMA no 357, from March 17, 2005. Diário Oficial União 53(1):58–63
Cooper CA, Shayeghi M, Techau ME, Capdevila DM, MacKenzie S, Durrant C, Bury NR (2007) Analysis of the rainbow trout solute carrier 11 family reveals iron import ≤pH 7.4 and a functional isoform lacking transmembrane domains 11 and 12. FEBS Lett 581:2599–2604
Fish JT (2009) Groundwater water treatment for iron and manganese reduction and fish rearing studies applied to the design of the Ruth Burnett Sport Fish Hatchery, Fairbanks, Alaska. Aquat Eng 41:97–108
Gonzalez RJ, Preest MR (1999) Ionoregulatory specializations for exceptional tolerance of ion-poor acidic waters in the neon tetra (Paracheirodon innesi). Physiol Biochem Zool 72:156–163
Gonzalez RJ, Wilson RW (2001) Patterns of ion regulation in acidophilic fish native to the ion-poor, acidic Rio Negro. J Fish Biol 58:1680–1690
Gonzalez RJ, Wood CM, Wilson RW, Patrick ML, Bergman HL, Narahara A, Val AL (1998) Effects of water pH and calcium concentration on ion balance in fish of the Rio Negro, Amazon. Physiol Zool 71:15–22
Gonzalez RJ, Wilson RW, Wood CM, Patrick ML, Val AL (2002) Diverse strategies for ion regulation in fish collected from the ion-poor, acidic Rio Negro. Physiol Biochem Zool 75:37–47
Graham J (1997) Air-breathing fishes: evolution, diversity, and adaptation. Academic Press, London
Grippo RS, Dunson WA (1996) The body ion loss biomarker. 1. Interactions between trace metals and low pH in reconstituted coal mine-polluted water. Environ Toxicol Chem 15:1955–1963
Grosell MH, Hogstrand C, Wood CM (1997) Cu uptake and turnover in both Cu-acclimated and non-acclimated rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 38:257–276
Hoffmann EK, Hoffmann E, Lang F, Zadunaisky JA (2002) Control of Cl–transport in the operculum epithelium of Fundulus heteroclitus: long- and short-term salinity adaptation. Biochim Biophys Acta-Biomemb 1566:129–139
Hogstrand C, Wilson RW, Polgar D, Wood CM (1994) Effects of zinc on the kinetics of branchial uptake in freshwater rainbow trout during adaptation to waterborne zinc. J Exp Biol 186:55–73
Holdway DA (2002) The acute and chronic effects of wastes associated with offshore oil and gas production on temperate and tropical marine ecological processes. Mar Pollut Bull 44:185–203
Holth TF, Nourizadeh-Lillabadi R, Blaesbjerg M, Grung M, Holbech H, Petersen GI, Alestrom P, Hylland K (2008) Differential gene expression and biomarkers in zebrafish (Danio rerio) following exposure to produced water components. Aquat Toxicol 90:277–291
Hunn JB (1985) Role of calcium in gill function in freshwater fishes. Comp Biochem Physiol A 82:543–547
Jackson RE, Reddy KJ (2007) Trace element chemistry of coal bed natural gas produced water in the Powder River Basin, Wyoming. Environ Sci Technol 41:5953–5959
Lie KK, Meier S, Olsvik PA (2009) Effects of environmental relevant doses of pollutants from offshore oil production on Atlantic cod (Gadus morhua). Comp Biochem Physiol C 150:141–149
Maco Garcia JT (1997) Influência da água de formação da extração de petróleo do Rio Urucu sobre aspectos hematológicos e conteúdo iônico de Colossoma macropomum e Glyptoperichthys joselimaianus. MSc thesis, Instituto Nacional de Pesquisa da Amazônia/Universidade do Amazonas, Manaus
Manfra L, Moltedo G, Lamberti CV, Maggi C, Finoia MG, Giuliani S, Onorati F, Gabellini M, Di Mento R, Cicero AM (2007) Metal content and toxicity of produced formation water (PFW): study of the possible effects of the discharge on marine environment. Arch Environ Contam Toxicol 53:183–190
McDonald DG, Tang Y, Boutilier RG (1989) Acid and ion transfer across the gills of fish: mechanisms and regulation. Can J Zool 67:3046–3054
Nevo Y, Nelson N (2006) The NRAMP family of metal-ion transporters. Biochim Biophys Acta-Mol Cell Res 1763:609–620
Pantaleão SD, Alcantara AV, Alves JDH, Spano MA (2006) The piscine micronucleus test to assess the impact of pollution on the Japaratuba River in Brazil. Environ Mol Mutagen 47:219–224
Petróbras (2008) Provincia petrolífera de Urucu. O desafio de produzir ouro negro na Amazônia. http://www2.petrobras.com.br/minisite/urucu/urucu.html. Accessed 28 Jan 2010
Prodocimo V, Galvez F, Freire CA, Wood CM (2007) Unidirectional Na+ and Ca2+ fluxes in two euryhaline teleost fishes, Fundulus heteroclitus and Oncorhynchus mykiss, acutely submitted to a progressive salinity increase. J Comp Physiol B 177:519–528
Rafii B, Coutinho C, Otulakowski G, O’Brodovich H (2000) Oxygen induction of epithelial Na+ transport requires heme proteins. Am J Physiol C 278:L399–L406
Reader JP, Morris R (1988) Effects of aluminum and pH on calcium fluxes, and effects of cadmium and manganese on calcium and sodium fluxes in brown trout (Salmo trutta L.). Comp Biochem Physiol C 91:449–457
Stephens SM, Frankling SC, Stagg RM, Brown JA (2000) Sub-lethal effects of exposure of juvenile turbot to oil produced water. Mar Pollut Bull 40:928–937
Sundt RC, Baussant T, Beyer J (2009) Uptake and tissue distribution of C4–C7 alkylphenols in Atlantic cod (Gadus morhua): relevance for biomonitoring of produced water discharges from oil production. Mar Pollut Bull 58:72–79
Val AL, Almeida-Val VMF (1995) Fishes of the Amazon and their environments. Physiological and biochemical features. Springer-Verlag, Heidelberg
Wood CM (1992) Flux measurements as indices of H+ and metal effects on freshwater fish. Aquat Toxicol 22:239–264
Woodall DW, Rabalais NN, Gambrell RP, DeLaune RD (2003) Comparing methods and sediment contaminant indicators for determining produced water fate in a Louisiana estuary. Mar Pollut Bull 46:731–740
Zhu SQ, King SC, Haasch ML (2008) Biomarker induction in tropical fish species on the Northwest Shelf of Australia by produced formation water. Mar Environ Res 65:315–324
Acknowledgments
The authors thank National Research Council of Brazil (CNPq; Conselho Nacional de Desenvolvimento Científico e Tecnológico) for fellowships to B. Baldisserotto, L.O. Garcia, L.C. Gomes, and A.L. Val and Rio Grande do Sul State Research Foundation (FAPERGS; Fundação de Amparo à Pesquisa no Rio Grande do Sul) for financial support to B. Baldisserotto. In addition, this work was funded by CNPq and Amazonas State Research Foundation (FAPEAM; Fundação de Amparo à Pesquisa do Estado do Amazonas)–INCT ADAPTA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baldisserotto, B., Garcia, L.O., Benaduce, A.P. et al. Sodium Fluxes in Tamoatá, Hoplosternum litoralle, Exposed to Formation Water from Urucu Reserve (Amazon, Brazil). Arch Environ Contam Toxicol 62, 78–84 (2012). https://doi.org/10.1007/s00244-011-9673-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-011-9673-z






