Abstract
We investigated resistance to metals in carabid beetles inhabiting metal-polluted and reference areas. Chronic multigeneration exposure to toxic metal concentrations may potentially result in adaptation through decreased metal uptake rate and/or increased excretion rate. The cost of resistance to pollution could be associated with increased metabolic rate. To test these predictions, laboratory cultured F1-generation beetles originating from metal-polluted and reference sites were exposed to food contaminated with zinc and/or cadmium for 10 weeks. After that, uncontaminated food was offered to the animals for another 3 weeks. During the experiment, internal concentrations of Cd and Zn were measured as were respiration rates of the animals. The results obtained show no significant differences in metal accumulation and excretion patterns or respiration rates between the populations. This may suggest that adaptation has not occurred in the beetles chronically exposed to toxic metal concentrations. The possible explanations for the lack of differences between the populations are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Chronic exposure to sublethal concentrations of toxic chemicals in the environment can lead to changes at all levels of biologic organization of soil and epigeic invertebrates: biochemistry and physiology of individuals, populations, and whole communities (e.g., Donker 1992; Posthuma and van Straalen 1993; Jones and Hopkin 1998; Lagisz et al. 2002c). Recently, one of the most actively discussed problems in ecotoxicology has been the cost of tolerance to pollution. The topic arises from the widely recognized theory of energetic trade-offs in life history assuming that the energetic budget of every organism is limited. Consequently, the available energy must be distributed to all processes in such a way as to maximize the organism’s fitness, and the selection pressure is expected to optimize this trade-off (Sibly and Calow 1989).
Detoxification of metals is connected to many energetically costly processes. For example, the production of the metal-binding proteins, metallothioneins, may cause an increase in metabolic rate (Hopkin 1989; Walker et al. 2001). It has been observed that respiration rate was increased by 51% in the shrimps Palaemonetes paludosus (Rowe 1998), by 175% in the tadpoles of frog Rana catesbeiana (Rowe et al. 1998), and by 32% in the water snakes Nerodia fasciata (Hopkins et al. 1999) collected in metal polluted environments compared with individuals of the same species originating from unpolluted sites. Similarly, the pupae of moths Spodoptera exigua fed with diet contaminated with zinc for a dozen generations exhibited an increase in respiration rate compared with the control animals (Kramarz and Kafel 2003).
At the same time, animals exposed to increased metal concentrations may suffer physiologic deterioration caused by direct toxicity of the metals, and this can be reflected in decreased respiration rate (Devi 1997). In fact, exposure to copper-contaminated food caused a decrease in oxygen consumption in centipedes Lithobius mutabilis (Laskowski et al. 1996). A significant decrease in respiration rate was also found in snails Turbo intercostalis (Pandeswara and Yallapragada 2000) and in mussels Mytilopsis sallei (Devi 1997) intoxicated with cadmium. Decreased metabolic rate was observed in house crickets Acheta domesticus exposed to cadmium but not when exposed to zinc or lead (Migula 1986).
Metals, being nondegradable, tend to accumulate in animals. However, numerous investigators have reported that soil invertebrates belonging to the same trophic level and exposed to the same environmental concentrations of metals often display different internal concentrations of metals as result of different strategies of decontamination (e.g., Laskowski 1991). Ground beetles, as predators, are potentially exposed to increased levels of toxicants. At the same time, some species are abundant even at heavy metal-polluted localities and are known for efficient metal-detoxification mechanisms (Janssen 1991; Kramarz 1999a).
We aimed to study accumulation and excretion of cadmium, zinc, and a mixture of both—as well as the cost of metal detoxification expressed as respiration rate, body mass change, and survival probability—in the carabid beetle Pterostichus oblongopunctatus F. This species was chosen because of its high abundance in the vicinity of the zinc- and lead smelter “Boleslaw” in Southern Poland, which has been highly polluted with zinc, cadmium, and lead for at least 50 years. P. oblongopunctatus is a generalist predator, and its diet consists of seasonally available invertebrates and plants; therefore this species is potentially exposed to a broad range of metal concentrations in its diet. Zinc and cadmium were chosen for the experiment on the basis of their different physiologic functions: cadmium is regarded as a xenobiotic (nonessential) metal, whereas zinc is a micronutrient (essential). Both are bound by metallothioneins; however, cadmium has higher affinity to them and may displace zinc. The concentrations of metals used in the experiment were chosen in accordance to the earlier study by Mozder et al. (2003) on the same species. To clarify if multigenerational intoxication with metals may lead to genetically based changes in mechanisms of metal regulation, we studied the F1-generation of beetles reared from the animals collected from two sites: unpolluted and metalpolluted.
Materials and Methods
Study Animals
Adult beetles were trapped at two sites: reference (Zn: 151 ± 35 mg kg−1 dry humus; Cd: 0.84 ± 0.4 mg kg−1 dry humus; Cu: 10.7 ± 1.0 mg kg−1 dry humus; Pb: 136.0 ± 8.8 mg kg−1 dry humus) and polluted (Zn: 5104 ± 729 mg kg−1 dry humus; Cd: 51.1 ± 19.3 mg kg−1 dry humus; Cu: 37.6 ± 3.7 mg kg−1 dry humus; Pb: 1832 ± 215 mg kg−1 dry humus). The contaminated site was located 2.5 km away from a zinc and lead smelter, whereas the control site was established at a 30-km distance from the source of pollution. Beetles were mated, and hatched larvae were reared on uncontaminated food (cat food Royal Canin Kitten 36). After emerging from the pupa, imagines were overwintered to reach sexual maturity for 2 months at 12°C ± 1°C in a photoperiod of 8 hours light to 16 hours of darkness. Adults of the F1-generation process were kept in plastic vials (approximately 150 ml volume) filled with a 1 cm-thick layer of wet peat, maintained at 20 ± 2°C at a photoperiod of 16 hours light to 8 hours of darkness.
At the beginning of the accumulation experiment the beetles were randomly assigned to one of four artificial mediums: (1) control, (2) contaminated with 50 mg Cd kg−1, (3) contaminated with or 500 mg Zn kg−1, (4) contaminated with a mixture of 500 mg Zn kg−1 and 50 mg Cd kg−1. Approximately the same number (n = 19 to 21) of female and male animals in each treatment from each site were used in the experiment. The artificial medium comprised dried chicken meat mixed with metal–salt solutions (CdCl2 * 2.5 H2O GR-pure [Merck, Poland], ZnCl2 anhydrous [a.r. Ubichem plc, UK], or mixture of both) to contaminate the food to the desired level. Nominal and actual concentrations of metals in the food for all treatments are listed in Table 1. Animals were fed every 3 days, all the remains of old food were removed; and survival of the beetles was checked and recorded.
Experimental Setup
During the accumulation period of the experiment at 0, 7, 28, 49, and 70 days, two male and female animals from each group (population/treatment) were randomly selected. After 70 days, the decontamination phase began. All of the remaining animals were offered only uncontaminated food and were selected for analysis at days 77 and 98. After 48 hours of starvation, respiration rates were measured in the selected animals. Each individual was weighed to the nearest 0.1 mg (Radwag WPA 180/k, Poland). Two individuals from each group (population/sex/treatment/day) were then placed in a separate 50-ml flask connected to an individual channel of a computer-controlled, closed-circuit, 30-channel respirometer (Micro-Oxymax, Columbus Instrument). Each flask (with an Eppendorf-type tube with distilled water and a hole in the lid to maintain high humidity) was put in a chamber with controlled conditions (20°C ± 0.5°C at a photoperiod of 16 hours light to 8 hours of darkness). The respiration rates were measured for 72 hours at 4-hour intervals. The animals were not provided with food during the measurements. After the measurement the beetles were refrigerated for further analyses.
For chemical analyses, individual animals were dried at 105°C for 12 hours and weighed and digested in 2 ml boiling Suprapur-grade Merck nitric acid. After cooling to room temperature, each sample was diluted to 10 ml with deionized water. Samples of artificial medium were prepared for analyses in a similar way. All samples were analyzed for Zn concentration using flame atomic absorption spectrometry (AAS), and Cd content was measured with graphite furnace AAS (Perkin-Elmer AAnalyst 800). Three blank samples and two samples of standard reference material (bovine liver BCR no. 185, reference material, Sweden; certified concentrations; Zn = 142 ± 3 mg kg−1 and Cd = 0.298 ± 0.035 mg kg−1) accompanied every run of analysis and were used for correction of obtained data.
Statistical Analysis
When analyzing survival, body mass, and metal kinetics, each specimen was treated as an independent replicate. Because we found no significant differences in survival between male and female animals data for both sexes were pooled together to increase the power of analyses. The treatments were compared for their effects on the animals’ survival using Gehan’s generalized Wilcoxon test for multiple samples (Statistica 6.0, StatSoft), whereas site of origin and treatment were treated as discrete/grouping variables. Body mass was analyzed using Generalized Linear Models (Statistica 6.0).
The kinetics of metals in the beetles during the accumulation and decontamination periods was described with the one-compartment model (Janssen 1991). It was assumed that a metal is assimilated at a constant rate a (mg day−1) and eliminated at a constant rate k a (day−1). The following equation was used to estimate the parameters,
where C t is the concentration of a metal in the beetle (mg kg−1) at time t; C0 (mg kg−1) is the average concentration of the metal before the start of the experiment (t0); t is time in days; and e is the base of the natural logarithm. For the decontamination period, only the excretion rate (k e ) was studied, and the simplified exponential model was used:
where C70 (mg kg−1) is the metal concentration at t = 70 days (end of accumulation period); k e is the excretion rate for the decontamination phase; and t e (days) is the time of switching to uncontaminated food (Kramarz 1999). The parameters were estimated from untransformed data using the Levenberg-Marquardt method as implemented in the Statistica 6.0 software package (StatSoft). Because most of the fitted models were statistically insignificant (p > 0.05), probably because of the high variation of the obtained data, and did not allow for comparisons between the populations, samples for male and female animals as well as populations were pooled to calculate uptake and excretion rates according to the models described.
Additionally, data on metal concentrations during days 7 to 70 (accumulation experiment) were pooled, log transformed to get normal distributions within groups (population/treatment/sex), and analyzed with analysis of variance (ANOVA). Similarly, data from days 77 and 98 (decontamination experiment) were pooled, log transformed, and also analysed with ANOVA. The influence of cadmium and zinc on each other’s accumulation and excretion rates were checked by comparing concentrations during the accumulation and decontamination periods between one-metal and two-metal treatments (Student t-test on pooled data).
The data concerning respiration rates were analyzed separately for the accumulation period (days 0, 7, 28, 49, and 70) and the decontamination period. In the case of decontamination period, statistical analyses included the last day of accumulation to check for possible increases or decreases in metabolic rate (i.e., days 70, 77, and 98). The analyzed variables were oxygen consumption, carbon dioxide production, and respiration quotient (RQ). RQ was calculated according to the following equation,
where VCO2 is produced carbon dioxide and VO2 consumed oxygen. Because the obtained values were measured for two individuals at the same time, oxygen consumption and carbon dioxide production were calculated per gram of body mass (and per hour). For the data representing the animals from each group (population/sex/treatment), simple regression was calculated (Statgraphics Plus 5.0, Statistical Graphics) to check if there was any trend in respiration rate during a given period.
Results and Discussion
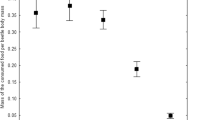
Out of the 315 animals used at the start of the experiment, 88 died before the end of it, and 224 were killed for AAS analysis, The initial dry body mass was significantly lower in the animals originating from the contaminated site (p = 0.0002), and female animals were larger than male animals in both populations (p < 0.0001). At the beginning of the experiment, there was no difference in body mass between the treatments (p > 0.05, N = 8). Survival analysis showed that neither sex nor treatment nor population type had any influence on the probability of death during the experiment. However, a general pattern was found showing that animals with larger initial body mass were more likely to survive longer (p = 0.0073, N = 24). During the experiment, average body mass tended to decrease during all treatments (p < 0.001, N = 224); however, this effect was most pronounced in the male animals originating from contaminated site (Fig. 1).
Changes in dry body weight in the carabid beetle P. oblongopunctatus originating from a contaminated site and exposed to four treatments: fed with uncontaminated food (0) and exposed for 70 days to food contaminated, respectively, with zinc (Zn), cadmium (Cd), and mixture of zinc and cadmium (Zn/Cd). After day 70, all animals were fed with uncontaminated food of the same type. (A) Male animals. (B) Female animals
The initial concentrations of zinc and cadmium in the animals were similar to those reported by Stone et al. (2002) for P. oblongopunctatus collected from unpolluted sites, i.e., on average 78.15 ± 13.92 mg kg−1 dry weight for Zn and 0.89 ± 0.39 mg kg−1 dry weight for Cd (N = 8). After the next 70 days of feeding with contaminated food, metal loads in animals treated with metals were significantly higher (p < 0.001, N = 128) and showed no differences between populations from different sites of origin (p = 0.151 for zinc and p = 0.424 for cadmium; N = 128) (Fig. 2). Average zinc concentration for zinc-treated animals was 125.50 ± 46.50 mg kg−1 dry weight (N = 32), and this result is consistent with numbers reported by Stone et al. (2002) for beetles captured at the metal-contaminated sites. This level was reached at the end of the first week, and the concentrations remained high but variable until the end of accumulation period. Female animals accumulated significantly more zinc per unit body mass than male animals (p = 0.003). The animals fed with cadmium-contaminated food during the accumulation period had average internal Cd concentrations of approximately 8.41 ± 5.61 mg kg−1 dry weight (N = 32), which was similar to the range reported by Stone et al. (2002) for animals collected from polluted sites. At the same time, there was no difference in cadmium concentrations between male and female animals (p = 0.512). Already after 7 days of feeding with uncontaminated food (decontamination period), the levels of metals in animals decreased to the values close to those observed at the beginning of the experiment (Fig. 2).
In the case of the accumulation period, all accumulation and elimination coefficients calculated from the pooled data were nonsignificant. The coefficient of elimination (k e ), calculated for the decontamination period for animals exposed to zinc, amounted to 0.008 ± 0.004 (asymptotic standard error [SE]) day−1 (p < 0.04), which equals an excretion rate of about 1 mg day−1 kg−1 dry weight. Cadmium k e was 0.047 ± 0.022 (asymptotic SE) day−1 (p < 0.05), which yields an excretion rate of approximately 1.5 mg day−1 kg−1 dry weight. In the closely related carabid Poecilius cupreus, Kramarz (1999) reported the following excretion coefficients (k e ): 0.002 ± 0.002 day−1 for zinc and 0.300 ± 0.019 day−1 for cadmium. Similar cadmium excretion rates those obtained by Kramarz (1999), i.e., 0.375 day−1, were also found by Janssen (1991) in Notiophilius biguttatus. The observed differences between species may result from species biology as well as from the experiment’s setup, e.g., the use of different medium for feeding beetles. However, the results obtained in our study showed that P. oblongopunctus is efficient in decontaminating metals and is able to control internal metal concentrations by excretion mechanism.
The differences between metal concentrations in carabids treated with single metals or with a mixture of zinc and cadmium were nonsignificant, which indicated that zinc and cadmium probably did not affect each other’s uptake at the levels tested in our study. These results are in agreement with the study by Kramarz (1999).
In our study, we did not find any significant differences in metal assimilation and excretion ability between animals originating from sites with different contamination histories. This suggests that no adaptation in terms of detoxification processes was developed in animals chronically exposed to increased levels of metals. On the contrary, the rapid weight loss observed during the experiment in F1-generation male animals originating from the metal-polluted site could be an indirect cue that male animals became more susceptible to additional stresses (e.g., nonnatural food). However, we still cannot exclude the existence of maternal effects, which could influence the characteristics of offspring from animals exposed to metals (Schmidt et al. 1992). At the same time, we observed higher levels of accumulated zinc in female than in male animals, which may indicate the existence of different accumulation patterns for this metal in both sexes of P. oblongopunctatus.
Comparison of metabolic rates between the two populations also did not show that adaptations were developed in the population from the metal-polluted site. In the case of either toxic effect or acclimation and adaptation to the metal-polluted environment, we should have observed differences in measured values between animals originating from the two sites. In fact, neither respiration rate nor RQ depended on site, treatment, or sex (p > 0.05, Figs. 3 through 5). Additionally, we did not observe any trend in respiration rate during the experiment (p > 0.05, Figs. 4 and 5). This outcome correspond well to the study by Lagisz and Laskowski (2002a) in which no differences in respiration rate of P. oblongopunctatus collected along the metal contamination gradient (including the sites studied here) were found. Similarly, no changes in metabolic rate were revealed in the populations of the isopod Porcellio scaber, which originated from metal-polluted sites (Khali et al. 1995) or in the pupae of the moths S. exigua exposed for 15 generations to cadmium (Kramarz and Kafel 2003).
Average oxygen consumption rates of beetles during the course of the experiment. After day, 70 all animals were fed with uncontaminated food of the same type. (A) Female animals from control site. (B) Male animals from control site. (C) Female animals from polluted site. (D) Male animals from polluted site. Square-control treatment; cross-zinc treatment; circle-cadmium treatment; triangle-mixture of zinc and cadmium
Average carbon dioxide production rates of beetles during the experiment. After day 70, all animals were fed with uncontaminated food of the same type. (A) Female animals from control site. (B) Male animals from control site. (C) Female animals from polluted site. (D) Male animals from polluted site. Square-control treatment; cross-zinc treatment; circle-cadmium treatment; triangle-mixture of zinc and cadmium
Average RQ values of beetles during the experiment. After day 70, all animals were fed with uncontaminated food of the same type. (A) Female animals from control site. (B) Male animals from control site. (C) Female animals from polluted site. (D) Male animals from polluted site. Square-control treatment; cross-zinc treatment; circle-cadmium treatment; triangle-mixture of zinc and cadmium, RQ-respiration quotient
This study was run in parallel with a larger project aimed at finding effects of both temporary and long-term pollution with metals on carabid beetles at the biochemical, physiologic individual, population, and community levels. Results obtained so far show that beetles trapped from contaminated sites exhibit higher susceptibility to additional stressors such as food deprivation and contamination as well as insecticide treatment (Stone et al. 2001). We observed the effects of contamination on population parameters in P-generation animals, and some differences in characteristics of the F1-generation animals, while working on populations originating from contaminated and uncontaminated sites (Lagisz et al. 2002; Kramarz et al. 2001; Lagisz and Laskowski 2002a, 2002b). We have also conducted experiments on metal dynamics on the F1-generation larval stage of P. oblongopunctatus originating from the same two sites as used in this study (Mozdzer et al. 2003). A significant difference was found between treatments in growth rate and metal accumulation, but not between populations, which is in agreement with the results of our previous experiment (Mozdzer et al. 2003). These results indicate that in terms of accumulation rates and resistance to metals in food, no adaptations have occurred in the beetles Pterostichus oblongopunctatus inhabiting chronically metal-polluted areas.
References
VM Devi (1997) ArticleTitleBioaccumulation and metabolic effects of cadmium on marine fouling dressinid bivalve, Mytilopsis sallei (Recluz) Arch Environ Contam Toxicol 31 47–53 Occurrence Handle10.1007/s002449900077
MH Donker (1992) Phsyiology of metal adaptation in the isopod Porcellio scaber Doctrol thesis, Vrije Universiteit Amsterdam, The Netherlands
SP Hopkin (1989) Ecophysiology of metals in invertebrates Elsevier Applied Science London, England
WA Hopkins CL Rowe JD Congdon (1999) ArticleTitleElevated trace element concentrations and standard metabolic rate in banded water snakes (Nerodia fascinata) exposed to coal combustion wastes Environ Toxicol Chem 18 1258–1263 Occurrence Handle1:CAS:528:DyaK1MXjt1SrtLY%3D
MPM Janssen (1991) Comparison of cadmium kinetics in four soil arthropod species Doctrol thesis, Vrije Universiteit Amsterdam, The Netherlands
DT Jones SP Hopkin (1998) ArticleTitleReduced survival and body size in the terrestrial isopod Porcellio scaber from a metal-polluted environment Environ Pollut 99 215–223 Occurrence Handle1:CAS:528:DyaK1cXktFeltLY%3D Occurrence Handle15093314
MA Khalil MH Donker NM Straalen ParticleVan (1995) ArticleTitleLong-term and short-term changes in the energy budget of Porcellio scaber Latreille (Crustacea) exposed to cadmium polluted food Eur J Soil Biol 31 163–172 Occurrence Handle1:CAS:528:DyaK28Xhs1Sns70%3D
P Kramarz (1999) ArticleTitleDynamic of accumulation and decontamination of cadmium and zinc in carnivorous invertebrates. 1. The ground beetle, Poecilus cupreus L Bull Environ Contam Toxicol 63 538–546 Occurrence Handle1:CAS:528:DyaK1MXmslOitrY%3D Occurrence Handle10501734
Kramarz P, Laskowski R, Stone D, Zygmunt P, Wojewodzic M (2001) Influence of chronic metal pollution on survival of carabid beetles exposed to additional intoxication. Proceedings of the Society of Environmental Toxicology and Chemistry Europe 11th Annual Meeting, Madrid, Spain, May 6–10, 2001, p 136
P Kramarz A Kafel (2003) ArticleTitleThe respiration rate of the beet armyworm pupae (Spodoptera exigua) after multi-generation intoxication with cadmium and zinc Environ Pollut 126 1–3 Occurrence Handle1:CAS:528:DC%2BD3sXlt1eitro%3D Occurrence Handle12860096
M Lagisz R Laskowski (2002a) ArticleTitleRespiratory metabolism in Pterostichus oblongopunctatus originating from metal contaminated and reference area Fresenius Environ Bull 11 74–77 Occurrence Handle1:CAS:528:DC%2BD38XisFOgtb0%3D
Lagisz M, Laskowski R (2002b) Effects of metal pollution on the ground beetle—Linking population parameters and metabolic characteristics. Proceedings of the Society of Environmental Europe 12th Annual Meeting, Vienna, Austria, May 12–17, 2002, p 177
M Lagisz R Laskowski P Kramarz M Tobor (2002) ArticleTitlePopulation parameters of the beetle Pterostichus oblongopunctatus F. from metal contamined and reference areas Bull Environ Contam Toxicol 69 243–249 Occurrence Handle1:CAS:528:DC%2BD38Xmt1WjurY%3D Occurrence Handle12107701
R Laskowski (1991) ArticleTitleAre the top carnivores endangered by heavy metal biomagnification? Oikos 60 387–390
R Laskowski M Maryański E Pyza J Wojtusiak (1996) Sublethal toxicity tests for long-lived invertebrates: Searching for a solution NM Straalen Particlevan DA Krivolutsky (Eds) Bioindicator Systems for soil pollution NATO ASI Series, 2: Environment. Volume 16 Kluwer Dordrecht/Boston/London 45–55
P Migula (1986) ArticleTitleCombined and separate effects of cadmium, lead and zinc on respiratory metabolism during the last larval stage of the house cricket, Acheta domesticus Biologia (Bratisl) 44 513–521
TJ Mozdzer P Kramarz A Piśkiewicz M Niklińska (2003) ArticleTitleEffects of cadmium and zinc on larval growth and survival in the ground beetle, Pterostichus oblongopunctatus Environ Int 28 737–742 Occurrence Handle1:CAS:528:DC%2BD3sXhsVymsLY%3D Occurrence Handle12605922
SL Pandeswara PR Yallaprogada (2000) ArticleTitleTolerance, accumulation and depuration in an intertidal gastropod, Turbo intercostalis, exposed to cadmium Mar Environ Res 50 103–106 Occurrence Handle10.1016/S0141-1136(00)00165-3
L Posthuma NM Straalen ParticleVan (1993) ArticleTitleHeavy-metal adaptation in terrestrial invertebrates: A review of occurrence, genetics, physiology and ecological consequences Comp Biochem Physiol 106 11–38
CL Rowe (1998) ArticleTitleElevated standard metabolic rate in a freshwater shrimp (Palaemonetes paludosus) exposed to trace element-rich coal combustion waste Comp Biochem Physiol 121 299–304 Occurrence Handle10.1016/S0305-0491(98)10103-7
CL Rowe OM Kinney RD Nagle JD Congdon (1998) ArticleTitleElevated maintenance costs in an anuran (Rana catesbeiana) exposed to a mixture of trace elements during the embryonic and early larval periods Physiol Zool 71 27–35 Occurrence Handle1:STN:280:DyaK1c7jt1WmsA%3D%3D Occurrence Handle9472810
GH Schmidt NMM Ibrahim MD Abdallah (1992) ArticleTitleLong-term effects of heavy metals in food on development of Aioolopus thalassinus (Saltatoria: Acrididae) Arch Environ Contam Toxicol 23 375–382 Occurrence Handle1:CAS:528:DyaK38XlvVKgsbc%3D Occurrence Handle1456784
RM Sibly P Calow (1989) ArticleTitleA life-cycle theory of responses to stress Biol J Linn Soc Lond 37 101–116
D Stone P Jepson P Kramarz R Laskowski (2001) ArticleTitleTime to death response in carabid beetles exposed to multiple stressors along a gradient of heavy metal pollution Environ Pollut 113 239–244 Occurrence Handle1:CAS:528:DC%2BD3MXjvFGhsLY%3D Occurrence Handle11383341
D Stone P Jepson R Laskowski (2002) ArticleTitleTrends in detoxification enzymes and heavy metal accumulation in ground beetles (Coleoptera: Carabidae) inhabiting a gradient of pollution Comp Biochem Physiol 32 105–112
CH Walker SP Hopkin RM Sibly DB Peakall (2001) Principles of ecotoxicology. Taylor Francis
Acknowledgments
The authors are grateful to R. Laskowski, M. Maryanski, A. Piskiewicz, and M. Wojewodzic for their assistance and advice. Financial support came from National Committee for Scientific Research (Grant no. 6 P04F 072 20) and Jagiellonian University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lagisz, M., Kramarz, P. & Niklinska, M. Metal Kinetics and Respiration Rates in F1 Generation of Carabid Beetles (Pterostichus oblongopunctatus F.) Originating From Metal-Contaminated and Reference Areas. Arch Environ Contam Toxicol 48, 484–489 (2005). https://doi.org/10.1007/s00244-004-0023-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-004-0023-2