Abstract
Calcium oxalate (CaOx) urolithiasis is a prevalent urinary disorder with significant clinical impact. This study investigates the therapeutic potential of Morin Hydrate (MH), a natural bioflavonoid, in preventing CaOx stone formation. Molecular docking studies revealed that MH binds strongly to glycolate oxidase (GO), suggesting its inhibitory effect on oxalate synthesis. In vitro assays demonstrated that MH effectively inhibits CaOx crystal nucleation, aggregation, and growth, altering crystal morphology to less stable forms. Diuretic activity studies in Wistar rats showed that MH substantially increased urine volume and ion excretion, indicating its moderate diuretic effect. In vivo experiments further supported these findings, with MH treatment improving urinary and serum markers, reducing oxidative stress, and protecting renal tissue, as evidenced by histopathological analysis. Notably, MH administration significantly decreased GO and lactate dehydrogenase activities in urolithiatic rats, indicating a reduction in oxalate production. These results suggest that MH is a promising candidate for the prevention and treatment of CaOx urolithiasis, with the potential for clinical application in reducing the risk and recurrence of kidney stones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urine is a supersaturated solution consisting of a variety of crystalloids and colloids, yet it does not precipitate under normal circumstances owing to the existence of stone-forming inhibitors. Urinary stones are made up of organic and inorganic matrix components that have been associated with proteins [1]. The most frequently found minerals in urinary stones are calcium, phosphate, oxalate, struvite, ammonium, and uric acid. Among these, calcium oxalate (CaOx) is the most prevalent, occurring in two forms: monohydrate (whewellite) and dihydrate (weddellite) [2]. The formation of calculi begins when the equilibrium between crystal promoters (such as the presence of high levels of urinary calcium, oxalate, phosphate, uric acid, low pH, and volume) and inhibitors (such as citrate, magnesium, and certain macromolecules) is altered [3].
In addition to lifestyle modifications like increasing fluid intake, reducing salt consumption, and adjusting animal protein and calcium intake, certain medications can significantly reduce the recurrence rate of CaOx stones. These drugs include thiazides for hypercalciuria, potassium citrate for hypocitraturia, and xanthine oxidase inhibitors for hyperuricosuria, adressing the underlying metabolic abnormalities [4]. However, dietary modifications or stone expulsive therapies are often ineffective for many patients with urinary stones, as the stones are too big or become lodged within the urinary tract. In these circumstances, patients require contemporary invasive methods. Unfortunately, most of these invasive techniques are distressing and the residual particles retained pose the risk of infection and seldom appropriate for individuals with a high frequency of urinary stone recurrence [1]. The limitation of chemical drugs in treating urolithiasis is due to the involvement of multiple factors in its etiology [2]. Therefore, natural products are multitargeted, efficacious, accessible, and affordable in the treatment of urolithiasis.
Flavonoids are a type of plant secondary metabolite with a polyphenolic structure, found in various fruits, vegetables, and beverages. They are widely used as nutraceuticals, pharmaceuticals, and cosmetic products. This is due to their antioxidant, anti-inflammatory, antimutagenic, and anticarcinogenic capabilities and their ability to influence important cellular processes [5]. Plant flavonoids exhibit anti-urolithiasis properties through their diuretic, antioxidant, anti-inflammatory, and antibacterial effects. They also protect microcirculation and modulate the synthesis and expression of endogenous stone activators or inhibitors [6].
Morin hydrate (MH), a bioflavonoid found in several plants of the Moraceae family, appears as a yellow crystalline pigment. It exhibits a wide range of pharmacological activities, including antioxidant, anti-inflammatory, neuroprotective, cardioprotective, anti-diabetic, hepatoprotective, antibacterial, antiviral, anticancer, and nephroprotective effects [7]. For the first time, we evaluated MH’s interaction with the target protein glycolate oxidase using molecular docking. Additionally, we assessed its diuretic and anti-urolithiatic potential both in vitro and in vivo.
Materials and methods
Molecular docking and visualization
Molecular docking is a computational method used to predict ligand-receptor interactions by simulating the binding of molecules to form stable complexes. In this study, 3D structures of MH and 4-carboxy-5-dodecylsulfanyl-1,2,3-triazole (CDST) were obtained from PubChem, and their torsional bonds were released and charges optimized using Auto Dock tools. The target protein, glycolate oxidase (PDB ID: 2RDT), was sourced from the RCSB protein data repository. The active site was identified using the CASTp 3.0 web server [8] which analyzes pocket characteristics and identifies contributing amino acids. The protein was prepared by removing water molecules and co-crystallized ligands using BIOVIA Discovery Studio and further optimized with Auto Dock tools. Molecular docking was performed using Autodock Vina software [9] to explore ligand-protein interactions, and the results were visualized with Discovery Studio Visualizer.
Drugs and chemicals
This study utilized analytical grade chemicals from Thermo Fisher Scientific India Pvt Ltd, Mumbai, India. Morin hydrate was obtained from Sigma-Aldrich Chemicals Pvt Ltd, Banglore, and Cystone from Himalaya Herbal Healthcare, India. Additional commercial kits were procured from Span Diagnostics and ERBA Diagnostics Mannheim, Mumbai, India.
In vitro anti-urolithiatic activity
The in vitro anti-urolithiatic activity of MH was evaluated through nucleation, aggregation, and oxalate depletion assays. Different concentrations (25–200 µg/ml) of the MH and Cystone were tested, and their optical densities were recorded to calculate the percentage inhibition in all three assays. Additionally, the morphology of CaOx crystals was examined microscopically with and without MH treatment. Cystone, a widely studied and clinically used polyherbal formulation for managing kidney stones, was used as the standard due to its multi-targeted approach, which includes inhibiting stone formation and promoting diuresis, mechanisms that align with the proposed effects of morin hydratet.
Nucleation assay
The nucleation assay assessed the effect of MH on CaOx crystal formation. Calcium chloride (5 mmol/L) and sodium oxalate (7.5 mmol/L) were mixed with MH and Cystone in a Tris-HCl buffer (pH 6.5). After 30 min of incubation at 37ºC, the optical density (OD) was measured at 620 nm to calculate the percentage inhibition of nucleation.
Aggregation assay
The aggregation assay evaluated MH’s impact on CaOx crystal aggregation. COM crystals were prepared and resuspended in Tris-HCl buffer (pH 6.5). MH and Cystone were added, followed by a 30-minute incubation at 37ºC. The OD was measured at 620 nm to determine the percentage inhibition of aggregation.
Oxalate depletion assay
The oxalate depletion assay examined MH’s effect on CaOx crystal growth. A CaOx slurry was prepared and combined with MH and Cystone in a sodium acetate buffer (pH 5.7). Oxalate depletion was monitored at 214 nm, and the percentage inhibition of crystal growth was calculated based on OD changes [10].
Formula for percentage inhibition
% Inhibition = [1 - (Optical Density of Sample / Optical Density of Control)] × 100.
Assessment of diuretic activity
Wistar rats (150–200 g) were housed in separate polypropylene cages under controlled conditions (12-hour light/dark cycle, 22–25 °C temperature, 50–60% humidity) with free access to food and water. This study received approval from the Institutional Animal Ethics Committee (IAEC Approval No. HCOP/IAEC/PR-8/2021) and followed the Committee for Control and Supervision of Experiments on Animals (CCSEA) guidelines.
To evaluate the diuretic activity using the Lipschitz model, rats were fasted for 18 h with water only. They were divided into four groups (six rats each): Group I (control) received 0.1% DMSO, Group II (standard) received 10 mg/kg furosemide, and Groups III and IV received 50 and 100 mg/kg of MH in 0.1% DMSO, respectively. All groups were also given 0.9% sodium chloride solution (5 ml/100 gm body weight) to maintain salt and water balance. The bladder of the rats was emptied before treatment and placed in metabolic cages equipped for urine collection [11]. Urine output was measured at 5 and 24 h, and parameters such as total urine volume, diuretic index, and Lipschitz value were recorded. Urinary electrolytes (Na+, K+, Cl-) were assessed using an Ion-Selective Electrode analyzer to determine saluretic, natriuretic, and carbonic anhydrase inhibitory activities [12].
Assessment of anti-urolithiatic potential
Urolithiasis was induced in rats by administering 0.75% ethylene glycol and 1% ammonium chloride in drinking water for the first five days, followed by 0.75% ethylene glycol alone for up to 28 days to promote crystal formation. The study involved 42 rats divided into seven groups. Group I (control) received 0.1% DMSO orally. Group II (disease control) underwent urolithiatic treatment for 28 days. Group III (positive control) received urolithiatic treatment plus 750 mg/kg cystone from days 15 to 28. Groups IV and V (prophylactic) received urolithiatic treatment plus MH at 50 and 100 mg/kg, respectively. Groups VI and VII (therapeutic) received urolithiatic treatment plus MH at 50 and 100 mg/kg from days 15 to 28 [13].
Urine samples were collected on days 14 and 28, and urine volume and parameters like calcium, phosphate, magnesium, uric acid, total protein, oxalate, and citrate were estimated. The pH and microscopic structure of urinary crystals were examined on day 28. Serum levels of calcium, creatinine, urea, and uric acid were measured post-blood collection. For histopathology, rats were euthanized, and kidneys were removed for histological examination with hematoxylin and eosin staining. The left kidney was homogenized and centrifuged to evaluate antioxidant parameters and liver homogenates were used to assess glycolate oxidase [14] and lactate dehydrogenase (LDH) activities.
Statistical analysis
Statistical analysis was conducted using one-way Analysis of Variance (ANOVA), followed by Tukey’s multiple comparison test with a significance level set at p < 0.05. Data were expressed as the mean with standard error of the mean (SEM), and differences were considered statistically significant if p < 0.05.
Results
Molecular docking
Docking studies were conducted to evaluate the binding energies of MH and the co-crystallized ligand 4-carboxy-5-dodecylsulfanyl-1,2,3-triazole (CDST) with glycolate oxidase. The Auto Dock Vina algorithm generated nine binding poses with an exhaustiveness value of 8 and a grid box size of 30Å x 30Å x 30Å, centered at coordinates (x = 35.059, y = 6.330, z = 11.784). Multiple docking runs were performed to analyze the binding affinity scores for each ligand. The binding energies for MH and CDST were − 8.7 and − 7.6 kcal/mol, respectively. CDST interacted with key amino acids such as ALA81, MET82, TRP110, LEU164, LEU205, VAL209 HIS260, GLY261, GLY293, ARG295, GLY314, ARG315, and PRO316, forming hydrogen bonds with GLY293, ARG295, GLY314, and ARG315. MH formed hydrogen bonds with TYR26, ALA81, GLY261, ARG263, ARG315 and TRP110 sharing active site interactions with CDST at ALA81, TRP110, HIS260, GLY261, and ARG315 (Figs. 1 and 2).
In vitro anti-urolithiatic activity
Microscopic examination revealed that the control group exhibited (Fig. 3A), numerous hexagonal and rectangular calcium oxalate monohydrate (COM) crystals with sharp edges. MH altered the morphology of COM crystals (Fig. 3F-I), at lower concentrations, and Cystone (Fig. 3B-E), at higher concentrations, encouraging the formation of tetrahedral and tetragonal calcium oxalate dihydrate (COD) crystals with smoother structures. MH and cystone significantly inhibited CaOx crystal nucleation and aggregation by 87%, 89%, 86.3%, and 80.3%, respectively, at a concentration of 200 µg/ml. At the same concentration, MH and Cystone reduced free oxalate by 66.5% and 59.7%, respectively (Fig. 4). MH significantly reduced CaOx crystal formation compared to cystone, demonstrating the anti-crystallization properties in a dose-dependent manner.
Diuretic activity
Furosemide at 10 mg/kg and MH at 100 mg/kg significantly increased urine volume (p < 0.001) compared to control at 5 and 24 h. MH at 50 and 100 mg/kg resulted in 25.1% and 27.9% of urine volume excreted within 5 h of administration. In contrast, furosemide resulted in 76.9% of urine volume excretion within five hours of administration indicating a shorter duration of action. At the end of 24 h, MH at 50 and 100 mg/kg showed a 4% and 25% increase in urine volume compared to the control (Table 1).
After 5 h, urinary pH measurements revealed significant differences in Groups II and III, with pH values of 5.00 (p < 0.001) and 5.50 (p < 0.01) respectively, compared to the control (pH 6.50). Additionally, a higher dose of MH showed a significant difference, with a pH of 6.00 (p < 0.01) compared to the standard furosemide. No statistically significant changes in urinary pH levels were observed among the rats after 24 h, except in Group II, which had a pH of 5.50 (p < 0.01) compared to the control.
Urine Samples were analyzed for electrolyte content after 5 and 24 h, with the results detailed in Tables 2 and 3. Group II, III (p < 0.05), and IV at 5 h, and Group II and Group IV at 24 h showed a significant increase in urinary sodium (p < 0.001) compared to the control. MH at 50 and 100 mg/kg increased sodium excretion by 23% and 54% after 5 h, and 40% for the higher dose after 24 h, compared to the control. Furosemide resulted in the highest sodium excretion, with an increase of 166% after 5 h and 72% after 24 h, significantly higher than MH.
A significant increase in potassium ion excretion was shown in Groups II and IV compared to the control at 5 h. Furosemide and MH at doses 10 and 100 mg/kg significantly increased (p < 0.001) potassium excretion by 387% and 58% respectively, compared to the control. Urinary potassium levels gradually increased in all groups except Group II after 24 h. MH at 100 mg/kg showed a 9% increase in urinary potassium excretion compared to the control. Chloride ion excretion decreased in all groups except the control between 5 and 24 h. However, both furosemide and the higher dose of MH significantly increased chloride ion excretion compared to the control, with furosemide showing a 91% (p < 0.001) increase and MH an 18% (p < 0.001) increase at 5 h, and a 42% (p < 0.001) increase for furosemide and a 15% (p < 0.05) increase for MH at 24 h.
In vivo anti-urolithiatic potential
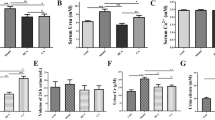
The urolithiatic treatment led to a significant reduction in percent body weight change in the urolithiatic group (p < 0.001) compared to the control, while other groups showed a notable weight gain by the end of the experiment. Water intake significantly increased (p < 0.001) in both the prophylactic and therapeutic MH groups compared to the urolithiatic group. Conversely, the urolithiatic group experienced a significant decrease (p < 0.001) in water intake due to disease progression compared to the control. The study revealed that urolithiatic group had a significantly lower urine output (p < 0.001) than the control (Table 4). The animals prophylactically and therapeutically treated with MH significantly increased urine volume (p < 0.001) compared to the disease control.
Inducing CaOx stones caused a significant decrease (p < 0.001) in pH (5.67) in the urolithiatic group compared to the control (pH 6.33). However, animals in the prophylactic groups treated with MH and those treated with cystone showed only a slight and non-significant rise in urinary pH, ranging from 6.5 to 7.5, compared to the urolithiatic group whereas therapeutic groups of MH showed a significant rise in pH.
Photomicrographs (40x) of calcium oxalate crystals in urine. (A) Group I: normal control, (B) Group II: Disease control, (C) Group III: cystone 750 mg/kg, (D) Group IV: MH 50 mg/kg (prophylactic), (E) Group V: MH 100 mg/kg (prophylactic), (F) Group VI: MH 50 mg/kg (therapeutic), (G) Group VII: MH100mg/kg (therapeutic)
Microscopic examination of urine of normal control and treatment groups was presented in Fig. 5. Control rats (Group I) had no observable crystals in their urine samples. In contrast, the urine samples of urolithiatic rats (Group II) contained numerous calcium oxalate monohydrate (COM) crystals. Groups treated with cystone and therapeutic doses of MH exhibited a shift from COM to calcium oxalate dihydrate (COD) crystals, resulting in reduced crystal size, formation, and urinary excretion. Prophylactic doses of MH (Groups IV and V) significantly decreased crystal formation.
All groups, except IV and V, demonstrated a significant increase (p < 0.001) in urinary calcium, phosphate, oxalate, uric acid, and total protein, along with decreased magnesium and citrate levels (Table 5), indicating the formation of CaOx stones and disease progression over 14 days of treatment. Conversely, groups treated prophylactically with MH (Groups IV and V) showed a slight but significant decrease in urinary calcium, phosphate, oxalate, uric acid, and total protein, and an increase in magnesium and citrate levels after 14 days of treatment.
At the end of 28 days, urine calcium excretion was significantly lower in the cystone (p < 0.001), prophylactic (p < 0.001), and therapeutic (p < 0.01) groups treated with MH compared to the urolithiatic group (Table 6), possibly reducing the probability of CaOx crystal formation. In addition, when compared to the urolithiatic group, Groups III to VII showed a substantial rise in magnesium and citrate and a substantial fall in the urine output of phosphate, oxalate, uric acid, and total protein (Table 7).
In Groups I–VII, serum levels of calcium, creatinine, urea, and uric acid were measured in order to evaluate renal function (Table 6). A significant (p < 0.001) increase in these markers was observed in the urolithiatic group when compared to the control (Group I), suggesting renal injury. Nevertheless, in both the preventive and therapeutic groups, treatment with the MH considerably decreased (p < 0.001) the serum concentrations of calcium, creatinine, urea, and uric acid compared to urolithiatic group (Group II), returning them to nearly normal levels similar to the standard group.
Effect of MH on renal biochemical markers (A) Catalase and (B) Superoxide dismutase (C) Reduced glutathione, GSH (D) Lipid peroxidation in ethylene glycol and aluminum chloride-induced urolithiasis in rats. Group I: normal control, Group II: Disease control, Group III: cystone 750 mg/kg, Group IV: MH 50 mg/kg (prophylactic), Group V: MH 100 mg/kg (prophylactic), Group VI: MH 50 mg/kg (therapeutic), Group VII: MH100mg/kg (therapeutic). The values were expressed in Mean±SEM; ***p<0.001, **p<0.01, *p<0.05 when compared to Group I; ###p<0.001, ##p<0.01, #p<0.05 when compared to Group II; SEM: Standard error of mean; H2O2: Hydrogen peroxide; MDA: Malondialdehyde
The impact of MH on antioxidant levels in kidney tissue homogenates of the treatment and control groups is shown in Fig. 6. When compared with the urolithiatic group to the normal control group, there was a substantial increase (p < 0.001) in LPO and a significant decrease (p < 0.001) in CAT, SOD, and GSH levels, likely due to oxidative stress caused by high free radical concentrations. Groups treated with prophylactic and therapeutic doses of MH, and cystone significantly increased (p < 0.001) the antioxidant parameters and significantly decreased (p < 0.001) the elevated levels of malonaldehyde, an indirect marker of LPO, demonstrating their effectiveness as antioxidants in preventing kidney injury.
Effect of MH on the liver (A) Lactate dehydrogenase (LDH) and (B) Glycolate Oxidase in ethylene glycol and aluminum chloride-induced urolithiasis in rats. Group I: normal control, Group II: Disease control, Group III: cystone 750 mg/kg, Group IV: MH 50 mg/kg (prophylactic), Group V: MH 100 mg/kg (prophylactic), Group VI: MH 50 mg/kg (therapeutic), Group VII: MH100mg/kg (therapeutic). The values were expressed in Mean ± SEM; ***p < 0.001, **p < 0.01, *p < 0.05 when compared to Group I; ###p < 0.001, ##p < 0.01, #p < 0.05 when compared to Group II; SEM: Standard error of mean; H2O2: Hydrogen peroxide
The effects of MH on biochemical markers in liver tissue homogenates on treated and control animals are shown in Fig. 7. The levels of GO and LDH in the urolithiatic group were considerably higher (p < 0.001) when compared to the control. In comparison to the urolithiatic group, the rise of LDH was dramatically reduced (p < 0.001) by prophylactic doses of MH. Similarly, when compared to the urolithiatic group, LDH levels were considerably lowered by cystone (p < 0.01) and greater therapeutic dosages of MH (p < 0.05). Furthermore, Groups III to VII displayed a substantial (p < 0.001) decrease in Go levels compared to the urolithiatic group.
Normal control rats revealed intact glomeruli and tubules with epithelial linings, showing no CaOx crystal deposits or inflammatory changes in histopathological examination of kidney tissue. Conversely, the urolithiatic group exhibited renal epithelial damage, inflammation, and intratubular and interstitial crystal deposits. Treatment with cystone and therapeutic doses of MH resulted in tubule dilation and minimal crystal formation. Remarkably, the kidney morphology of the prophylactic MH groups was similar to the normal control group (Fig. 8), with no evidence of crystal deposition.
Photomicrographs of renal tissue stained with hematoxylin and eosin under a light microscope (40x). (A) Group I: normal control, (B) Group II: Disease control, (C) Group III: cystone 750 mg/kg, (D) Group IV: MH 50 mg/kg (prophylactic), (E) Group V: MH 100 mg/kg (prophylactic), (F) Group VI: MH 50 mg/kg (therapeutic), (G) Group VII: MH100mg/kg (therapeutic)
Discussion
Kidney stone cases have surged globally over 30 years, with little progress in understanding or treating them effectively [15]. However, hyperoxaluria is one of the major risk factors for calcium oxalate stones. The oxalate concentration in urine is key to the balance between calcium and oxalate ions. Even minor increases in urinary oxalate can significantly impact the formation of Caox crystals. Hyperoxaluria not only encourages the formation of these crystals but also causes damage to the renal tubular epithelial cells. Therefore, reducing both exogenous and endogenous oxalate is essential to decrease the formation of calcium oxalate stones [16]. Glyoxylate is a key precursor for oxalate, converted by liver enzymes such as glycolate oxidase and lactate dehydrogenase [17].
In our study, we utilized molecular docking to explore the potential of morin hydrate, a natural flavonoid, against GO enzyme activity. This computational technique predicts the affinity between ligands and receptors, playing a crucial role in drug discovery and nutraceutical research. Our findings indicate that morin hydrate docked at the active site of the GO enzyme exhibited lower energy conformations compared to the co-crystallized ligand. This suggests a plausible molecular mechanism for reducing urinary oxalate levels, highlighting MH potential in this context.
The formation of kidney stones, irrespective of their type, is a complex, multistep process, that involves urinary supersaturation, crystal nucleation, aggregation, and growth [18]. The development of CaOx urolithiasis starts with nucleation, a thermodynamic phase transition in which dissolved substances in a supersaturated solution rapidly precipitate. This process is observable in nucleation assays. Preventing the nucleation of crystals or subsequent crystallization phases can thus reduce urolithiasis. The ability of a test compound to inhibit these processes is beneficial as it promotes the expulsion of tiny particles from the kidney, reducing the risk of urinary tract retention and stone formation.
Crystals of CaOx nucleate, grow and begin to interact, eventually forming aggregates that can be retained in the kidney. Among the two crystalline forms, COM crystals are more stable and tend to aggregate more than COD crystals, which have lower adhesion properties. The strong adhesion and aggregation tendency of COM crystals leads to their retention in the kidney, causing renal cell injury. In contrast, due to their low adhesion, COD crystals are more likely to be expelled in urine, reducing kidney stone development [19]. Increasing concentrations of MH inhibited crystallization by preventing CaOx nucleation and breaking down into tiny particles.
Crystal aggregation, the process by which numerous crystals bind to form large agglomerates, favors stone formation as these aggregates are retained in renal tubular cells [20]. The growth of calcium oxalate (CaOx) crystals in a supersaturated solution is driven by the adsorption of crystal-forming ions onto the CaOx crystal lattice, which reduces the overall free energy. This process is crucial for the formation of kidney stones. The test compound significantly inhibited CaOx crystal growth compared to the cystone, suggesting that MH significantly impede nucleation, aggregation and hinder CaOx crystal growth.
The diuretic index value measures the diuretic potential of a substance. MH at 50 and 100 mg/kg demonstrated diuretic index values of 1.02 and 1.36 at 5 h and 1.04 and 1.25 at 24 h, respectively. These values suggest that MH has moderate diuretic potential (Diuretic index > 1.5 is considered good; 1.0 to 1.5 moderate; 0.72 to 1.0 mild; <0.72 no effect) [21]. Lipschitz values of 1.0 or higher indicate a positive effect, with 2.0 or higher showing strong diuretic potency [22]. MH showed a nearly positive effect with a value of 0.80 at the higher dose after 24 h.
The Na+/K + ratio serves as an indicator of natriuretic activity and is increasingly recognized as a biomarker for mineralocorticoid receptor antagonism, with its blockade elevating the urinary Na+/K + ratio in rats [23]. A ratio above 2 signifies effective natriuretic activity, while a ratio above 10 indicates potassium-sparing effects, essential for diuretic efficiency and preventing hypokalemia [22]. MH demonstrated natriuretic activity with Na+/K + ratios of 2.80 and 2.61 at 5 h, and 2.05 and 2.41 at 24 h for lower and higher doses, respectively, indicating favorable natriuretic activity.
Urinary excretion of sodium and chloride ions was measured to assess saluretic activity. MH significantly increased the excretion of these ions at both 5 and 24 h compared to the control, indicating a saluretic diuretic effect. The Cl-/Na + + K + ratio, or carbonic anhydrase (CA) index, was calculated to assess CA inhibition activity. Ratios between 0.8 and 1.0 suggest unlikely CA inhibition, while lower ratios indicate varying degrees of CA inhibition [22]. Thus, except for furosemide, all groups likely exert their diuretic effects through mechanisms other than CA inhibition. Furosemide showed slight CA inhibition with a ratio of 0.73 after 5 h of administration [24]. The urinary elimination of Na+, K+, Cl-, and urine volume remained stable among the MH-treated groups. Conversely, furosemide exhibited increased urine volume and electrolyte excretion during the initial 5 h, followed by a decline, suggesting its rapid onset and shorter duration of action compared to MH [25].
According to Ghelani et al. (2016), the urolithiatic group exhibited a decrease in the percentage changes in body weight, water intake, urine volume, and pH when compared to the control group [26]. This suggests that the treatment with ethylene glycol and ammonium chloride led to the deposition of CaOx crystals in renal tissue. On the other hand, the MH-treated groups exhibited notable increases in body weight, water consumption, and urine volume, all of which helped to maintain the pH of the urine. Numerous pointed, rectangular COM crystals were found in the urine of the urolithiatic group in the current investigation. On the other hand, there were fewer circular and octahedral-shaped COD crystals in the urine of the groups treated with cystone and MH. The development of CaOx kidney stones is frequently accelerated by high urine levels of calcium, oxalate, and uric acid, as well as insufficient levels of citrate and magnesium. Elevated phosphate levels serve as a nidus for CaOx crystals, and excess protein in the urine is indicative of renal tubular injury [27].
Reducing variables that promote stone formation and enhancing inhibitors of stone formation were both greatly aided by the prophylactic MH groups. The groups receiving cystone and therapeutic MH, on the other hand, demonstrated a small but considerable reduction in stone promoters and a rise in stone inhibitors. The urolithiatic group builds up nitrogenous waste products, including urea, creatinine, and uric acid, as a result of urinary blockage caused by calcium oxide stones. Serum analysis showed that giving MH to hyperoxaluric animals significantly reversed their electrolyte imbalance. According to earlier research, rats treated with ethylene glycol had lower levels of catalase (CAT), superoxide dismutase (SOD), and reduced glutathione (GSH), but their levels of lipid peroxidation (LPO) were noticeably higher [28]. These results were supported by our research, which showed that prophylactic dosages of MH substantially decreased LPO levels and demonstrated antioxidant action. According to Goyal et al. (2017) and Khan (2018), ethylene glycol rapidly promotes urolithiasis in rats by activating the liver enzymes LDH and GO [29, 30], leading to the deposition of CaOx crystals observable under microscopic examination of kidney sections in rats. MH has demonstrated significant GO inhibitory efficacy in vivo, as evidenced by the fact that prophylactic dosages of MH inhibited crystal deposits and normalized GO and LDH levels in a manner comparable to the control group.
Conclusion
The study evaluated the potential of morin hydrate, a natural bioflavonoid, as a therapeutic agent for calcium oxalate urolithiasis through in silico, in vitro, and in vivo models. Molecular docking showed MH’s strong binding to glycolate oxidase, suggesting inhibition of oxalate synthesis. MH exhibited substantial inhibition of calcium oxalate crystal nucleation, growth, and aggregation, alongside the promotion of CaOx crystal transformation from the more adhesive monohydrate form to the less adhesive dihydrate form. Furthermore, MH showed moderate diuretic activity by significantly increasing urine volume and electrolyte excretion in vivo, MH improved urinary and serum markers, reduced oxidative stress, and protected renal tissue, as evidenced by histopathological analysis. Furthermore, MH significantly reduced GO and LDH activities in urolithiatic rats, indicating reduced oxalate production. These findings indicate that MH could serve as a promising therapeutic agent for the prevention and treatment of urolithiasis, offering a natural alternative to existing treatments with notable efficacy and a favorable safety profile.
Data availability
No datasets were generated or analysed during the current study.
References
Kasote DM, Jagtap SD, Thapa D, Khyade MS, Russell WR (2017) Herbal remedies for urinary stones used in India and China: a review. J Ethnopharmacol 203:55–68. https://doi.org/10.1016/j.jep.2017.03.038
Jena SC, Panigrahi PN, Dey S (2016) Urolithiasis: critical analysis of mechanism of Renal Stone formation and use of Medicinal plants as Antiurolithiatic agents. Asian J Anim Veterinary Adv 11(1):9–16. https://doi.org/10.3923/ajava.2016.9.16
Patel PK, Patel MA, Vyas BA, Shah DR, Gandhi TR (2012) Wendl. (Solanaceae) against ethylene glycol induced urolithiasis in rats. J Ethnopharmacol 144(1):160–170. https://doi.org/10.1016/j.jep.2012.08.043. Antiurolithiatic activity of saponin rich fraction from the fruits of Solanum xanthocarpum Schrad
Lai Y, Zheng H, Sun X, Lin J, Li Q, Huang H, Hou Y, Zhong H, Zhang D, Fucai T, He Z (2022) The advances of calcium oxalate calculi associated drugs and targets. Eur J Pharmacol 935:175324. https://doi.org/10.1016/j.ejphar.2022.175324
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutritional Sci e47. 5. https://doi.org/10.1017/jns.2016.41
Zeng X, Xi Y, Jiang W (2019) Protective roles of flavonoids and flavonoid-rich plant extracts against urolithiasis: a review. Crit Rev Food Sci Nutr 59(13):2125–2135. https://doi.org/10.1080/10408398.2018.1439880
Rajput SA, Wang X, Yan H-C (2021) Morin hydrate: a comprehensive review on novel natural dietary bioactive compound with versatile biological and pharmacological potential. Biomed Pharmacother 138:111511. https://doi.org/10.1016/j.biopha.2021.111511
Tian W, Chen C, Lei X, Zhao J, Liang J (2018) CASTp 3.0: computed atlas of surface topography of proteins. Nucleic Acids Res 46(W1):W363–W367. https://doi.org/10.1093/nar/gky473
Trott O, Olson AJ (2009) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):NA–NA. https://doi.org/10.1002/jcc.21334
Bawari S, Negi Sah A, Tewari D (2018) Antiurolithiatic activity of Daucus carota: an in vitro study. Pharmacognosy J 10(5):880–884. https://doi.org/10.5530/pj.2018.5.148
Korah M, Rahman J, Rajeswari R, Sherief H, Lalitha V, Sengottavelu S, Sivakumar T (2020) Evaluation of diuretic efficacy and antiurolithiatic potential of ethanolic leaf extract of Annona squamosa Linn. In experimental animal models. Indian J Pharmacol 52(3):196. https://doi.org/10.4103/ijp.ijp_92_18
Fekadu N, Basha H, Meresa A, Degu S, Girma B, Geleta B (2017) Diuretic activity of the aqueous crude extract and hot tea infusion of Moringa stenopetala (Baker f.) cufod. Leaves in rats. J Experimental Pharmacol Volume 9:73–80. https://doi.org/10.2147/jep.s133778
Sweta Bawari, Sah AN, Tewari D (2020) Anticalcifying effect of Daucus carota in experimental urolithiasis in Wistar rats. J Ayurveda Integr Med 11(3):308–315. https://doi.org/10.1016/j.jaim.2018.12.003
Hall NP, Reggiani R, Lea PJ (1985) Molecular weights of glycollate oxidase from C3 and C4 plants determined during early stages of purification. Phytochemistry 24(8):1645–1648. https://doi.org/10.1016/s0031-9422(00)82527-3
Stamatelou K, Goldfarb DS (2023) Epidemiol Kidney Stones Healthc 11(3):424. https://doi.org/10.3390/healthcare11030424
Chen T, Qian B, Zou J, Luo P, Zou J, Li W, Chen Q, Zheng L (2023) Oxalate as a potent promoter of kidney stone formation. Front Med 10:1159616. https://doi.org/10.3389/fmed.2023.1159616
Huang Y, Zhang Y, Chi Z, Huang R, Huang H, Liu G, Zhang Y, Yang H, Lin J, Yang T, Cao S (2019) The handling of Oxalate in the body and the origin of Oxalate in Calcium Oxalate stones. Urol Int 104(3–4):167–176. https://doi.org/10.1159/000504417
Wang Z, Zhang Y, Zhang J, Deng Q, Liang H (2021) Recent advances on the mechanisms of kidney stone formation (review). Int J Mol Med 48(2):149. https://doi.org/10.3892/ijmm.2021.4982
Wesson JA, Ward MD (2006) Role of crystal surface adhesion in kidney stone disease. Curr Opin Nephrol Hypertens 15(4):386–393. https://doi.org/10.1097/01.mnh.0000232879.50716.6f
Chaiyarit S, Thongboonkerd V (2017) Defining and systematic analyses of aggregation indices to evaluate degree of Calcium Oxalate Crystal Aggregation. Front Chem. 5https://doi.org/10.3389/fchem.2017.00113
Hailu W, Engidawork E (2014) Evaluation of the diuretic activity of the aqueous and 80% methanol extracts of Ajuga remota Benth (Lamiaceae) leaves in mice. BMC Complement Altern Med 14:135. https://doi.org/10.1186/1472-6882-14-135
Hock FJ (ed) (2016) Drug Discovery and Evaluation: Pharmacological Assays. https://doi.org/10.1007/978-3-319-05392-9
Eudy RJ et al (2011) Oct. The Use of Plasma Aldosterone and Urinary Sodium to Potassium Ratio as Translatable Quantitative Biomarkers of Mineralocorticoid Receptor Antagonism. Journal of Translational Medicine, vol. 9, no. 1, 21 https://doi.org/10.1186/1479-5876-9-180
CUI J, ZHAO T, JIANG Y, ZHOU H (2006) Inhibitory effect of Furosemide on Carbonic anhydrase. Tsinghua Sci Technol 11(4):391–394. https://doi.org/10.1016/s1007-0214(06)70206-1
Khan TM, Patel R, Siddiqui AH (2023) Furosemide. National Library of Medicine; StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK499921/
Ghelani H, Chapala M, Jadav P (2016) Diuretic and antiurolithiatic activities of an ethanolic extract of Acorus calamus L. rhizome in experimental animal models. J Traditional Complement Med 6(4):431–436. https://doi.org/10.1016/j.jtcme.2015.12.004
Alelign T, Petros B (2018) Kidney stone disease: an update on current concepts. Adv Urol 20183068365:1–12. https://doi.org/10.1155/2018/3068365
Ly HT, Kim T, Khoi Nguyen M, Minh Le (2022) Diuretic efficacy and prophylactic effects of hydroethanolic extract from Musa balbisiana fruits against urolithiasis. Adv Traditional Med 22(4):823–836. https://doi.org/10.1007/s13596-022-00629-3. Van
Goyal PK, Verma SK, Sharma AK (2017) Antilithiatic potential of Vernonia cinerea against calcium oxalate calculi in experimental rats. J Phytopharmacology 6(2):149–155. https://doi.org/10.31254/phyto.2017.6213
Khan A (2018) Prevalence, pathophysiological mechanisms and factors affecting urolithiasis. Int Urol Nephrol 50(5):799–806. https://doi.org/10.1007/s11255-018-1849-2
Author information
Authors and Affiliations
Contributions
M. P and C. G developed the research concept and design, data collection and processing, performed the biological experiments, and conducted the biochemical and statistical analyses, wrote the manuscript, and interpreted the data. N. M managed the methods, docking, results, and statistical analysis. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ponugoti, M., Guntupalli, C. & Malothu, N. Morin hydrate mitigates calcium oxalate urolithiasis by inhibiting oxalate synthesis and modulating crystal formation. Urolithiasis 52, 127 (2024). https://doi.org/10.1007/s00240-024-01628-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00240-024-01628-6