Abstract
Introduction
This article attempts to provide a comprehensive review of the learning objectives and importance of the supine percutaneous nephrolithotomy (PCNL) technique.
Material method
We retrospectively reviewed the cases of Supine PCNL between January 2018 and January 2024. We divided the groups into 3: residents between 2 and 3 years (Group 1), residents between 4 and 5 years (Group 2), and endourologist (Group 3). The 2–3-year resident started to perform PCNL for the first time, while the 4–5-year resident started to perform Supine PCNL for the first time while previously performing prone PCNL.
Results
Access, fluoroscopy, and operation time were higher in Group 1, shorter in Group 2, and shortest in Group 3 (p < 0.001). Postoperative length of stay and the need for additional treatment were found to be shorter (p < 0.001), and the stone-free rate (SFR) increased (p < 0.001) from Group 1 to Group 3. The highest complication rates were observed in Group 1 (p = 0.002). SFR rate increased as the number of cases increased in Group 1 patients. Success was stable after 46–60 cases in terms of SFR. In Group 2, the SFR rate was stable after 31–45. cases. The most complications were observed in Group 1 and the least in Group 3.
Conclusion
In 2-3-year residents, access time and fluoroscopy time decrease with experience. In 4-5-year residents, due to their expertise in prone PCNL, the operation time and fluoroscopy time decrease with the number of cases performed. SFR is higher after 46–60 cases for 2-3-year residents and 31–45 cases for 4-5-year residents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the evolving environment of urology, the adoption of supine percutaneous nephrolithotomy (PCNL) is a significant evolution in managing renal stone disease. Its potential to improve stone-free rate (SFR) is closely linked to the skill level of the urologist performing the operation [1, 2]. This technique, distinguished by the patient’s positioning and the approach’s complexity, requires a rigorous analysis of the learning curve to master this technique within the framework of urological training programs [3]. Given the challenges associated with high stone scores and variable stone complexity, understanding the curve of skill acquisition in supine PCNL is crucial for the training of urologists and patient outcomes regarding safety and efficacy [4,5,6]. This article attempts to provide a comprehensive review of the learning objectives and importance of the supine PCNL technique, assesses the learning curve, and provides a comparative analysis of supine PCNL, highlighting the nuances of each approach in the context of urological training.
Material method
This study was approved by our institutional ethical review committee (Decision No: 2023/09–46 Date: 10.10.2023). All patients gave their written consent before participating in the study. We retrospectively reviewed the cases of Supine PCNL between January 2018 and January 2024.
The procedure of the PCNL resident education and training program in our hospital is as follows: The first-year resident does not primarily perform PCNL but observes and assists the senior resident and endourologist in fluoroscopy-assisted PCNL. Second-year residents assist the senior resident or the attending endourologist in the first ten to fifteen cases. Then, they can perform PCNL primarily under the supervision of the attending endourologis. Second- and third-year residents can perform procedures alone but always consult the attending endourologist in the operational steps, and the endourologist is involved in the procedure’s steps if necessary. 4-5th year residents perform the primary PCNL operation; the attending endourologist is present in the operating room, enters the case, and assists if necessary.
Supine PCNL has been performed in our clinic since 2017. Before 2017, Prone PCNL was performed in patients with stones larger than 2 cm. In our study, we divided the groups into 3: residents between 2 and 3 years (Group 1), residents between 4 and 5 years (Group 2), and endourologists (experienced specialists with > 5 years after board certification [Group 3]). In all three groups, the first 105 cases eligible for inclusion criteria were evaluated since 2017, when supine PCNL was started to be performed. Demographic data, intraoperative data, and stone characteristics were compared between the three groups. Each group was then divided into subgroups as the first 15 cases, 16-30th, 31-45th, 46-60th, 61-75th, 76-90th, and 91-105th cases, and comparative analysis was performed again. In this study, the 1–3-year resident started to perform PCNL for the first time, while the 4–5-year resident started to perform Supine PCNL for the first time while previously performing prone PCNL. A single surgeon performed supine PCNL in each group.
The most important point we paid attention to in the patients we included in our study was the following: If the surgeon in one group performed the case primarily, while the surgeon in the other group joined in and completed the case primarily instead, we excluded these patients from the study. Exclusion criteria were as follows: Patients undergoing Prone PCNL, patients with < 2 cm stones, history of neuromuscular disease, congenital renal anomaly, coagulopathy, morbid obesity, and skeletal deformity.
Demographic data including age, gender, body mass index (BMI), history of previous PCNL; preoperative evaluations including the degree of hydronephrosis, stone characteristics on a kidney-ureter-bladder radiograph, stone complexity (using Guy Stone Score [GSS]), stone density, stone volume, stone-skin distance; intraoperative data such as access, fluoroscopy, total operation time, number of accessories; postoperative data such as length of hospital stay (LOS), residual stone status and complications, need for additional treatment at 1-month follow-up and SFR were obtained from electronic patient folders and recorded in a database. The stone skin distance was calculated by measuring the distance from the skin to the leading edge of the stone at its largest diameter at a 45 angle from the horizontal [7]. All patients had detailed medical history, physical examination data, and laboratory data, including complete blood count, serum biochemistry, coagulation profiles, urinalysis, and urine culture results in the patient files. All patients received prophylactic antibiotherapy with second-generation cephalosporins. Antibiotic therapy was given to patients with growth in preoperative urine culture until sterile urine culture results were obtained. Stone complexity was classified using GSS as grade I, II, III, and IV [8]. Stone density (Hounsfield Unit [HU]) was calculated by the point value in the center of the stone. Stone volume was determined using the ellipsoid formula (0.167 × π × L × W × H) [9]. Access time is defined as the time from anesthesia induction to successful placement of access sheath. Operation time was determined as the time from the puncture of the collecting system to the nephrostomy insertion [10].
Renal access time was defined as the time from retrograde pyelography to puncture of the desired calyx, expansion of the tract with fascial dilators and placement of the Amplatz sheath. Fluoroscopy time was measured as the total fluoroscopy time used during the operation. The operation time was measured as the period starting with the needle puncture and finishing with the placement of the nephrostomy tube or JJ catheter. SFR was defined as the absence of a residual stone on Non-Contrast Computer Tomography (NCCT) 1 month after the operation or the largest stone size of the residual stone < 4 mm. Clavien-Dindo Classification was used for complications [11].
Operation technique
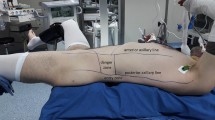
After induction of general anesthesia, a 5 Fr ureteral catheter was inserted into the renal pelvis with fluoroscopy in the Galdakao-modified Valdivia position. The differences between this position and complete supine are that the patient’s side to be operated on is elevated and one leg is opened to the side (Fig. 1A and B) [12]. Renal access was obtained through the renal calyx with a fluoroscopy-guided puncture needle (18 Gauge, Boston Scientific Corporation, Natick, MA). After placement of the guide wire (SensorTM Guide Wire, Boston Scientific), the tract was dilated using Amplatz dilators and a 30 Fr Amplatz sheath (Karl Storz, Tutlingen, Germany) was inserted. A 28 Fr nephroscope was inserted through the Amplatz. Stone was directly identified and fragmented into smaller fragments using a 3.4 Fr Pneumatic Lithotriptor (Vibrolity, Elmed, Ankara, Turkey) (Fig. 1C and D). After the decision to end the operation, an 8Fr nephrostomy tube was placed in all patients. In cases of residual stone or suspicion, or collector system perforation or suspicion, a 4.8Fr JJ catheter was inserted with the decision of the attending endourologist.
Statistical analysis
The data were evaluated in the statistical package program IBM SPSS Statistics 25.0 (IBM Corp., Armonk, New York, USA). The normal distribution of the data of numerical variables was evaluated with the Shapiro Wilk normality test and Q-Q graphs. Categorical variables were given as frequency and percentage. Descriptive statistics are given as Mean ± Standard Deviation and Median (IQR) values. Homogeneity of group variances was evaluated with the Levene test. Mann Whitney Utest was used to compare two groups of independent continuous variables where the normal distribution assumption was not met. Comparisons of more than two independent groups were performed by Kruskal Wallis test. In case of a difference as a result of Kruskal Wallis test, Dunn-Bonferroni’s multiple comparison test was used. The relationship between categorical variables was evaluated with the Pearson Chi-Square test in r x c tables. A value of p < 0.05 was considered statistically significant.
Results
No difference was observed between the groups in terms of the patients’ age, gender, BMI, and American Society of Anesthesiologists (ASA) scores. There was also no difference between the groups in terms of stone structure, degree of hydronephrosis, localisation of the stone, laterality of the stone, stone volume and density, and stone-skin distance among the findings obtained in preoperative radiological evaluations. Quantitative data on the demographic and radiological findings of the patients are shown in Table 1.
Among the intraoperative results, access time, fluoroscopy time, and operation time were higher in Group 1, shorter in group 2, and shortest in Group 3 (p < 0.001). While the number of access was less in Group 1, it was statistically higher in Group 2 and Group 3 (p = 0.002). JJ stent placement was higher in group 1 patients but statistically decreased from group 1 to group 3 (p < 0.001). Postoperative LOS and the need for additional treatment were found to be shorter (p < 0.001), and SFR increased (p < 0.001) from Group 1 to Group 3. Regarding complications, the highest complication rates were observed in Group 1, while fewer complications were observed in Group 3 (p = 0.002). Quantitative data on intraoperative and postoperative results of the patients are shown in Table 2.
When the relationship between the learning curve groups (groups of first 15 cases) and intraoperative times was analyzed, no statistical difference was observed in terms of the access time. However, the access time decreased as the number of cases increased in Group 1 and Group 3 patients. In Group 2, the access time was 10.5 ± 3.5 min in cases 0–15 and 7.2 ± 1.2 min in cases 91–105, and it was observed that it decreased between each subgroup and stabilized after cases 91–105 (p = 0.023). The operation time in Group 1 was 142.2 ± 29 min in 0–15. cases, 110.9 ± 31.2 min in 46–60. cases, 98.8 ± 15.5 min in 91–105. cases and although the operation time decreased after 46–60. cases, it was statistically stable (p < 0.001). Fluoroscopy time decreased as the number of cases increased in all groups; we could not find a statistically significant decrease in the first 105 cases. The findings of the operation times and learning curve groups are shown in Table 3; Figs. 2 and 3-4.
When the learning curve groups and SFR rates were analyzed, the SFR rate progressively increased as the number of cases increased in Group 1 patients. Success was stable after 46–60. cases in terms of SFR. In Group 2, the SFR rate was stable after 31–45. cases. There was no statistical difference between the number of cases and SFR in Group 3 patients. The relationships between the groups regarding SFR and the learning curve are shown in Table 4; Fig. 4.
More complications were found in Group 1 than in Group 2 and more in Group 2 than in Group 3. According to the Clavien-Dindo classification, complication grades (1-2–3 A-3B) decreased with experience. Grade 3 A-3B-4 and 5 complications were not observed in Group 3, and Grade 3B-4 and 5 complications were not observed in Group 2. In the learning curve groups, it was observed that complication rates varied in each group in grade 1 complications, but grade 2–3 A-3B complications decreased with the number of cases in the learning curve groups. The findings related to complications are shown in Tables 5 and 6.
Discussion
A limited number of articles in the literature regarding the supine PCNL learning curve exist. These studies were performed by surgeons with no previous PCNL experience. Our study is the first in the literature to include data on the learning curve of surgeons with no previous PCNL experience and data on the learning curve of surgeons with prone PCNL experience but no supine PCNL experience.
Although the supine position has gained popularity due to its potential advantages, performing this operation requires a significant learning curve [6, 13]. Surgeons must acquire the necessary skills to navigate the complex anatomy of the kidney, use appropriate instruments, and manage potential complications [14]. The learning curve for PCNL is a topic of ongoing research, as it may affect patient outcomes and surgical efficiency [15]. Different outcomes have been obtained in various studies examining the learning curve for PCNL. Still, most of the studies are related to the prone position and studies related to the learning curve in the supine position are quite limited [6, 15,16,17,18,19,20,21,22,23].
The most important parameters considered in the PCNL learning curve are the operation time (accessory time, fluoroscopy time and total operation time), SFR and complication [16, 23, 24]. The operation’s main step affecting surgical success is providing access to the calyx of the kidney [26]. Urologists in training programs need to learn how to perform percutaneous renal access safely; however, there is still a need for defined parameters for renal access training [27]. These parameters are the access methods and time. Fluoroscopy- and ultrasound-guided access methods are available [27]. In the literature on puncture and access time, Negrete-Pulido et al. [21] showed that access time decreased from 7.67 min for the first 20 cases to 4.69 min for the 61st to 82nd cases and reported that the success rate increased from 82.5 to 97.6% after 40 PCNL accesses. Sahan et al. [24] reported in their study on supine mini PCNL that the access time was 14 min in the first 15 cases and decreased to 6.5 min after 61-75th cases. Birowo et al. [6] related to supine PCNL access [6], on the other hand, showed that the access time decreased significantly and surgical competence increased after 20 urologist interventions. In our study, while the access time was the longest in Group 1 cases, it was the shortest in Group 3. In the learning curve groups, although the accessory time decreased with experience in the 2–3-year resident group, we could not obtain a predictive value related to the learning curve in the first 105 cases. In the 4–5-year resident group, accessory times decreased significantly with experience, and having previous Prone PCNL experience made it easier to provide access in supine PCNL. It was observed that they completed their learning curve for access from 16 to 30 cases.
The fluoroscopy time of Ziaee et al. [22] decreased from an average of 17.5 min in the first 15 cases to an average of 8.9 min in the 60th case. Allen et al. [15] decreased the fluoroscopy time from 9.93 min at the beginning to 3.85 min in 100–115 cases in their series of 155 PCNL cases. Negrete-Pulido et al. [21] reported that the fluoroscopy time decreased from 4.73 min in the first 20 cases to 2.03 min after 40 punctures and plateaued after 40 cases. Tanrıverdi et al. [16] observed that the mean fluoroscopy time was 17.5 min in 0–15 cases and decreased to 8.92 min after 46-60th cases and stated that although the fluoroscopy time decreased as the number of cases increased, it was not statistically significant. Sahan et al. [24] found that the fluoroscopy time decreased as the number of cases increased, but this was not statistically significant. In our study, there was a statistical difference between the groups regarding fluoroscopy time (Group 1: 8.63 ± 2.99 min vs. Group 2: 5.21 ± 2.23 min vs. Group 3: 2.02 ± 0.95 min). Still, in terms of the relationship between the learning curve and fluoroscopy time, similar to Sahan et al. [24], it was observed that the fluoroscopy time decreased as the number of cases increased, but this was not statistically significant. Our study did not obtain any cut-off value for fluoroscopy time in the first 105 cases in all three groups.
The most important clinical factor for the patient and the surgeon in stone surgery is achieving SFR. In studies evaluating SFR in patients undergoing PCNL related to the learning curve, Ziaee et al. [22] reported that SFR was stable after 105 cases, Jessen et al. [28] after 30–40 cases, and Yu et al. [29] after 120 cases. Bucuras et al. [23] studied 149 cases related to the supine PCNL learning curve and found a significant difference in SFR between 0 and 40 and the last 40 cases. After 40 cases, they reported that supine PCNL can be performed safely. Sahan et al. [24] reported a 93.3% SFR in cases 46–75. In our study, SFR rates were 48.6% in Group 1, 67.6% in Group 2, and 92.4% in Group 3, and it was observed that SFR increased significantly with experience. In the learning curve groups regarding SFR, in Group 1, SFR was stable after 46-60th cases, and SFR increased again after 76–90 cases, while in Group 2, SFR was stable after 31–45 cases. Our study also observed that because of more experience in Group 3, more accesses were performed to obtain SFR (two accesses rate: 19%). The surgeon needed less JJ placement because of the high SFR obtained (7.6%). In 2-3-year residents, the rate of two accesses was 3.8%, and the rate of JJ stent placement was 41.9%. In 4-5-year residents, these rates were 9.5% and 32.4%, respectively. The rates of patients who could not achieve SFR and required additional treatment were 28.6%, 25.7% and 6.7% from Group 1 to Group 3, respectively. The most common additional treatment modality in each group was ureterorenoscopic procedures.
Complication rates may be higher at the beginning of the learning process, and the complication rate tends to decrease with experience. Complications are essential to evaluate the safety and efficacy of the training process [6, 25]. In PCNL-learning curve studies [15,16,17,18,19,20,21,22,23,24], more articles have been written on hemoglobin level drop and the need for blood transfusion, and the Clavien-Dindo classification has been used less. We evaluated our complications using the Clavien-Dindo classification. Garg et al. [18] reported that complications decreased according to the Clavien-Dindo classification as experience increased in their study on complications developing during the PCNL learning curve. Yu et al. [29] reported that all Clavien > 2 complications occurred in their study’s first 60 cases in the patient groups. Zieaa et al. [22] reported that complications decreased significantly after the first 45 cases. Sahan et al. [24] reported that Clavien > 2 complications occurred in the first 30 cases in supine PCNL. In our study, complication rates were lowest in Group 3 patients and highest in Group 1 patients (Group 1: 45.8%, Group 2: 31.5%, Group 3: 15.3%). Clavien 3 A and complications were not seen in 61–75 cases for 1–3-year residents and 46–60 cases for 4–5-year residents. Clavien 3B complications were not seen in 4-5-year residents and the endourologist group, while in 2-3-year residents, they started to be seen from the 16-30th cases. Clavien 4 and 5 complications were not detected in any group. In a meta-analysis, the incidence of complications such as fever and sepsis in PCNL was found to be 9.5% [29]. In our study, fever was observed in 23 (7.3%) patients and sepsis was observed in 8 (2.5%) patients. In addition, LOS was 3.7 ± 1.6 days in Group 1, 2.8 ± 1.3 days in Group 2 and 1.6 ± 1.1 days in Group 3. The longer hospitalization in Group 1 may be due to more complications.
Our study has some limitations. The first is that the results belong to the learning curve of a single surgeon in each group and may not be valid for surgeons in other operating environments. The second limitation is the small patient volume. The third limitation is that we did not record the number of attempts to access the kidney during the accessory procedure. Of course, our most important limitation is the retrospective nature of the study. Prospective randomized designed studies would be much more appropriate to evaluate resident education and learning curve. In addition, when a surgeon from another group is involved and completes the primary case, excluding these patients shows that the SFR and complication rates are relatively high in Groups 1 and 2. However, this situation also shows how important patient-surgeon selection is. In patients with more complicated stones and comorbidities, having more experienced surgeons perform the procedure primarily will improve the results of the surgery. This principle should always remain a situation that should be considered in the resident training process. The results of our study have changed our clinical approaches in this direction and have the potential to change them in others.
Conclusion
Our study is the first in the literature in which urologists who started PCNL training first and urologists with previous prone PCNL experience who started supine PCNL training for the first time were evaluated and compared. In 2-3-year residents, access time and fluoroscopy time decrease with experience. Still, we think they do not reach competence in the first 105 cases but reach adequacy after 46–60. cases in terms of operation time. In 4-5-year residents, due to their experience in prone PCNL, the operation time and fluoroscopy time decrease with the number of cases performed supine PCNL. We think they reached an adequate access time after the 16-30th case. SFR is higher after 46–60 cases for 2-3-year residents and after 31–45 cases for 4-5-year residents.
Data availability
No datasets were generated or analysed during the current study.
References
Birowo P, Tendi W, Widyahening IS et al (2020) Supine versus prone position in percutaneous nephrolithotomy: a systematic review and meta-analysis. F1000Res 9:231. https://doi.org/10.12688/f1000research.22940.3
Zhang X, Xia L, Xu T et al (2014) Is the supine position superior to the prone position for percutaneous nephrolithotomy (PCNL)? Urolithiasis 42:87–93. https://doi.org/10.1007/s00240-013-0614-3
Choudhury S, Sinha Roy PP, Pal DK (2024) Calcutta position: a new modified supine decubitus for supine PCNL. Urologia 91:125–130. https://doi.org/10.1177/03915603231191268
Batagello CA, Barone Dos Santos HD, Nguyen AH et al (2019) Endoscopic guided PCNL in the prone split-leg position versus supine PCNL: a comparative analysis stratified by Guy’s stone score. Can J Urol 26:9664–9674
Lim EJ, Tanidir Y, Ganesan S et al (2022) Influence of Webinar-based learning on practice of Percutaneous Nephrolithotomy: outcomes of a global survey. J Endourol 36:279–286. https://doi.org/10.1089/end.2021.0445
Birowo P, Rustandi R, Risky Raharja PA et al (2022) The learning curve for a single surgeon using ultrasonography to guide supine percutaneous nephrolithotomy with an alken metal telescopic dilator. Heliyon 2022 8:e12524. https://doi.org/10.1016/j.heliyon.2022.e12524
Pareek G, Hedican SP, Lee FT Jr, Nakada SY (2005) Shock wave lithotripsy success determined by skin-to-stone distance on computed tomography. Urology 66(5):941–944. https://doi.org/10.1016/j.urology.2005.05.011
Thomas K, Smith NC, Hegarty N et al (2011) The Guy’s stone score–grading the complexity of percutaneous nephrolithotomy procedures. Urology 277–281. https://doi.org/10.1016/j.urology.2010.12.026
Heidar NA, Labban M, Nguyen DD et al (2022) Does volume matter? Incorporating estimated stone volume in a nomogram to predict ureteral stone passage. Can Urol Assoc J 16:E150–E154. https://doi.org/10.5489/cuaj.7364
Özlü DN, Ekşi M, Şahin S et al (2024) Effect of access sheath diameter used in percutaneous nephrolithotomy on renal function: a prospective randomized study. Urolithiasis 52:100. https://doi.org/10.1007/s00240-024-01582-3
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Falahatkar S, Moghaddam AA, Salehi M et al (2008) Complete supine percutaneous nephrolithotripsy comparison with the prone standard technique. J Endourol 22:2513–2517. https://doi.org/10.1089/end.2008.0463
Sabnis R, Desai MR, Singh A (2022) Supine Percutaneous Nephrolithotomy. J Endourol 36:S35–S40. https://doi.org/10.1089/end.2022.0299
Wang X, Zhang Y, Zhao F et al (2022) Learning curves of flexible ureteroscopy (275 cases) and Prone Percutaneous Nephrolithotomy (73 cases) in Pediatric stones: data from 348 children. J Endourol 36:1502–1508. https://doi.org/10.1089/end.2021.0757
Allen D, O’Brien T, Tiptaft R et al (2005) Defining the learning curve for percutaneous nephrolithotomy. J Endourol 19:279–282. https://doi.org/10.1089/end.2005.19.279
Tanriverdi O, Boylu U, Kendirci M et al (2007) The learning curve in the training of percutaneous nephrolithotomy. Eur Urol 52:206–211. https://doi.org/10.1016/j.eururo.2007.01.001
Jang WS, Choi KH, Yang SC et al (2011) The learning curve for Flank Percutaneous nephrolithotomy for kidney calculi: a single surgeon’s experience. Korean J Urol 52:284–288. https://doi.org/10.4111/kju.2011.52.4.284
Garg A, Yadav SS, Tomar V et al (2016) Prospective evaluation of learning curve of Urology residents for Percutaneous Nephrolithotomy. Urol Pract 3:230–235. https://doi.org/10.1016/j.urpr.2015.06.009
Reddy SVK, Shaik AB, Srinivas K (2016) Surgical Training in Percutaneous Nephrolithotomy: The Learning Curve. Kidney Urol Res 2016;2:008
Quirke K, Aydin A, Brunckhorst O et al (2018) Learning curves in Urolithiasis surgery: a systematic review. J Endourol 32:1008–1020. https://doi.org/10.1089/end.2018.0425
Negrete-Pulido O, Molina-Torres M, Castaño-Tostado E et al (2010) Percutaneous renal access: the learning curve of a simplified approach. J Endourol 24:457–460. https://doi.org/10.1089/end.2009.0210
Ziaee SA, Sichani MM, Kashi AH et al (2010) Evaluation of the learning curve for percutaneous nephrolithotomy. Urol J 7:226–231
Bucuras V, Bardan R, Muresan A et al (2011) The role of the learning curve in supine percutaneous nephrolithotomy. J Endourol 1:A292–A292
Sahan M, Sarilar O, Savun M et al (2020) Adopting for Supine Percutaneous Nephrolithotomy: analyzing the learning curve of Tertiary Academic Center Urology Team. Urology 140:22–26. https://doi.org/10.1016/j.urology.2020.03.022
Falahatkar S, Esmaeili S, Rastjou Herfeh N et al (2022) The safety of continued low dose aspirin therapy during complete supine percutaneous nephrolithotomy (csPCNL). Prog Urol 32:458–464. https://doi.org/10.1016/j.purol.2021.04.005
Ayranci A, Caglar U, Cakir H et al (2024) Inter-observer Reliability and Reproducibility of CROES, Guy’s and STONE Nephrolithometry Scoring systems for Predicting Percutaneous Nephrolithotomy outcomes. New J Urol 19:85–89. https://doi.org/10.33719/nju1388671
Watterson JD, Soon S, Jana K (2006) Access related complications during percutaneous nephrolithotomy: urology versus radiology at a single academic institution. J Urol 176:142–145. https://doi.org/10.1016/S0022-5347(06)00489-7
Jessen JP, Honeck P, Knoll T et al (2013) Percutaneous nephrolithotomy under combined sonographic/radiologic guided puncture: results of a learning curve using the modified Clavien grading system. World J Urol 31:1599–1603. https://doi.org/10.1007/s00345-012-1016-9
Yu W, Rao T, Li X et al (2017) The learning curve for access creation in solo ultrasonography-guided percutaneous nephrolithotomy and the associated skills. Int Urol Nephrol 49:419–424. https://doi.org/10.1007/s11255-016-1492-8
Falahatkar R, Falahatkar S, Khajavi Gaskarei MA et al (2024) The global, prevalence, and risk factors of postoperative fever after percutaneous nephrolithotomy: a systematic review and meta-analysis. Asian J Urol 11:253–260. https://doi.org/10.1016/j.ajur.2022.04.008
Author information
Authors and Affiliations
Contributions
Conception: YA, YOD, DNODesign: YA, MZK, OKSupervision: DNO, YA, BE, OKFundings: -Materials: -Data Collection: YOD, YA, DNOAnalysis: BELiterature Review: YA, YODWriter: YA, YOD, DNOCritical Review: MZK, YOD, BE.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by The University of Health Sciences, Izmir Tepecik Training and Research Hospital Ethical Committee, Izmir, Turkey (Decision No: 2023/09–46, Date: 10.10.2023).
Research involving human participants and/or animals
This article does not contain any studies with animals performed by any of the authors. All procedures performed in studies involving human participants were by the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Formal consent is not required for this type of retrospective study.
Inform of publication
The study’s results were not published in full or in part as an abstract.
Conflict of interest
The authors declare no competing interests.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arıkan, Y., Danacioğlu, Y.O., Özlü, D.N. et al. Analyzing learning curve for supine percutaneous nephrolithotomy in urology resident training programme: comparative analysis. Urolithiasis 52, 129 (2024). https://doi.org/10.1007/s00240-024-01624-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00240-024-01624-w