Abstract
Fig wasps (Agaonidae; Hymenoptera) are the only pollinating insects of fig trees (Ficus; Moraceae), forming the most closely and highly specific mutualism with the host. We used transcriptome sequences of 25 fig wasps from six genera to explore the evolution of key molecular components of fig wasp chemosensory genes: odorant-binding proteins (OBPs) and chemosensory proteins (CSPs). We identified a total 321 OBPs and 240 CSPs, with each species recording from 6 to 27 OBP genes and 6–19 CSP genes. 318 OBP genes are clustered into 17 orthologous groups and can be divided into two groups: PBP sensitive to pheromone and GOBP sensitive to general odor molecules, such as alcohols, esters, acids, ketones, and terpenoids. 240 CSP genes are clustered into 12 orthologous groups, which can be divided into three major groups and have functions, such as olfactory, tissue formation and/or regeneration, developmental, and some specific and unknown function. The gene sequences of most orthologous groups vary greatly among species and are consistent with the phylogenetic relationships between fig wasps. Strong purifying selection of both OBP and CSP genes was detected, as shown by low ω values. A positive selection was detected in one locus in CSP1. In conclusion, the evolution of chemosensory proteins OBPs and CSPs in fig wasps is relatively conservative, and they play an indispensable role in the life activities of fig wasps. Our results provide a starting point for understanding the molecular basis of the chemosensory systems of fig wasps.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fig wasps (Agaonidae; Chalcidoidea; Hymenoptera) are the only pollinating insects of fig trees (Ficus; Moraceae) with special morphology and life history. The chemical perception of fig wasps runs through their entire life cycle and plays an important role in foraging, laying eggs, mating, avoiding natural enemies, and searching for hosts. The chemical perception of external chemical substances is a key link in the life history of fig wasps, especially after mating, the females must accurately identify the species-specific volatile odor emitted by the receptive syconia (inflorescences) of the host fig tree within their shortest oviposition time. Even in complex natural forests with dozens of fig trees coexisting, they can accurately identify extremely small amounts of specific odor substances released by host and then locate them (Hossaert-Mckey et al. 1994; Grison-Pigé et al. 2002). So, the chemosensory system of fig wasps, like other insects, should be highly specialized in the long-term co-evolution with their host fig tree (Jacquin-Joly and Merlin 2004), forming a highly sensitive and specific chemical detector that can even lead to reproductive isolation between species and then speciation. The chemosensory system of fig wasps is an important model for studying the role of natural selection in the evolutionary adaptation of them. This process mainly involves the participation of Odor-Binding Proteins (OBPs), Chemosensory Proteins (CSPs), Olfactory Receptors (ORs), Ionotropic Receptors (IRs), and Gustatory Receptors (GRs).
Insect OBPs are a class of water-soluble acidic proteins mainly distributed in the lymph of olfactory receptors that play an important role in olfactory system, although they also exist in nonolfactory tissues and may have other functions (Paesen and Happ 1995). The OBP family members of insects are highly variable, and the consistency between sequences is even as low as 16.7%, and the length of amino acids of OBPs varies from 85 to 329 (Vieira and Rozas 2011). However, almost all OBP proteins have conservative domains, including the N-terminal containing signal peptide, 6 α-helix and 6 highly conserved cysteine profiles form 3 disulfide bonds (Cys1-Cys3, Cys2-Cys5, Cys4-Cys6) to maintain the tertiary structures for binding ligands (Zhou et al. 2009; Pelosi and Maĭda 1995; Vogt and Riddiford 1981). OBPs can be further subdivided into pheromone-binding proteins (PBPs) and general odorant-binding proteins (GOBPs). PBPs are sensitive and highly specific proteins whose main function is to identify and bind sex pheromones. They can effectively strengthen the communication between insects, promote mating, and courtship of insects (Zhang et al. 2018). The GOBPs are typically used to bind common odorant molecules in the environment.
CSPs are another water-soluble acidic proteins that have similar functions to OBPs, but only have four conservative cysteine profiles, forming two disulfide bonds (Jacquin-Joly et al. 2001). This arrangement is different from OBPs, where the disulfide bonds are inter-helical and make two small loops to form a more rigid structure (Angeli et al. 1999; Sánchez-Gracia et al. 2009). CSPs are widely expressed in the olfactory organs of insects, including the antennae, maxillary palps, and labial palps (Maleszka et al. 1997; Jin et al. 2005; Sheng et al. 2017), as well as nonolfactory tissues, such as legs, wings, and pheromone glands (Jacquin-Joly et al. 2001; Pelletier and Leal 2011). CSPs can play multiple physiological functions in insects, such as dissolving and transporting lipophilic ligands of different chemoreceptors, regulating insect growth and development, nutrient absorption, and participating in drug resistance in vivo (Ban et al. 2003; Maleszka et al. 2007). In insects, CSPs are more conservative than OBPs, with an average amino acid identity of 34% for arthropod (Vieira and Rozas 2011). They maintain high sequence consistency not only among the same species, but also among different orders and families. The sequence conservation in CSPs reflects the adaptation and evolution of insects to a large number of different types of environmental chemical factors during the long-term evolution (Wanner et al. 2004).
The main function of OBPs and CSPs in the process of chemoreception is to act as selective filters, combining specific odors or chemical signaling compounds in the external environment and transport them to receptors (Pelosi and Maida 1990; Zhou et al. 2009), thereby triggering neural impulses and triggering insect behavioral responses, such as foraging, finding spawning sites, and mating. They play an important role in receiving and recognizing chemical signals (Leal 2013). The pollination, egg laying, development, and mating of fig wasps in their host syconia require the participation of various chemosensory proteins, which are crucial for maintaining this symbiotic system. However, there is little research on the genes related to the chemical acceptance of fig wasps.
Before the fig wasp lands on a suitable fig, the host mainly attracts pollinators through volatile cues, but the final stages of host acceptance may also involve other sensory senses that has been rarely studied, such as touch, gustation, and vision (Ware et al. 1993; Wang et al. 2013). In previous studies, we identified and analyzed the OR and GR genes of 25 fig wasps belonging to 6 genera based on transcriptome data (Yu et al. 2023). Compared with the genome data of fig wasps, the number of OR (44–67) and GR (4–5) genes expressed in the female adults is relatively small, with 5–30 and 1–4, respectively, which is consistent with the discrepancy between genome and transcriptome data (Karpe et al. 2016; Legan et al. 2021). The amino acid sequences of fig wasp receptor genes vary greatly among different species, which is consistent with the phylogenetic divergence among them (Yu et al. 2023). This is consistent with the phenomenon that fig wasps of the same genus usually pollinate the same section and subsection of figs and that closely related fig species are more likely to share closely related pollinators (Wiebes 1979; Cruaud et al. 2012). Strong purifying selection of OR and GR receptors was detected, although positive selection loci can be detected from orthologous groups. Despite being the first link in the chain of chemoreception and playing an important role in insect life history, there is little research on OBPs/CSPs. It is necessary to understand their diversity and evolution in fig wasps (Wang et al. 2014).
Here, we identify and compare the OBP and CSP genes present in a taxonomically diverse range of fig wasps based on the putative OBPs and CSPs previously identified from 25 fig wasp transcriptomes (Chen et al. 2021). Our aim is to further characterize the genes by recording their sequence alignment and motif structures. Then, we provide a phylogenetic analysis to investigate whether sequence variation is associated with fig wasp phylogenetic proximity and host relationships. Specifically, we compare sequence variation (1) within a fig wasp species reared from two different host trees, (2) among closely related fig wasps that share the same host, (3) among congeneric fig wasp species associated with different host species, and (4) among fig wasp species belonging to different genera. The results are discussed in terms of the role that OBPs and CSPs have played in the diversification of fig wasps and co-evolution with their host fig trees.
Materials and Methods
Fig Wasp Samples
We sequenced the transcriptomes of 25 adult female fig wasps belonging to six genera (Agaonidae) reared from 22 Ficus host species (Table 1 and 2; Chen et al. 2021). Genera of the fig wasps included Blastophaga (five species), Ceratosolen (five species), Eupristina (one species), Kradibia (one species), Platyscapa (three species), and Valisia (10 species). For some authors, four of the five Blastophaga taxa (Blastophaga 1, 2, 3, and 5) collected from four different host species in Guangdong Province are considered as one species (Su et al. 2022; Yu et al. 2023). Four different Valisia species (Valisia javana sp1, sp2, sp7, and sp8) collected from one fig species F. hirta are closely related and share a recent common ancestor (Yu et al. 2019).
From each Ficus host species, we collected nearly mature figs and extracted 50–100 female fig wasps. Total RNA of their whole bodies was extracted using a modified CTAB method (Murray and Thompson 1980). The methods of mRNA-Seq library construction, illumina sequencing, assembly, and annotation have been explained in Chen et al. (2021). The proportion of unigenes annotated in the 25 fig wasps exceeded 75%, indicating that the transcriptomes of all 25 fig wasps were well assembled and relatively complete (Chen et al. 2021).
Gene Identification
Unlike previous studies that only used Hmmer (http://www.hmmer.org/) to search for chemosensory genes (Chen et al. 2021), the candidate OBP and CSP genes in this study was also used Blastp. The amino acid sequences of OBP and CSP genes from extensively studied reference species, such as Tribolium castaneum, Apis mellifera, Nasonia vitripennis, Anopheles gambiae, and Drosophila melanogaster, available in Nr, UniProt, and other databases, were used as query sequences to find the predicted genes by blastp (E-values < 10–5). Based on the structural characteristics of OBPs (PF01395) and CSPs (PF03392) from the Pfam database (http://pfam.sanger.ac.uk/), we searched for candidate OBP and CSP genes in the 25 fig wasp transcriptomes using the hmmscan command in HMM v3.3.2. The results were then filtered using a GA bitScore threshold with E-value < 10–5 and 25% HMM coverage. Finally, candidate genes with conserved domains were retained detected by both Blastp and Hmmer using interproscan (Quevillon et al. 2005) and the NCBI CDD web server (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
The most striking conservation is the presence of six and four cysteine residues in insect OBPs and CSPs, respectively (Pelosi and Maida 1995; Jacquin-Joly et al. 2001), that are also conserved in the fig wasp sequences described here. Multiple alignments of derived amino acid sequences from the OBP and CSP genes were constructed with MAFFT v7.475 program using default parameters (Katoh and Standley 2013). The position of cysteine residues was determined by Jalview v2 (Waterhouse et al. 2009) and the number of aromatic acids between adjacent cysteine sites was calculated. The cysteine patterns of OBPs and CSPS for each species and genus were summarized. N-terminal signal peptide was speculated by SignalP 4.1 Server (Petersen et al. 2011).
Orthologous Analysis of the OBPs and CSPs
The orthologous groups of OBPs and CSPs were predicted using orthoMCL with default parameters (Li et al. 2003). According to the number of fig wasp taxa in each orthologous group, the OBP groups were numbered from 1 to 17, and the CSP groups were numbered from 1 to 12. Sequence similarity in the genes within orthologous groups is important when assessing relationships between species. MegAlign in DNAStar was used to compare the divergence of protein sequences in orthologous groups at the levels of conspecific Blastophaga taxa from different hosts, as well as different Valisia species (all related to V. javana) that were reared from a single fig tree host, congeneric species, and species belonging to different genera (Clewley and Arnold 1997).
Phylogenetic Analyses of the OBPs and CSPs
Phylogenetic analyses of the OBPs and CSPs were performed with IQ-tree 2.12 using the maximum likelihood method based on VT + R7 and JTT + R6 models, respectively. The model selection was based on Bayesian Information Criterion (BIC). A total of 51 OBPs and 4 CSPs of Drosophila melanogaster (Hekmat-Scafe et al. 2002; Mckenna et al. 1994) and 21 OBPs and 6 CSPs of Apis mellifera (Forêt and Maleszka 2006; Vieira and Rozas 2011) were included as outgroups. The reliability of each tree node was evaluated by bootstrap proportions based on 1000 runs.
Recognition of Motifs
Motifs are shared three-dimensional structures that are conserved in functional proteins to maintain function. The multiple Em-for-Motif-Elicitation (MEME) web server was used for motif analysis (Bailey and Elkan 1994). The parameter settings followed Wang et al. (2014): distribution of motif occurrences = 0 or 1 per sequence, minimum width = 6, maximum width = 50, and maximum number of motifs to find = 6 or 8. Only motifs identified in input sequences with P < 0.0001 were included. The drawings of motifs were constructed using TBtools v1.09854 (Chen et al. 2020).
Tests for Positive Selection
Selection pressure on genes is assessed using the ratio (ω) of the normalized nonsynonymous (dN) to the synonymous (dS) substitution rates. The ω value infers evolution mode, ω > 1 is considered to provide evidence of positive selection for amino acid substitution, while ω < 1 represents purifying selection and ω = 1 indicates a lack of selection, i.e., neutral evolution. We used the branch model and branch-site model in the CodeML program from PAML package version 4.6 (Yang 2007) to study the different types of selection pressures acting on the fig wasp OBPs and CSPs. This analysis was based on the phylogenetic tree of 25 fig wasps reconstructed using 625 single-copy orthologous genes (Chen et al. 2021). Each orthologous gene was compared across at least four of the fig wasps.
For the analysis of branch models (Yang 2007), it was assumed each branch in the phylogenetic tree has independent ω. The free-ratio model was used to calculate ω of each branch as H1 hypothesis. Next, a one-ratio model was used as H0 hypothesis to calculate ω of the whole tree. This model assumes that all branches have ω equal. Finally, the significance of chi-square tests comparing the likelihood values of the two models was assessed. This was used to detect any significant heterogeneity between ω values to evaluate selection pressure on a specific branch.
Selection can act at specific amino acid sites or on entire branches. Branch-site models can simultaneously analyze the ω value of branches and amino acid sites in a phylogenetic tree, so that positive selection acting on both specific branches and specific sites can be detected. According to the phylogenetic tree, we choose null hypothesis model A (model = 2, nssites = 2, fix_omega = 1, omega = 1) as the no selection and model A (model = 2, nssites = 2, fix_omega = 0, omega = 2) as an indicator of positive selection alternative (Yang 2007). We tested these two models using likelihood ratio tests (LRTs). When the LRT was significant, a Bayes Empirical Bayes (BEB) analysis was used to identify positively selected sites with greater than 95% confidence.
Results
Identification of OBP and CSP Genes
A total of 321 OBPs and 240 CSPs were identified in the 25 fig wasp transcriptomes. Mean OBPs per species were 12.91 ± S.E. 6.28 (range = 6–27; Table 1) and mean CSPs per species were 9.6 ± 3.46 (range = 6–19; Table 2). The length of OBPs ranged from 94 to 233 aa with an average of 138 aa (Table S1). The proportion of OBPs with six conserved cysteine residues was 93.8% and all sequences contain at least three of them. The length of CSPs ranged from 99 to 249 aa with an average of 133 aa (Table S2). The proportion of CSPs with four conserved cysteine residues was 99.2%, with two sequences containing two and three cysteine residues, respectively.
The cysteine patterns of OBPs and CSPs in 25 fig wasps are C1-X20-33-C2-X3-C3-X27-43-C4-X6-12-C5-X4-8-C6 and C1-X6-8-C2-X17-19-C3-X2-C4 (C1–6 represents the conserved six cysteines and Xn represents any n amino acids), respectively. For OBPs, there are certain differences in the number of amino acids between adjacent cysteines among different genera and species. While for CSPs, the cysteines pattern of fig wasps is relatively conservative.
Analysis of Orthologous OBP and CSP Genes
Among the 321 OBPs, 318 were clustered into 17 orthologous groups (labeled OBPs 1–17) using orthoMCL (Table 1; Figs. 1, 2). The OBPs of each fig wasp taxon clustered into between 6 and 16 orthologous groups (mean = 10.44 ± 2.72 SE). Only OBP1 orthologous group was recorded in all fig wasps, and OBPs 2 through 10 were present in more than half of the fig wasps (Table 1). For orthologous OBP2, 4, 5, 7, and 8, the similarity of protein amino acid sequences between fig wasps was between 53.3% and 100%, while in the other orthologous groups varied from as low as 17.2% up to 48.8% (Table 1). From OBPs 1 through 10 were present in more than half of the sample species, sequence similarities for Blastophaga considered to belong to a single species were similar to those among V. javana-related species with the median of 100% and 98.6%, respectively (Fig. 3A). Lower-sequence similarity values were detected between congeneric species (median = 83.8%), and the lowest ones were found between genera (median = 67.6%).
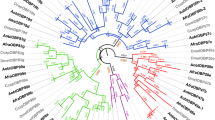
Phylogenetic tree of OBP genes from fig wasps, Drosophila melanogaster and Apis mellifera. Clades with black color indicate OBPs of fig wasps. These can be divided into two types: PBP and GOBP. OBP1-17 with different colors represent the orthologous groups of fig wasps. Clades with a yellow color indicate OBP genes of Drosophila melanogaster and those with a purple color indicate OBP genes of Apis mellifera. The branch labels are bootstrap values (%), which are based on 1000 replicates. The scale bar refers to a phylogenetic distance of 1 nucleotide substitutions per site
Sequence similarity of OBPs (A) and CSPs (B) in 25 fig wasps associated with four levels of taxonomic proximity. I (blue). The same Blastophaga species reared from four different hosts; II (red). Related species across different sites; III (gray). Species in different genera; IV (yellow). Between genera. Q1 represents the lower whisker limit and is the minimum data point extending to 1.5 times the frame height from the bottom of the frame; Q2 indicates that 25% of the data is less than this value; Q3 is the median data; Q4 indicates that 75% of the data is less than or equal to this value; Q5 represents the upper whisker limit and is the maximum data point extending to 1.5 times the frame height from the top of the frame. Values exceeding the upper or lower limit of the whiskers are represented by a dot “·”
All 240 CSPs were clustered into 12 orthologous groups (Table 2; Figs. 4, 5). The CSPs of each fig wasp taxon clustered into between 4 and 12 orthologous groups (mean = 7.36 ± 1.57). CSP1-8 were widespread, being present in from 25 to 17 fig wasps (Table 2). The remaining groups were each present in fewer fig wasp from 4 to 2. The lowest sequence similarity in CSPs 1–4 and 7–8 was higher than 50%, while in the other orthologous groups varied from as low as 39.5% up to 49.1% (Table 2). From CSPs 1 to 8 which were present in more than half of sample species, the sequence similarities among same species of Blastophaga were similar to those among V. javana-related species with the median of 100% and 97.5%, respectively (Fig. 3B). Lower-sequence similarities were found between congeneric species (median = 83.1%) and the lowest between genera (median = 73.6%).
Phylogenetic tree of CSPs genes from fig wasps, Drosophila melanogaster, and Apis mellifera. Clades with black color indicate CSPs of fig wasps and can be divided into group 1, 2, and 3 according to their motif structure. CSP1-17 with different colors represent the orthologous groups of fig wasps. Clades with yellow color indicate CSP genes of Drosophila melanogaster and the ones with purple color indicate the CSP genes of Apis mellifera. The branch labels are bootstrap values (%), which are based on 1,000 replicates. The scale bar refers to a phylogenetic distance of 0.5 nucleotide substitutions per site
Motif structure of CSP genes from 25 fig wasps. A Schematic representation of the 6 conserved motifs. Different colored boxes represent different motifs. The lengths of box do not correspond to lengths of motif. B Motif analyses of the CSP genes for 25 fig wasps. Three highly conserved sequences YTTKYDN (V/I) (N/D) (L/V) D at the N-terminal, DGKELKXX (I/L) PDAL at the central region, and KYDP at the C-terminal indicated by the red-dashed box were detected
Phylogenetic Analysis
The phylogenetic trees were reconstructed based on OBPs and CSPs of the fig wasps and using those of Drosophila melanogaster (Diptera) and Apis mellifera (Hymenoptera) as outgroups (Figs. 1, 4).
Based on the known functions of some OBPs in A. mellifera and D. melanogaster, the OBPs in the reconstructed phylogenetic tree could be divided into two major branches, PBP and GOBP (Fig. 1). The PBP branch included OBP1, 3–5, 8, 13, and 17 as well as OBPs of A. mellifera and D. melanogaster, whose functions have been predicted to be involved in the detection of pheromones. The other fig wasp OBPs clustered into the GOBP branch with OBPs of A. mellifera and D. melanogaster that are more liable to bind with general odors.
CSPs of fig wasps were clustered into 12 orthologous groups (Fig. 4). In the phylogenetic tree of CSPs, some orthologous groups clustered with some genes of A. mellifera whose functions have been predicted previously: CSP3, 6, and 10–12 grouped with AmelCSP1; CSP7 grouped with AmelCSP6 and CSP8 grouped with AmelCSP4; and CSP2 and 9 were related with AmelCSP2 and AmelCSP5.
Motif Analysis of OBP and CSP Genes
Although the motif structure of OBPs of different fig wasps is distinct (Fig. 2A and B), most members of the same orthologous group have similar motif structure, indicating that the same group of OBPs may share similar functions. These motif structures can be roughly divided into three types: 2-1-3, 5-2-1-4, and 6-1-4. The first type is PBPs, while the other two types are GOBPs. Among them, motif 1 is highly conserved and almost exists in all OBPs of fig wasps (Fig. 2A).
According to the motif structure, a total of 240 CSPs of 25 fig wasps can be further divided into three groups (Fig. 5A and B): CSP1, 3, 4, 6–8, and 10–12 belong to group1 with a motif structure of 4-2-(5)-1-3; CSP5 belong to group 2 with a motif structure of 2-5-1-3; and CSP2 and 9 belong to group 3 with a motif structure of 6-2-1–3. The highly conserved regions of other insect CSPs (Wanner et al. 2004), including YTTKYDN (V/I) (N/D) (L/V) D at the N-terminal and DGKELKXX (I/L) PDAL at the central region, and KYDP at the C-terminal, can also be found in the CSPs motifs of the 25 fig wasps (Fig. 5B).
Selective Pressures on OBP and CSP Genes
The ratio of normalized nonsynonymous to synonymous substitution rates (ω or dN/dS) of genes in 16 OBP (OBPs 1 to 16) and 9 CSP orthologous groups (CSPs 1 to 9) was calculated (Table 3.). In the branch model, the estimated ω value of OBPs and CSPs from a one-ratio model was from 0.019 to 0.392 and 0.019 to 0.265, respectively, indicating a strong purifying selection (Table 3). Further comparison of ω for each group between free-ratio and one-ratio models, showed significant (P < 0.05) differences in evolutionary rates for OBP1, OBP2, OBP4, OBP6, OBP8, OBP9, OBP13, OBP14, and CSP6.
In the branch-site model, in 7 groups, namely OBP1, OBP4, OBP9, OBP10, OBP12, CSP1, and CSP5, the BEB posterior probability at amino acid sites in some branches was greater than 95% (Table 4). However, after comparing the likelihood values of their model A versus null model A, it was found that the differences between the two models were not significant (P > 0.05), except for CSP1. Therefore, sites other than CSP1 cannot be considered as sites under positive selection.
Discussion
Up to now, there has been little research on OBPs and CSPs in fig wasps (Wang et al. 2014). Our results provide a detailed comparison on the diversity and evolution of chemosensory genes in fig wasps. As a shuttle for external chemical substances to the underlying receptors, our research shows that the OBPs and CSPs of fig wasps share sequence similarity and motif structure to those in other insects. It is remarkable that although fig wasps mainly develop and mate in the enclosed fig inflorescence and their living environment is relatively simple, there are great differences in the number and sequence similarity of the OBP and CSP genes between fig wasps. The 25 fig wasps were collected from 22 fig species representing 6 genera. For some of the species, individuals were from different locations (or hosts). The inflorescence of each fig host is a specific microenvironment, containing different VOC compounds, non-pollinating wasps, pathogens, or endophytes. Therefore, fig wasps require specificity in finding hosts, information exchange, developmental regulation, mating, and immune regulation that is provided by the great diversity of OBPs and CSPs. Our results show that comparing sequence similarities based on BLAST searches are less efficient and reliable than using the HMM method based on conservative domains to search for target genes. Compared to the previous HMM results (Chen et al. 2021), we further removed some genes with domain deletions using interproscan (Quevillon et al. 2005) and NCBI CDD web server. In this study, the number of OBPs and CSPs for each fig wasp varied greatly from 6 to 27 and 6 to 19, respectively, which is the same in other insects in same order or different orders. The number of CSPs in Nasonia vitripennis (Hymenoptera) is up to 90, while those in Apis mellifera and Solenopsis invicta is 21 and 18, respectively. In other order insects, the number of OBPs in Lepidoptera Bombyx mori, Diptera Anopheles gambiae, and Drosophila melanogaster is 45, 57, and 51 (Hekmat-Scafe et al. 2002; Xiao et al. 2013; Forêt and Maleszka 2006; Gu et al. 2015; Gong et al. 2009). The number of CSPs in Aedes aegypti (Diptera) is up to 110, while those in Anopheles gambiae (Diptera), D. melanogaster (Diptera), Bombyx mori (Lepidoptera), and Tribolium castaneum (Coleoptera) is 8, 4, 19, and 22 (Mckenna et al. 1994; Croset et al. 2010; Vieira and Rozas 2011). Genomic data of four fig wasps have been published (Xiao et al. 2013; Zhang et al. 2020; Wang et al. 2021; Chen et al. 2022), and we have sequenced the transcriptome of two of them, namely Valisia javana and Ceratosolen solmi. For V. javana, the number of OBPs and CSPs in the genome is 19 and 8, respectively, while the number of OBPs and CSPs in the transcriptome is 13 and 8, respectively (Chen et al. 2021). For C. solmi, the number of OBPs and CSPs in the genome is 10 and 8, respectively, while the number of OBPs and CSPs in the transcriptome is 8 and 6, respectively. Unlike the ORs and GRs of fig wasps identified using transcriptome data, which are much lower than those identified using genomics (Yu et al. 2023), the OBP and CSP numbers between them are slightly lower or similar, indicating that most of them can express and perform functions in adulthood. Unlike ORs, the similarity of ORs sequence in the same Blastophaga species is usually higher than that of the related V. javana species, while the OBP and CSP sequence similarity in the same Blastophaga species is basically similar to and even lower than those of related V. javana species (Yu et al. 2019, 2023). Both OBP and CSP are selective filters that play an important role in receiving and recognizing chemical signals emitted by host or external environment (Leal 2013). The same Blastophaga species are collected from four different host species (subsection Frutescentiae; subgenus Ficus), and the odor composition and living condition between the hosts may be different. Therefore, compared to receptor genes, these binding proteins are more selected by the external environment rather than internal phylogenetic relationships.
We detected 17 orthologous groups of OBPs in the 25 fig wasps, and this diversity should enable them to bind to various odor molecules. In addition to molecules responsible for attracting volatile compounds released by fig hosts, these proteins may also participate in reproductive communication through pheromones and have other functions. For CSPs, their functions are more extensive and can perform various physiological functions, such as signal exchange, feeding, development, mating, and immunity (Maleszka et al. 2007). There are 12 orthologous groups, which enable them to bind to various chemical molecules and play a role throughout the entire life cycle of the fig wasp.
Nine OBP and eight CSP orthologous groups (52.9% and 66.7%, respectively) were detected in over half of 25 fig wasp species and their sequence similarity was also higher than those of the other orthologous groups. They may be key genes in the olfactory and chemosensory systems of fig wasps. The sequence similarity difference between orthologous OBP and CSP genes are greatly, as low as 17.2% and 39.5%, respectively. The cysteine pattern of OBPs is C1-X20-33-C2-X3-C3-X27-43-C4-X6-12-C5-X4-8-C6, which is slightly different from the conservative cysteine distribution pattern of Hymenoptera insects, C1-X23-35-C2-X3-C3-X27-45-C4-X7-14-C5-X8-C6 (Xu et al. 2009). Previous studies have found that there are eight amino acids between the 5th and 6th cysteine of Hymenoptera (Field et al. 2000; Xu et al. 2009). However, in this study, in addition to eight amino acids, there are also four amino acids between the 5th and 6th cysteine of four genera which may reflect ongoing evolution of the OBPs of fig wasps. The cysteine pattern of CSPs is C1-X6-8-C2-X17-19-C3-X2-C4. Except for some CSPs in genera Kradibia and Valisa, which have eight amino acids between the first and second cysteine, and Valisa with 17 amino acids between the second and third cysteine, the rest are the same in fig wasps, indicating that the cysteine pattern and function of CSPs are more conservative.
The OBP genes of fig wasps are divided into two major functional groups based on their phylogenetic relationship with the OBPs with known functions of A. mellifera and D. melanogaster. For the PBP group, we found that AmOBP1 in A. mellifera, which binds to the queen pheromone, clustered with OBP1 of fig wasps (Danty et al. 1999). AcerOBP11 in A. cerana, which is related to A. mellifera, could bind to bee pheromones and some floral volatiles (Song et al. 2018). DmelOBPlush and DmelOBP19a in D. melanogaster could detect male specific pheromones and enhance sensitivity to male specific pheromones, except for reaction to ethanol, propanol, and butanol (Galindo and Smith 2001). DmelOBP83a and DmelOBP83b are clustered with OBP5, and are mainly located in the antennal sensory organs of D. melanogaster, where they detect pheromone (Vieira and Rozas 2011). The function of DmelOBP69a is related to social interaction to regulate gender specific pheromone communication (Bentzur et al. 2018). DmelOBP8a has been found to be highly expressed in male accessory glands and may be involved in insect mating behavior (Arya et al. 2010). For the GOBP group, AmelOBP2 is more likely to bind to general odors, such as 2-isobutyl-3-methoxypyrazine, isoamyl acetate, 1,8-cineol, and 2-heptanone, except for β-ionone (Danty et al. 1997; Briand et al. 2001). AmelOBP13 showed a good binding specificity to oleic acid and other structurally similar compounds. AmelOBP14 is better tuned to monoterpenoid structures. AmelOBP21 binds to the main component of the queen mandibular pheromone and farnesol (Iovinella et al. 2011). Myrcene may be a ligand for AcerOBP15 (Du et al. 2021). For DmelOBP58d, 56a, 56d, and 56e in D. melanogaster, they can recognize odor molecules released by plants, such as trans-2-hexenal, myrcene, limonene, 4-heptylphenol, linalool, hexadecane acid, and linolenic acid. DmelOBP28a can combine with a large number of odor compounds in plants (e.g., β- ionone) and also has the function of buffering the rapid change of odor (Gonzalez et al. 2019). The OBP16 of fig wasps grouped with DmelOBP28a. Therefore, we speculate that OBP16 may have the similar function, and β-ionone may also be the key odor substance for fig wasps seeking their host fig trees. The motif structure of the PBP group (5-2-1-3) is different from the 5-2–1-4 and 6-1-4 structures of the GOBP groups, supporting this conclusion.
According to the phylogenetic tree of CSPs, CSP3, 6, and 10–12 are clustered with AmelCSP1 of A. mellifera and may detect linear chain alcohols and esters (Nagnan-Le Meillour et al. 2000; Jacquin-Joly et al. 2001; Forêt et al. 2007). CSP7 and 8 are clustered into one branch with AmelCSP6 and AmelCSP4, respectively. Therefore, these proteins of fig wasps may be involved in pheromone transport, recognition, and pollen collection, tissue formation, and/or regeneration and they also have the strongest ability to bind terpenoids (Briand et al. 2001; Forêt et al. 2007). All these CSPs mainly bind to odor molecules and belong to group 1, supported by motif structure of 4-2-(5)-1-3. AmelCSP2 of A. mellifera has the strongest ability to bind aromatic compounds and also has a conserved function in regulating insect development (Forêt et al. 2007). AmelCSP5 of A. mellifera is expressed in the queen’s ovaries and eggs, and RNA interference inhibition of AmelCSP5 indicates its involvement in embryonic development (Maleszka et al. 2007). The CSP2 and 9 clustered with AmelCSP2 and AmelCSP5, which have similar functions and belong to group 3, with a motif structure of 6-2-1-3. The group 2 including CSP5 with a motif structure of 2-5-1-3 is a specific clade of the fig wasp.
Our evolutionary analysis shows that both the OBP and CSP gene families evolved under strong purifying selection, and relaxation of purifying selection at some amino acid sites also led to sequence differences. CSP5 has the strongest purifying selection (ω = 0.019). It is the specific branch with the lowest sequence similarity. Therefore, CSP5 may perform some specific/important functions in fig wasps. Among the 25 orthologous groups tested, only CSP1 had a positive selection site 43Q, which is located in α1 helix, and generally considered as a key component of the active domain of CSPs. The adaptive evolution in α1 helix may help wasps recognize specific hosts, ultimately leading to species differentiation.
Conclusion
As the first link in the chain of chemoreception, we compared the diversity and evolution of OBPs and CSPs in the transcriptome of 25 fig wasps (six genera). Our survey detected 321 OBPs and 240 CSPs, clustered mainly into 17 and 12 orthologous groups, respectively. Comparisons with known functional OBP genes in Drosophila melanogaster and Apis mellifera suggest they may also detect a wide range of volatile cues, including pheromones and general odor molecules. The structure of the CSPs indicates that they can be divided into three groups and have extensive functions in odor detection, mating, and development regulation. The overall comparison of the dN/dS values of orthologous genes indicates that both OBPs and CSPs are under strong purifying selection, with only CSP1 having one amino acid site under positive selection. These results provide us a better understanding of the molecular basis of the peripheral chemosensory system of fig wasps.
References
Angeli S, Ceron F, Scaloni A, Monti M, Monteforti G, Minnocci A, Petacchi R, Pelosi P (1999) Purification, structural characterization, cloning and immunocytochemical localization of chemoreception proteins from Schistocerca gregaria. Eur J Biochem 262:745
Arya GH, Weber AL, Wang P, Magwire MM, Negron YLS, Mackay TFC, Anholt RRH (2010) Natural variation, functional pleiotropy and transcriptional contexts of odorant binding protein genes in Drosophila melanogaster. Genetics 186:1475–1485
Bailey TL, Elkan C (1994) Fitting a mixture model by expectation maximization to discover motifs in bipolymers. ISMB 2:28
Ban L, Scaloni A, Brandazza A, Angeli S, Zhang L, Pelosi P (2003) Chemosensory proteins of Locusta migratoria. Insect Mol Biol 12:125
Bentzur A, Shmueli A, Omesi L, Ryvkin J, Knapp JM, Parnas M, Davis FP (2018) Odorant binding protein 69a connects social interaction to modulation of social responsiveness in Drosophila. PLoS Genet 14:e1007328
Briand L, Nespoulous C, Huet JC, Takahashi M, Pernollet JC (2001) Ligand binding and physico-chemical properties of ASP2, a recombinant odorant-binding protein from honeybee (Apis mellifera L.). Eur J Biochem 268:752
Chen C, Chen H, Zhang Y, Thomas HR, Xia R (2020) TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 13:194
Chen LF, Simon ST, Chantarasuwan B, Wang DM, Wang R, Chen XY, Yu H (2021) Adaptation of fig wasps (Agaoninae) to their host revealed by large-scale transcriptomic data. InSects 12:815
Chen LF, Feng C, Wang R, Nong XJ, Deng XX, Chen XY, Yu H (2022) A chromosome-level genome assembly of the pollinating fig wasp Valisia javana. DNA Res 29:1
Clewley JP, Arnold C (1997) MegAlign. The multiple alignment module of LASERGENE. Methods Mol Biol 70:119
Croset V, Cummins SF, Benton R. (2010) Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. Plos Genet 6:e1001064
Cruaud A, Ronsted N, Chantarasuwan B, Chou LS, Clement WL, Couloux A, Cousins B, Genson G, Harrison RD, Hanson PE (2012) An extreme case of plant-insect codiversification: figs and fig-pollinating wasps. Syst Biol 61:1029
Danty E, Michard Vanhee C, Huet JC, Genecque E, Pernollet JC, Masson C (1997) Biochemical characterization, molecular cloning and localization of a putative odorant-binding protein in the honeybee Apis mellifera L. (Hymenoptem Apidea). FEBS Lett 414:595
Danty E, Briand L, Michard-Vanhee C, Perez V, Arnold G, Gaudemer O, Huet D, Huet J, Ouali C, Masson C (1999) Cloning and expression of a queen pheromone-binding protein in the honeybee: an olfactory-specific, developmentally regulated protein. J Neurosci 19:7468
Du YL, Xu K, Zhao HT, Jiang YS, Li HQ (2021) Identification and functional characterization of AcerOBP15 from Apis cerana cerana (Hymenoptera: Apidae). Apidologie 52:668
Field LM, Pickett JA, Wadhams LJ (2000) Molecular studies in insect olfaction. Insect Mol Biol 9:545
Forêt S, Maleszka R (2006) Function and evolution of a gene family encoding odorant binding-like proteins in a social insect, the honey bee (Apis mellifera). Genome Res 16:1404
Forêt S, Wanner KW, Maleszka R (2007) Chemosensory proteins in the honey bee: insights from the annotated genome, comparative analyses and expressional profiling. Insect Biochem Mol Biol 37:19
Galindo K, Smith DP (2001) A large family of divergent Drosophila odorant-binding proteins expressed in gustatory and olfactory sensilla. Genetics 159:1059
Gonzalez D, Rihani K, Neiers F, Poirier N, Briand L (2019) The Drosophila odorant-binding protein 28a is involved in the detection of the floral odour ss-ionone. Cell Mol Life Sci 77:1
Gong DP, Zhang HJ, Zhao P, Xia QY, Xiang ZH (2009) The odorant binding protein gene family from the genome of silkworm. Bombyx Mori BMC Genom 10:1
Grison-Pigé L, Bessiére JM, Hossaert-McKey M (2002) Specific attraction of fig-pollinating wasps: role of volatile compounds released by tropical figs. J Chem Ecol 28:283
Gu SH, Zhou JJ, Gao S, Wang DH, Li XC, Guo YY, Zhang YJ (2015) Identification and comparative expression analysis of odorant binding protein genes in the tobacco cutworm Spodoptera litura. Sci Rep 5:13800
Hekmat-Scafe DS, Scafe CR, McKinney AJ, Tanouye MA (2002) Genome-wide analysis of the odorant binding protein gene family in Drosophila melanogaster. Genome Res 12:1357
Hossaert-Mckey M, Gibernau M, Frey JE (1994) Chemosensory attraction of fig wasps to substances produced by receptive figs. Entomol Exp Appl 70:185
Iovinella I, Dani FR, Niccolini A, Sagona S, Michelucci E, Gazzano A, Turillazzi S, Felicioli A, Pelosi P (2011) Differential expression of odorant-binding proteins in the mandibular glands of the honeybee according to caste and age. J Proteome Res 10:3439
Jacquin-Joly E, Vogt RG, Francois MC, Meillour NL (2001) Functional and expression pattern analysis of chemosensory proteins expressed in antennae and pheromonal gland of Mamestra brassicae. Chem Senses 26:833
Jacquin-Joly E, Merlin C (2004) Insect olfactory receptors: contributions of molecular biology to chemical ecology. J Chem Ecol 30:2359
Jin X, Brandazza A, Navarrini A, Ban L, Zhang S, Steinbrecht RA, Zhang L, Pelosi P (2005) Expression and immunolocalisation of odorant-binding and chemosensory proteins in locusts. Cell Mol Life Sci 62:1156
Karpe SD, Jain R, Brockmann A, Sowdhamini R (2016) Identification of complete repertoire of Apis florea odorant receptors reveals complex orthologous relationships with Apis mellifera. Genome Biol Evol 8:2879
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780
Leal WS (2013) Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Ann Rev Entomol 58:373–391
Legan AW, Jernigan CM, Miller SE, Fuchs MF, Sheehan MJ (2021) Expansion and accelerated evolution of 9-exon odorant receptors in Polistes paper wasps. Mol Biol Evol 38:3832
Li L, Stoeckert CJ, Roos DS (2003) OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res 13:2178
Maleszka J, Foret S, Saint R, Maleszka R (2007) RNAi-induced phenotypes suggest a novel role for a chemosensory protein CSP5 in the development of embryonic integument in the honeybee (Apis mellifera). Dev Genes Evol 217:189
McKenna MP, Hekmat-Scafe DS, Gaines P, Carlson JR (1994) Putative Drosophila pheromone-binding proteins expressed in a subregion of the olfactory system. J Biol Chem 269:16340
Meillour NL, Cain AH, Jacquinjoly E, Francois MC, Ramachandran S, Maida R, Steinbrecht RA (2000) Chemosensory proteins from the proboscis of Mamestra brassicae. Chem Senses 25:541
Murray M, Thompson W (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321
Paesen G, Happ G (1995) The B proteins secreted by the tubular accessory sex glands of the male mealworm beetle, Tenebrio molitor, have sequence similarity to moth pheromone-binding proteins. Insect Biochem Mol Biol 25:401
Pelletier J, Leal WS (2011) Characterization of olfactory genes in the antennae of the southern house mosquito. Culex Quinquefasciatus J Insect Physiol 57:915
Pelosi P, Maida R (1995) Odorant-binding proteins in insects. Comp Biochem Physiol B 111:503
Pelosi P, Maida R (1990) Odorant-binding proteins in vertebrates and insects: similarities and possible common function. Chem Senses 15:205
Petersen TN, Brunak S, Von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786
Quevillon E, Silventoinen V, Pillai S, Harte N, Mulder N, Apweiler R, Lopez R (2005) InterProScan: protein domains identifier. Nuc Acids Res 33:116
Sánchez-Gracia A, Vieira FG, Rozas J (2009) molecular evolution of the major chemosensory gene families in insects. Heredity 103:208
Sheng S, Liao CW, Zheng Y, Zhou Y, Xu Y, Song WM, He P, Zhang J, Wu FA (2017) Candidate chemosensory genes identified in the endoparasitoid Meteorus pulchricornis (Hymenoptera: Braconidae) by antennal transcriptome analysis. CBPD 22:20
Song XM, Zhang LY, Fu XB, Wu F, Tan J, Li HL (2018) Various bee pheromones binding affinity, exclusive chemosensillar localization, and key amino acid sites reveal the distinctive characteristics of odorant-binding protein 11 in the eastern Honeybee. Apis Cerana Front Physiol 9:422
Su ZH, Sasaki A, Kusumi J, Chou PA, Tzeng HY, Li HQ, Yu H (2022) Pollinator sharing, copollination, and speciation by host shifting among six closely related dioecious fig species. Commun Biol 5:284
Vieira FG, Rozas J (2011) Comparative genomics of the odorant-binding and chemosensory protein gene families across the Arthropoda: origin and evolutionary history of the chemosensory system. Genome Biol Evol 3:476–490
Vogt RG, Riddiford LM (1981) Pheromone binding and inactivation by moth antennae. Nature 293:161
Wang G, Compton SG, Chen J (2013) The mechanism of pollinator specificity between two sympatric fig varieties: a combination of olfactory signals and contact cues. Ann Bot 111:173
Wang N, Wang NX, Niu LM, Bian SN, Xiao JX, Huang DW (2014) Odorant-binding protein (OBP) genes affect host specificity in a fig-pollinator mutualistic system. Insect Mol Biol 23:621
Wang R, Yang Y, Jing Y, Segar ST, Zhang Y, Wang G, Chen J, Liu QF, Chen S, Chen Y, Cruaud A (2021) Molecular mechanisms of mutualistic and antagonistic interactions in a plant-pollinator association. Nat Ecol Evol 5:974
Wanner KW, Willis LG, Theilmann DA, Isman MB, Feng QL, Plettner E (2004) Analysis of the insect os-d-like gene family. J Chem Ecol 30:889
Ware AB, Kaye PT, Compton SG, Noort SV (1993) Fig volatiles: their role in attracting pollinators and maintaining pollinator specificity. Plant Syst Evol 186:147
Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ (2009) Jalview Version 2-a multiple sequence alignment editor and analysis workbench. Bioinformatics 25:1189–1191
Wiebes JT (1979) Coevolution of figs and their insect pollinators. Ann Rev Ecol Syst 10:1
Xiao JH, Yue Z, Jia LY, Yang XH, Niu LH, Wang Z, Zhang P, Sun BF, He SM, Li Z (2013) Obligate mutualism within a host drives the extreme specialization of a fig wasp genome. Genome Biol 14:R141
Xu YL, He P, Zhang L, Fang SQ, Dong SL, Zhang YJ, Li F (2009) Large-scale identification of odorant-binding proteins and chemosensory proteins from expressed sequence tags in insects. BMC Genom 10:632
Yang Z (2007) PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24:1586
Yu H, Tian EW, Zheng LN, Deng XX, Cheng YF, Chen LF, Wu W, Tanming W, Zhang DY, Compton SG, Kjellberg F (2019) Multiple parapatric pollinators have radiated across a continental fig tree displaying cliincal genetic variation. Mol Ecol 28:2391
Yu H, Nong XJ, Fan SL, Bhanumas C, Deng XX, Wang R, Chen XY, Compton SG (2023) Olfactory and gustatory receptor genes in fig wasps: evolutionary insights from comparative studies. Gene 850:146953
Zhang GH, Chen J, Yu HL, Tian XL, Wu JX (2018) Molecular and functional characterization of pheromone binding protein 1 from the oriental fruit moth, grapholita molesta (Busck). Sci Rep 8:1–11
Zhang XT, Wang G, Zhang SC, Chen S, Wang YB, Wen P, Ma XK, Shi Y, Qi R, Yang Y (2020) Genomes of the banyan tree and pollinator wasp provide insights into fig-wasp coevolution. Cell 183:875
Zhou JJ, Robertson G, He X, Dufour S, Hooper AM, Pickett JA, Keep Nicholas H, Field LM (2009) Characterisation of Bombyx mori odorant-binding proteins reveals that a general odorant-binding protein discriminates between sex pheromone components. J Mol Biol 389:529
Acknowledgements
This study was funded by supported by the National Key R & D Program of China (2023YFE0100540), Science and Technology Projects in Guangzhou (E33309), and Guangzhou Collaborative Innovation Center on Science-tech of Ecology and Landscape (202206010058).
Author information
Authors and Affiliations
Contributions
All experimental data was collected by HY and analyzed by HY, XJN, WCH, and YMD. The manuscript was drafted by XJN and HY and all authors contributed to subsequent editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
No approvals required.
Consent of Publish
All authors read and approved the final manuscript.
Additional information
Handling editor: Rafael Zardoy.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, H., Nong, X., Huang, W. et al. Odorant-Binding and Chemosensory Proteins in Fig Wasps: Evolutionary Insights From Comparative Studies. J Mol Evol 92, 42–60 (2024). https://doi.org/10.1007/s00239-023-10152-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-023-10152-x