Abstract
The ATP-binding cassette (ABC) transporter gene family is ubiquitous in the living world. ABC proteins bind and hydrolyze ATP to transport a myriad of molecules across various lipid-containing membrane systems. They have been studied well in plants for transport of a variety of compounds and particularly, in vertebrates due to their direct involvement in resistance mechanisms against several toxic molecules/metabolites. ABC transporters in insects are found within large multigene families involved in the efflux of chemical insecticides and toxic/undesired metabolites originating from food and endogenous metabolism. This review deals with ABC transporter subfamilies of few agronomically important Lepidopteran pests. The transcriptional dynamics and regulation of ABC transporters during insect development emphasizes their functional diversity against insecticides, Cry toxins, and plant specialized metabolites. To generate insights about molecular function and physiological roles of ABCs, functional and structural characterization is necessary. Also, expansion and divergence of ABC transporter gene subfamilies in Lepidopteran insects needs more systematic investigation. We anticipate that newer methods of insect control in agriculture can benefit from an understanding of ABC transporter interactions with a vast range of natural specialized molecules and synthetic compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Annually, around 590,000 tons of insecticides are used worldwide for safe-guarding crops in the field and during storage (Sharma et al. 2019). It is well known that some of the target insect species have developed resistance against insecticides such as organophosphates, dichlorodiphenyltrichloroethane, pyrethroids, and carbamates (reviewed by Dawkar et al. 2013). The phenomenon of resistance in insects is due to their diverse and efficient adaptive mechanisms. These mechanisms include detoxification, target site modifications, and nerve insensitivity (McCaffery et al. 1997). Detoxification of specific molecules is one of the mechanisms to adapt to resistance. It involves reduction of substrate toxicity by cytochrome P450 monooxygenases (Scott and Wen 2001; Wu et al. 2021), conversion of hydrophobic toxic compounds to hydrophilic products by glutathione-S-transferases, uridine diphosphate glucuronosyl transferases and carboxyl esterases (Pan et al. 2019; Hilliou et al. 2021; Meng et al. 2022), and efflux of conjugated xenobiotics from the cell mainly by the ATP-binding cassette (ABC) transporters (Dawkar et al. 2013; Hilliou et al. 2021).
ABC transporters are widespread across all species and represent one of the most prominent transmembrane protein families (Hull et al. 2014). ABC transporters comprise an intracellular domain to bind and hydrolyze ATP to transport molecules like inorganic ions, sugars, amino acids, lipids, peptides, specialized metabolites, and xenobiotic agents across membranes (Higgins 1992). These transporters are divided into three categories based on their functions: (i) importers—found exclusively in prokaryotes, transport mainly ions, sugars, amino acids, peptides, and other hydrophilic molecules, (ii) exporters—present in prokaryotes and eukaryotes, involved in the transport of hydrophobic compounds like lipids, fatty acids, drugs, endogenous and exogenous toxins, and (iii) non-transport proteins—act as ion channels or their regulators and receptors, function in the mechanism of DNA repair, participate in the assembly of ribosomes and in protein translation (Beek et al. 2014). A functional ABC transporter comprises two domains: Nucleotide-Binding Domain (NBD) and Transmembrane Domain (TMD) (Higgins 1992). Further, the ABC transporter superfamily is classified into eight subfamilies (ABCA to H), based on NBD sequence similarity and the arrangement of NBD and TMD (Dean et al. 2001; Dermauw and Van Leeuwen 2014).
Insect ABC transporters are also required for several physiological and metabolic processs such as (i) molting and cuticle tissue differentiation (Broehan et al. 2013), (ii) egg development and germ cell migration (Ricardo and Lehman 2009; Broehan et al. 2013), (iii) transport of eye pigment molecules, and (iv) biochemical processes like uric acid uptake (Wang et al. 2013). An overview of Lepidopteran ABC transporters and their varied functions has been provided in Table 1. While insect ABC transporters, in general, have been discussed earlier, this review aims to focus especially on the order, Lepidoptera.
Lepidopteran larvae damage economically important plants and their parts, causing heavy agronomical losses especially at the pre-harvest level. For example, the cotton bollworm (Helicoverpa armigera Hubn., Lepidoptera: Noctuidae) is a devastating pest of several important crops such as chickpea (Cicer arietinum L.), cotton (Gossypium species), tomato (Solanum lycopersicum) feeding on pods, bolls, and fruits, respectively. Further, H. armigera is known to infest over 300 different plants, including important food and cash crops (Kotkar et al. 2009). The diamondback moth (Plutella xylostella L., Lepidoptera: Plutellidae), a specialist insect of Brassicaceae members, is a severe threat to the worldwide production of cauliflower and cabbage. Its short life cycle and development of resistance against almost all known insecticides poses a challenge to control its infestation. Other members of Lepidoptera include silk moth (Bombyx mori L.), Asian cotton leafworm (Spodoptera litura F.), tobacco hawkmoth (Manduca sexta L.), navel orangeworm moth (Amyelois transitella W), Asiatic rice borer moth (Chilo suppressalis W.), fall armyworm (Spodoptera frugiperda S.), cabbage looper (Trichoplusia ni Hubn.), cotton pink bollworm (Pectinophora gossypiella S.), and small white butterfly (Pieris rapae L.). In this review, detoxification mechanisms employed by Lepidopteran insects using ABC transporters have been discussed emphasizing their sequence and structural complexity, evolutionary attributes, and their key functions.
Structural Facets of Lepidopteran ABC Proteins
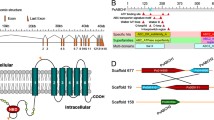
Lepidopteran ABC proteins exhibit structural features similar to other eukaryotic ABC transporters. ABC transporters couple movement of substrate(s) across the membrane by hydrolysis of a phosphate bond between α and β-phosphate of ATP (Higgins 1992). Two TMDs with multiple membrane-spanning α-helices together form specific binding site(s) and a pathway that transports substrate(s) across a lipid bilayer. They couple conformational changes induced by ATP binding, its hydrolysis, and ADP release. A full transporter consists of two TMDs and two NBDs. These domains are structurally similar and generate an internal twofold (or pseudo twofold) symmetry to form a functional unit (Higgins and Linton 2004). As mentioned previously, eukaryotic ABC transporters are exporters. Few of these belong to the Multidrug-Resistant Protein (MRP) class that mediate resistance to xenobiotics (Labbé et al. 2011). On the contrary, prokaryotic importers are selective for a single or a few related water-soluble substrates. Eukaryotic exporters either have a single polypeptide chain containing all the domains or a dimer of two polypeptides, each having one NBD and one TMD as found in bacterial exporters (Beek et al. 2014). NBD is responsible for the hydrolysis of ATP needed to provide energy for transport. Also, NBDs share a common evolutionary origin as they are structurally similar (Linton 2007). They are a subgroup of the diverse superfamily of P-loop NTPase (Vetter and Wittinghofer 2001) and require Mg2+ ions for catalysis. Each NBD has a core of 200 amino acids and two sub domains, the RecA-like domain and a more diverse α-helical domain unique to ABC proteins (Beek et al. 2014). Conserved motif analysis for Lepidopteran ABC proteins was carried out using MEME server (Fig. 1; Supplementary File 1). NBDs are identified based on a signature loop (LSGGQ; Fig. 1A) and seven conserved motifs, A-loop, P-loop, Walker A (Fig. 1B), Walker B, D-loop, H-loop, and a Q-loop (Fig. 1C). The Walker A motif is a phosphate-binding loop with highly conserved lysine residues. Amide nitrogen and ε group of lysine residues form interactions with β- and γ-phosphate of ATP. The Walker B motif identified by ϕϕϕϕDE (where ϕ is a hydrophobic amino acid) coordinates with Mg2+ via an aspartate residue. Glutamate acts as a general base, polarizing the attack of the water molecule. LSGGQ is located at the N-terminus of the α-helical subdomain that directs positive charge of the helical dipole toward γ-phosphate of ATP (Beek et al. 2014). The Walker B motif is followed by the D-loop (SALD motif). Alteration in the confirmation of D-loop affects conformation of the catalytic site and formation of ATP hydrolysis site. The A-loop contains a conserved aromatic residue, usually a tyrosine that helps to position ATP via stacking with the adenine ring. The Q-loop is eight residues long and is identified by the presence of glutamine. It is located at the interface between the RecA-like and the α-helical subdomain. This site of NBDs undergoes interaction with the TMDs. The Q-loop undergoes conformational changes allowing the conserved glutamine residue to move in and out of the active site during the hydrolysis cycle. The site is activated when Mg-ATP is bound (Beek et al. 2014).
Motif analyses of Lepidopteran ABC transporters. ABC transporter protein sequences of Bombyx mori L., Spodoptera litura F., Helicoverpa armigera Hub., Manduca sexta L., Plutella xylostella L., Amyelois transitella W., Chilo suppressalis W., Spodoptera frugiperda S., Trichoplusia ni Hub., Pectinophora gossypiella S., and Pieris rapae L. were retrieved from the National Center for Biotechnology Information (NCBI). The criterion for selection of these sequences was the presence of the ABC transporter signature motif (LSGGQ) in the nucleotide-binding domain (NBD). A total of 72 protein sequences (refer Supplementary File 1) were used to generate conserved motifs using default parameters on the web-based tool, MEME suite (Multiple Em for Motif Elicitation; version 5.0.4; https://meme-suite.org/meme/tools/meme) as described by Bailey et al. 2015. Five motifs were selected in the data submission form. Out of these, top three motifs have been presented based on their e-value (LSGGQ: 5.7e-1289, GXXGXGK (S/T) 4.6e-727), xQx: 2.4e-323). A P-loop/Walker A with GXXGXGK(S/T) conserved residues (where X can be any amino acid), B Q-loop with conserved glutamine, and C Signature motif with LSGGQ. D represents Manduca sexta ABCC transporter protein structure. Protein sequence was uploaded on SWISS-MODEL (https://swissmodel.expasy.org; Waterhouse et al. 2018). Protein Data Bank (PDB) ID 5UJ9 was used as template with 34.45% sequence identity and 90% query coverage. Representative 3D structure of Manduca sexta ABCC transporter depicts Trans Membrane Domain (TMD; red); Nucleotide-Binding Domain (NBD; cyan; Signature motif (LSGGQ; magenta), and Walker A GXXGXGK (S/T: blue) motif. The start and end amino acid numbers of TMDs and NBDs of M. sexta have been mentioned
TMDs are α-helices embedded in membranes (Dermauw and Van Leeuwen 2014; Locher 2016). They can show diversity in the number of alpha-helices and are therefore highly variable. NBDs are coupled to different TMDs. Such coupling creates a vast number of combinations generating diversity of ABC proteins (Higgins and Linton 2004). Lepidopteran ABC proteins show a topology similar to other eukaryotes, possessing either half or full transporters (Xiong et al. 2015). They can be single (either NBD or TMD), ABC2 (NBD-NBD), half (TMD-NBD or NBD-TMD) or full (TMD-NBD-TMD-NBD or NBD-TMD-NBD-TMD) transporters (Fig. 1D). TMDs from ABC proteins are classified into nine categories according to the Pfam database, out of which four have been reported in eukaryotes (Xiong et al. 2015). TMDs reported in Lepidoptera are as follows: ABC2_membrane TMDs (PF01061) in ABCA subfamily, ABC_membrane TMDs (PF00664) in ABCB and ABCC subfamily, ABC_membrane_2 TMDs (PF06472) (Supplementary Table 1) in ABCD subfamily, and ABC2_membrane_3 (PF12698) in ABCG subfamily. ABCA proteins exhibit TMD-NBD-TMD-NBD and is a full transporter (Dermauw and Van Leeuwen 2014; Liu 2011; Xiong et al. 2015; Tian et al. 2017). Some ABC transporters encode half structures and need to dimerize to form fully functional transporters (Dermauw and Van Leeuwen 2014). The subfamilies ABCA-C domain structures showed the presence of TMD-NBD-TMD-NBD full transporters (Sturm et al. 2009; Dermauw and Van Leeuwen 2014). Our analyses depict that Lepidopteran ABC transporters have structural diversity across their subfamilies. ABCE protein contains four highly conserved motifs present selectively in this subfamily. In the Pfam database, PF00005 class of NBD is present across all subfamilies (Supplementary Table 1). ABCE and ABCF transporters do not have TMDs, but their NBDs are linked, suggesting an atypical characteristic. ABCG subfamily members are half transporters, also showing reverse orientation (NBD-TMD) with NBD at the N-terminal of TMD and dimerize to a functional transporter. ABCH proteins show inverse half-transporter architecture similar to the ABCG proteins. This immense structural variability within ABC sub-families leads further to their divergence and expansion.
Divergence and Lineage-Specific Expansion of Lepidopteran ABC Transporters
We have constructed a phylogenetic tree using 72 ABC protein sequences from 11 candidate Lepidopteran insects retrieved from the National Centre for Biotechnology Information (NCBI) (Fig. 2; Supplementary File 1). The criterion for selecting these insects was based on their importance in agriculture and as model systems. As expected, the phylogenetic analysis depicts that Lepidopteran ABC proteins form distinct 8 clades corresponding to ABCA to H subfamilies. The evolution of eukaryotic ABCs, in general, has been studied using a sequence analysis of NBDs and TMDs separately. We have presented a phylogeny based on the full-length sequences of representative Lepidopteran ABC proteins. As observed in eukaryotes, ABCB, ABCC, and ABCD classify into one group, while ABCB and ABCC cluster together. ABCE and ABCF subfamilies that do not possess TMDs group together and are involved in functions other than transport (Xiong et al. 2015). ABCA and ABCG group together in eukaryotic NBD- and TMD-based tree, but we find a dissimilar trend in our analysis. We noticed that ABCA and ABCH subfamilies cluster together to form a clade; similarly, ABCB and ABCC form a different clade (Fig. 2). This depicts that in Lepidopteran ABC transporters, ABCA and ABCH are closely related. As observed in other eukaryotes, gene duplication events are dominant in ABCC and ABCG clades, implicating expansion in the number of genes in these subfamilies compared to other ABC subfamilies (Sturm et al. 2009; Labbé et al. 2011). Interestingly, Lepidopteran P-glycoprotein (P-gp) expansion is not species-specific, suggesting gene expansion across all its members (Denecke et al. 2021). Specifically, ABCC and ABCG families involved in insecticide resistance have diversified TMDs adapting to the transport of different substrates (Dermauw and Van Leeuwen 2014).
Phylogenetic analyses of select Lepidopteran ABC transporters. Phylogenetic analysis of ABC transporters from Bombyx mori L., Spodoptera litura F., Helicoverpa armigera Hub., Manduca sexta L., Plutella xylostella L., Amyelois transitella W., Chilo suppressalis W., Spodoptera frugiperda S., Trichoplusia ni Hub., Pectinophora gossypiella S., and Pieris rapae L. was carried out using the IQTREE web server (http://www.iqtree.org) (Trifinopoulos et al. 2016). Full-length ABC transporter protein sequences (refer Supplementary File 1) were aligned using the MUltiple Sequence Comparison by Log- Expectation (MUSCLE) tool (Edgar 2004). Maximum Likelihood (ML) method employing VT + G4 model with 1701 informative sites and gamma shape alpha = 2.46 was used for constructing the phylogenetic tree (Kalyaanamoorthy et al. 2017). Further, FigTree (https://tree.bio.ed.ac.uk/software/figtree/) version 1.4.2 was used for visualization of the consensus tree. Clade colors are as follows: ABCA-cyan blue; ABCB-red; ABCC-golden yellow; ABCD-purple; ABCE-green; ABCF-blue-gray; ABCG-blue; and ABCH-orange (Color figure online)
Differential Expression of ABC Transporters Indicates Their Role in Efflux of Plant Specialized Metabolites
The selection of host plants by insects is dependent on nutritional and ecological features. This eventually directs adaptation to particular plants or plant families, grouping insects as monophagous, oligophagous, and polyphagous, or broadly, generalist and specialist feeders. However, during feeding on different food, polyphagous Lepidopteran larvae face several challenges. Various proteinaceous plant defensive molecules and non-proteinaceous plant specialized metabolites are encountered throughout the larval (feeding) stages. Such specialized plant defensive molecules include phenolics, quinones, flavonoids, tannins, terpenoids, proanthocyanidins, lignins, glucosinolates, amino acids, proteins (digestive enzyme inhibitors, lectins, defensins), malondialdehyde, and herbivore-induced plant volatiles (HIPVs) (War et al. 2012).
Intense selection pressure by these deleterious dietary compounds might have led to the rapid evolution of transport mechanisms in insects, such as the development of specific detoxification pumps (Labbé et al. 2011). In herbivores, gut efflux transporters as counter-mechanisms to plant specialized metabolites have been reported (Sorenson and Dearing 2006). An important example is the blood–brain barrier (BBB) in M. sexta, a specialist feeder of Nicotiana spp. A BBB is a protective epithelium that excludes nicotine from the sensitive neuropile using detoxifying enzymes and a nicotine pump. This nicotine pump is an ABCB1 transporter homolog that excretes plant metabolites like nicotine, vinblastine, morphine, and atropine (Maddrell and Gardiner 1975; Murray et al. 1994; Gaertner and Morris 1999). Further, P-gp has also been reported to be involved in the efflux of non-polar cardenolides. For example, access of digoxin to the nerve cord is prevented by an active efflux carrier (Petschenka et al. 2013).
Further, when insects are fed on different plant metabolites, expression of ABC transporters during various stages of their life cycle and in different tissues varies considerably. For example, when H. armigera larvae were reared on an artificial diet supplemented with host (atropine-scopolamine, nicotine, and tomatine) and non-host (taxol) plant metabolites, the highest number of differentially expressed ABC transporter genes was found in the gut as compared to the other tissues (Bretschneider et al. 2016). However, these results indicated that several ABC transporters were upregulated, while compound-specific expression pattern was not apparent. Conversely, members of subfamilies B and C reported for xenobiotic detoxification were upregulated in M. sexta gut upon feeding on different Solanaceous plants and a non-host, rapeseed (Brassica napus), highlighting the importance of ABC transporters in larval plasticity and adaptability on host plants (Koenig et al. 2015). Preferential expression of ABC transporters in P. xylostella Malpighian tubules and midgut predicted their involvement in detoxifying specialized plant metabolites (Qi et al. 2016). In vivo and in vitro evidence of HaABCB6 in the metabolism of gossypol in H. armigera has been presented (Jin et al. 2020). In a nutshell, toxic metabolites and their intermediates travel through various metabolic pathways, potentially mediated by ABC transporters, to be either used for a physiological function or then, to be excreted (Fig. 3). Thus, studies on ABC transporters will provide a platform for interrogating whether they show specificity for a particular class of plant specialized metabolites, mainly in polyphagous insects that survive in a complex ecological niche, for example, comprising even host plant-associated microbiota.
Schematic representation of potential fate of ingested metabolites in the Lepidopteran gut and transport via ABC transporters. The Lepidopteran gut has three distinct regions for digestion: foregut, midgut, and hindgut. During feeding xenobiotics (pink), plant specialized metabolites (red) and Cry toxins (yellow) are generally ingested by the larvae. Action on such molecules begins in the mouth parts until they reach the anus or are completely metabolized. Some of these can be potentially metabolized in the gut (golden yellow) and transported as metabolic intermediates to the hemolymph. Malpighian tubules transport (green arrows) such toxic wastes back to the hindgut where resorption takes place and they are excreted through the anus. Several detoxification enzymes and transporters are involved in such processes. Here, we focus on full ABC transporters, the structure of which is redrawn (Dermauw and Van Leeuwen 2014) to show two transmembrane domains (TMDs; purple) each containing 6 transmembrane (TM) segments, and two nucleotide-binding domains (NBDs; yellow). The mechanism of transport begins by binding of substrates (pink, red, and yellow) to a binding pocket (white pentagon) formed by the TMDs inducing a conformational change in the NBDs (yellow). This helps ATP (green circles) binding and formation of a closed NBD-dimer. In turn, this induces a major conformational change in the TMDs following which they open outside and translocate the substrate. Hydrolysis of ATP results in dissolution of the closed NBD-dimer and induces further changes in the conformation of TMDs. Phosphate (Pi) and ADP are released which bring back open NBD-dimer conformation (Color figure online)
ABC Transporter Gene Expression in Insects and Their Correlation with Insecticide Resistance
P-gp is a permeability glycoprotein also known as MDR1; multi-drug resistance protein 1 or ABCB1 (Srinivas et al. 2004). It has been found that insecticides such as monocrotophos, endosulfan, cypermethrin, fenvalerate, and methyl parathion stimulate ATPase activity of P-gp in H. armigera (Aurade et al. 2006). Susceptibility of H. armigera larvae to indoxacarb, tebufenozide, chlorpyrifos, lambda-cyhalothrin, and abamectin upon treatment with a P-gp inhibitor, verapamil, has been reported (Jin et al. 2019). Expression of other subfamilies such as ABCC and ABCG increased in response to several insecticides like pirimicarb, thiodicarb, abamectin, avermectin, emamectin benzoate, ivermectin, and thiamethoxam (Dermauw and Van Leeuwen 2014). In the red flour beetle (Tribolium castaneum), silencing of two TcABCC genes by RNAi resulted in an increase in susceptibility to malathion. On the contrary, there was no significant increase in insecticide-induced mortalities upon knocking down TcABC genes in the larvae treated with cyfluthrin and diacylhydrazine tebufenozide (TBF insecticides) (Rosner and Merzendorfer 2021).
Consider an example of P. xylostella, the first reported insect to develop resistance against DDT in 1950s and later to Bacillus thuringiensis toxins in 1990s (Ankersmit 1953; Shelton et al. 1993). ABC transporters are upregulated more frequently in insecticide-resistant strains of P. xylostella than the expression of GSTs, COEs or P450s (You et al. 2013). Also, ABCA, ABCC, ABCF, ABCG, and ABCH subfamily members were over-expressed in chlorpyrifos-resistant strain of P. xylostella (Qi et al. 2016). Nevertheless, such observations cannot be extrapolated to other Lepidopteran members. Further, synergism studies in C. suppressalis larvae showed that treatment with verapamil, a potent inhibitor of ABCs, resulted in significantly increased toxicity of chlorantraniliprole involving upregulation of CsABCC8, CsABCG1C, and CsABCH1 (Meng et al. 2020). However, upregulation of genes alone is insufficient to demonstrate resistance, demanding an insight into its mechanism (Denecke et al. 2017; Figuera-Mansur et al. 2013). So further functional studies are needed to confirm involvement of upregulated candidate ABCs in insecticide resistance. The development of new molecules for the control of agricultural pests demands a thorough investigation into their binding efficiency with ABC transporters to avoid the pitfall of resistance.
ABCs Play Key Role in Bt Resistance Strategies Employed by Insects
A recent review by Heckel (2021) summarizes advancements in the role of ABC transporters in Bt resistance of Lepidoptera. Cry toxins from Bacillus thuringiensis (Bt) toxin form pores in the larval midgut epithelium to eventually lyse the cells. These toxins are known as PFTs (pore-forming toxins), produced during the sporulation phase of growth. The sequential binding model for Cry toxin proposes binding to cadherin and further to receptor proteins (aminopeptidase N; APN and alkaline phosphatase; ALP) in the membrane. Membrane pores are formed leading to osmotic stress and cell death (Gomez et al. 2014; Andrés-Garrido et al. 2020). Cry1 toxins are effective against polyphagous noctuid and non-noctuid pests. However, P. xylostella, T. ni, Plodia interpunctella, P. gossypiella, and H. armigera have long been reported for resistance against Bt toxin (Alvi et al. 2012; Fabrick et al. 2015; Janmaat and Myers 2003; Lei et al. 2014; McGaughey 1985; Tabashnik et al. 1994). Bt resistance in Lepidoptera comprises mutations in toxin receptors such as aminopeptidase N (APN), cadherin, alkaline phosphatase (ALP), and ABC transporters (Knight et al. 1994; Vadlamudi et al. 1995; Jurat-Fuentes and Adang 2004; Gahan et al. 2010; Tanaka et al. 2016).
Several ABC subfamilies are linked with Bt resistance. ABCC1 acts as a functional receptor to Cry2Ab toxin (Chen et al. 2018), while the ABCC2 helps Cry1Ac in oligomerization and membrane insertion (Ocelotl et al. 2017). CRISPR/Cas9 single and double knockouts of HaABCC2 and HaABCC3 have demonstrated resistance against Cry1Ac and Cry1Fa (Wang et al. 2020; Zhao et al. 2021). Similarly, a single amino acid change in ABCC2 loop 1 is responsible for difference in toxicity of Cry1Ac in S. frugiperda and S. litura (Liu et al. 2018). Enhanced metabolic resistance to chemical insecticides in P. xylostella has been speculated to increased ABCC2 expression (Xu et al. 2020). Not only ABCC and ABCG subfamilies, but also the ABCA2 subfamily corroborates with Cry1A and Cry2A resistance in H. armigera and H. punctigera (Tay et al. 2015). This has been further confirmed by reports on mutations in ABCA2 that confer resistance to Cry2Ab in T. ni and P. gossypiella (Mathew et al. 2018; Yang et al. 2019; Fabrick et al. 2021). Cry1Fa and Cry1A.105 resistance are due to loss-of-function mutations in the ABCC2 gene in S. frugiperda (Flagel et al. 2018; Boaventura et al. 2020; Banerjee et al. 2017). Also, regulation of resistance to Cry1Ac in Lepidoptera has been attributed to a conserved target site of microRNA-998–3p, identified from the coding sequence of ABCC2 (Zhu et al. 2020). Many conclusions are drawn from laboratory experiments. However, it is essential to examine insects from the field to analyze naturally occurring mutations that impart resistance. Conclusively, it is interesting to note that down regulation of ABCG (Pxwhite) from P. xylostella is linked to Cry1Ac resistance (Guo et al. 2015a). Whereas, in yet another example, PxABCH is an essential gene with a mechanism independent of Cry1Ac resistance independent of Cry1Ac resistance suggesting its significance as a potential target for pest control (Guo et al. 2015b; Zuber et al. 2018). Genetically engineered crops harboring Bt insecticidal proteins have been demonstrated to be a boon in agriculture and are therefore cultivated worldwide. Investigations into ABC-Cry protein interactions provide a strong platform for the development of future genetically engineered crops.
Lepidopteran ABC Transporters Demonstrate Functional Diversity
Several reports indicate functional diversity of ABC transporters in Lepidoptera. To understand the physiological functions of ABC transporters, gene expression-based studies, RNA interference (RNAi), and CRISPR/Cas9 have been widely used.
Apart from their role in detoxification, functional analysis of ABC transporters in H. armigera using CRISPR/Cas9-induced mutations established their role in pigment transport to the cytoplasm (Khan et al. 2017). The role of ABC transporters in development is evident in the case of silkworm, where homolog of the white gene, Bmwh3, a member of ABCG subfamily, transports ommochrome precursors and uric acid into pigment and urate granules, respectively. The white, brown, and scarlet proteins transport guanine or tryptophan in Drosophila melanogaster Meigen. (Fruit fly) (Diptera: Drosophilidae) (Ewart et al. 1994; Komoto et al. 2009). Other subfamilies such as ABCE and ABCF are not considered as transporters, due to the absence of the TMD (Dean et al. 2001; Bretschneider et al. 2016). Their function in transcription, translation, and ribosome assembly has been reported (Tyzack et al. 2000). In yet another interesting study, larval and pupal mortality was demonstrated in P. xylostella by suppressing the expression of PxABCH1 using RNAi (Guo et al. 2015b). Thus, the involvement of Lepidopteran ABC transporters ranging from the transport of molecules required in several important physiological processes and in the detoxification of xenobiotics implies their functional diversity.
Conclusion and Future Perspectives
Insect ABCs are essential for detoxifying xenobiotics and the transport of substances involved in critical physiological processes. In particular, ABC transporters from Lepidopteran insect pests exposed to various chemicals are involved in the efflux of plant metabolites, chemical insecticides, and Bt toxins. Available literature on insect ABC transporters reveals diversification and evolution for various substrates transported by them. The expression profiles of candidate ABC transporter genes during different stages of insect development explain their functional diversity. For example, in insects, ABC transporters have a role in transport of pigment molecules responsible for eye color. As a future perspective, based on the vast knowledge resource available on this topic, we suggest following studies on ABC transporters:
-
1.
ABC transporter-plant specialized metabolite interaction using in silico analysis and further validation by experiments to unravel binding specificity.
-
2.
Elucidating three-dimensional crystal structures of insect ABC transporters with and without ligands (binding partners).
-
3.
Deciphering the rationale for the presence of large number of ABC transporter subfamily members in general, and in particular, in Lepidopteran insects.
-
4.
Evaluate their roles in transport of different substrates.
-
5.
Provide functional evidence(s) to support development of resistance against new chemicals used for control of insects as well as metabolites from host and non-host plants.
-
6.
Interaction of ABC transporters with Bt toxin for the formation of a membrane pore.
-
7.
Demonstrate sequential metabolism (if any) of xenobiotics and whether it is general or specific.
-
8.
Exploring the potential of ABCH for the control of agricultural insect pests.
Since ABC transporters are important especially in agricultural insects, it is necessary to understand their function to design counter-strategies for minimizing crop losses and enhancing productivity.
Abbreviations
- ABC:
-
ATP-binding cassette
- NBD:
-
Nucleotide-binding domain
- TMD:
-
Transmembrane domain
- AChE:
-
Acetylcholinesterase
- APN:
-
Aminopeptidase N
- ALP:
-
Alkaline phosphatase
- HIPV:
-
Herbivore-induced plant volatiles
- Bt:
-
Bacillus thuringiensis
- MDR:
-
Multidrug-resistance
References
Alvi AHK, Sayyed AH, Naeem M, Ali M (2012) Field evolved resistance in Helicoverpa armigera (Lepidoptera: Noctuidae) to Bacillus thuringiensis toxin Cry1Ac in Pakistan. PLoS ONE 7:e47309. https://doi.org/10.1371/journal.pone.0047309
Andrés-Garrido A, González-Martínez RM, Ramos S, Escriche B (2020) Cadherin fragments of Lepidopteran and Coleopteran species do not enhance toxicity of Cry1Ca and Vip3Aa proteins to Spodoptera exigua (Hübner) (Lepidoptera:Noctuidae). Biocontrol Sci Technol 30:941–950. https://doi.org/10.1080/09583157.2020.1772960
Ankersmit GW (1953) DDT resistance in Plutella maculipennis (Curt.) (Lepidoptera) in Java. Bull Entomol Res 44:421–425
Aurade R, Jayalakshmi SK, Sreeramulu K (2006) Stimulatory effect of insecticides on partially purified P-glycoprotein ATPase from the resistant pest Helicoverpa armigera. Biochem Cell Biol 84:1045–1050. https://doi.org/10.1139/O06-194
Banerjee R, Hasler J, Meagher R et al (2017) Mechanism and DNA-based detection of field-evolved resistance to transgenic Bt corn in fall armyworm (Spodoptera frugiperda). Sci Rep 7:10877. https://doi.org/10.1038/s41598-017-09866-y
Beek J, Guskov A, Slotboom DJ (2014) Structural diversity of ABC transporters. J Gen Physiol 143:419–435. https://doi.org/10.1085/jgp.201411164
Boaventura D, Ulrich J, Lueke B et al (2020) Molecular characterization of Cry1F resistance in fall armyworm, Spodoptera frugiperda from Brazil. Insect Biochem Mol Biol 116:103280. https://doi.org/10.1016/j.ibmb.2019.103280
Bretschneider A, Heckel DG, Vogel H (2016) Know your ABCs: Characterization and gene expression dynamics of ABC transporters in the polyphagous herbivore Helicoverpa armigera. Insect Biochem Mol Biol 72:1–9. https://doi.org/10.1016/j.ibmb.2016.03.001
Broehan G, Kroeger T, Lorenzen M, Merzendorfer H (2013) Functional analysis of the ATP-binding cassette (ABC) transporter gene family of Tribolium castaneum. BMC Genom 14:6. https://doi.org/10.1186/1471-2164-14-6
Chen L, Wei J, Liu C et al (2018) Specific binding protein ABCC1 is associated with Cry2Ab toxicity in Helicoverpa armigera. Front Physiol 9:745. https://doi.org/10.3389/fphys.2018.00745
Coates BS, Siegfried BD (2015) Linkage of an ABCC transporter to a single QTL that controls Ostrinia nubilalis larval resistance to the Bacillus thuringiensis Cry1Fa toxin. Insect Biochem Mol Biol 63:86–96. https://doi.org/10.1016/j.ibmb.2015.06.003
Dawkar VV, Chikate YR, Lomate PR et al (2013) Molecular insights into resistance mechanisms of lepidopteran insect pests against toxicants. J Proteome Res 12:4727–4737. https://doi.org/10.1021/pr400642p
Dean M, Rzhetsky A, Allikmets R (2001) The human ATP-Binding Cassette transporter superfamily. Genome Res 11:1156–1166. https://doi.org/10.1101/gr.184901
Denecke S, Fusetto R, Batterham P (2017) Describing the role of Drosophila melanogaster ABC transporters in insecticide biology using CRISPR-Cas9 knockouts. Insect Biochem Mol Biol 91:1–9. https://doi.org/10.1016/j.ibmb.2017.09.017
Denecke S, Rankić I, Driva O et al (2021) Comparative and functional genomics of the ABC transporter superfamily across arthropods. BMC Genom 22:553. https://doi.org/10.1186/s12864-021-07861-2
Dermauw W, Van Leeuwen T (2014) The ABC gene family in arthropods: Comparative genomics and role in insecticide transport and resistance. Insect Biochem Mol Biol 45:89–110. https://doi.org/10.1016/j.ibmb.2013.11.001
Edgar RC (2004) MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. https://doi.org/10.1093/nar/gkh340
Ewart GD, Cannells D, Cox B, Howells AJ (1994) Mutational analysis of the Traffic ATPase (ABC) transporters involved in uptake of eye pigment precursors in Drosophila melanogaster. J Biol Chem 269:10370–10377
Fabrick JA, Unnithan GC, Yelich AJ et al (2015) Multi- toxin resistance enables pink bollworm survival on pyramided Bt cotton. Sci Rep 5:16554. https://doi.org/10.1038/srep16554
Fabrick JA, LeRoy DM, Mathew LG et al (2021) CRISPR-mediated mutations in the ABC transporter gene ABCA2 confer pink bollworm resistance to Bt toxin Cry2Ab. Sci Rep 11:10377. https://doi.org/10.1038/s41598-021-89771-7
Figueira-Mansur J, Ferreira-Pereira A, Mansur JF et al (2013) Silencing of P-glycoprotein increases mortality in temephos-treated Aedes aegypti larvae. Insect Mol Biol 22:648–658. https://doi.org/10.1111/imb.12052
Flagel L, Lee YW, Wanjugi H et al (2018) Mutational disruption of the ABCC2 gene in fall armyworm, Spodoptera frugiperda, confers resistance to the Cry1Fa and Cry1A.105 insecticidal proteins. Sci Rep 8:7255. https://doi.org/10.1038/s41598-018-25491-9
Gaertner LS, Morris CE (1999) Accumulation of daunomycin and fluorescent dyes by drug-transporting Malpighian tubule cells of the tobacco hornworm Manduca Sexta. Tissue Cell 31(2):185–194. https://doi.org/10.1054/tice.1999.0025
Gahan LJ, Pauchet Y, Vogel H, Heckel DG (2010) An ABC transporter mutation is correlated with insect resistance to Bacillus thuringiensis Cry1Ac toxin. PLoS Genet 6(12):e1001248. https://doi.org/10.1371/journal.pgen.1001248
Gómez I, Sánchez J, Muñoz-Garay C et al (2014) Bacillus thuringiensis Cry1A toxins are versatile proteins with multiple modes of action: Two distinct pre-pores are involved in toxicity. Biochem J 459:383–396. https://doi.org/10.1042/BJ20131408
Guo Z, Kang S, Zhu X et al (2015a) Down-regulation of a novel ABC transporter gene (Pxwhite) is associated with Cry1Ac resistance in the diamondback moth, Plutella xylostella (L.). Insect Biochem Mol Biol 59:30–40. https://doi.org/10.1016/j.ibmb.2015.01.009
Guo Z, Kang S, Zhu X et al (2015b) The novel ABC transporter ABCH1 is a potential target for RNAi-based insect pest control and resistance management. Sci Rep 5:13728. https://doi.org/10.1038/srep13728
Guo Z, Sun D, Kang S et al (2019) CRISPR/Cas9-mediated knockout of both the PxABCC2 and PxABCC3 genes confers high-level resistance to Bacillus thuringiensis Cry1Ac toxin in the diamondback moth, Plutella xylostella (L.). Insect Biochem Mol Biol 107:31–38. https://doi.org/10.1016/j.ibmb.2019.01.009
Heckel DG (2021) The essential and enigmatic role of ABC transporters in Bt resistance of noctuids and other insect pests of agriculture. Insects 12:389. https://doi.org/10.3390/insects12050389
Higgins CF (1992) ABC TRANSPORTERS: from microrganisms to man. Annu Rev Cell Biol 8:67–113. https://doi.org/10.1146/annurev.cb.08.110192.000435
Higgins CF, Linton KJ (2004) The ATP switch model for ABC transporters. Nat Struct Mol Biol 11:918–926. https://doi.org/10.1038/nsmb836
Hilliou F, Chertemps T, Maïbèche M, Le Goff G (2021) Resistance in the genus Spodoptera: key insect detoxification genes. Insects 12(6):544. https://doi.org/10.3390/insects12060544
Hull JJ, Chaney K, Geib SM et al (2014) Transcriptome-based identification of ABC transporters in the western tarnished plant bug Lygus hesperus. PLoS ONE 9:e113046. https://doi.org/10.1371/journal.pone.0113046
Janmaat AF, Myers J (2003) Rapid evolution and the cost of resistance to Bacillus thuringiensis in greenhouse populations of cabbage loopers, Trichoplusia ni. Proc R Soc B Biol Sci 270:2263–2270. https://doi.org/10.1098/rspb.2003.2497
Jin M, Liao C, Chakrabarty S et al (2019) Transcriptional response of ATP-binding cassette (ABC) transporters to insecticides in the cotton bollworm, Helicoverpa armigera. Pestic Biochem Physiol 154:46–59. https://doi.org/10.1016/j.pestbp.2018.12.007
Jin M, Cheng Y, Guo X et al (2020) Down-regulation of lysosomal protein ABCB6 increases gossypol susceptibility in Helicoverpa armigera. Insect Biochem Mol Biol 122:103387. https://doi.org/10.1016/j.ibmb.2020.103387
Jurat-Fuentes JL, Adang MJ (2004) Characterization of a Cry1Ac-receptor alkaline phosphatase in susceptible and resistant Heliothis virescens larvae. Eur J Biochem 271:3127–3135. https://doi.org/10.1111/j.1432-1033.2004.04238.x
Kalyaanamoorthy S, Minh BQ, Wong TKF et al (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589. https://doi.org/10.1038/nmeth.4285
Khan SA, Reichelt M, Heckel DG (2017) Functional analysis of the ABCs of eye color in Helicoverpa armigera with CRISPR/Cas9-induced mutations. Sci Rep 7:40025. https://doi.org/10.1038/srep40025
Knight PJK, Crickmore N, Ellar DJ (1994) The receptor for Bacillus thuringiensis CrylA(c) delta endotoxin in the brush border membrane of the lepidopteran Manduca sexta is aminopeptidase N. Mol Microbiol 11:429–436. https://doi.org/10.1111/j.1365-2958.1994.tb00324.x
Koenig C, Bretschneider A, Heckel DG et al (2015) The plastic response of Manduca sexta to host and non-host plants. Insect Biochem Mol Biol 63:72–85. https://doi.org/10.1016/j.ibmb.2015.06.001
Komoto N, Quan GX, Sezutsu H, Tamura T (2009) A single-base deletion in an ABC transporter gene causes white eyes, white eggs, and translucent larval skin in the silkworm w-3(oe) mutant. Insect Biochem Mol Biol 39:152–156. https://doi.org/10.1016/j.ibmb.2008.10.003
Kotkar HM, Sarate PJ, Tamhane VA et al (2009) Responses of midgut amylases of Helicoverpa armigera to feeding on various host plants. J Insect Physiol 55:663–670. https://doi.org/10.1016/j.jinsphys.2009.05.004
Labbé R, Caveney S, Donly C (2011) Genetic analysis of the xenobiotic resistance-associated ABC gene subfamilies of the Lepidoptera. Insect Mol Biol 20:243–256. https://doi.org/10.1111/j.1365-2583.2010.01064.x
Lei Y, Zhu X, Xie W et al (2014) Midgut transcriptome response to a Cry toxin in the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Gene 533:180–187. https://doi.org/10.1016/j.gene.2013.09.091
Li Q, Sun Z, Shi Q, Wang R, Xu C, Wang H, Song Y, Zeng R (2019) RNA-Seq analyses of midgut and fat body tissues reveal the molecular mechanism underlying Spodoptera litura resistance to tomatine. Front Physiol. https://doi.org/10.3389/fphys.2019.00008
Linton KJ (2007) Structure and function of ABC transporters. Physiology 22:122–130. https://doi.org/10.1152/physiol.00046.2006
Liu S (2011) Genome-wide identification and characterization of ATP-binding cassette transporters in the silkworm, Bombyx mori. BMC Genom 12:491
Liu L, Chen Z, Yang Y et al (2018) A single amino acid polymorphism in ABCC2 loop 1 is responsible for differential toxicity of Bacillus thuringiensis Cry1Ac toxin in different Spodoptera (Noctuidae) species. Insect Biochem Mol Biol 100:59–65. https://doi.org/10.1016/j.ibmb.2018.06.004
Liu Z, Fu S, Ma X, Baxter SW, Vasseur L, Xiong L, Huang Y, Yang G, You S, You M (2020) Resistance to Bacillus thuringiensis Cry1Ac toxin requires mutations in two Plutella xylostella ATP-binding cassette transporter paralogs. PLoS Pathog 16(8):e1008697. https://doi.org/10.1371/journal.ppat.1008697
Locher KP (2016) Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat Struct Mol Biol 23:487–493. https://doi.org/10.1038/nsmb.3216
Maddrell SH, Gardiner BO (1975) Excretion of alkaloids by Malpighian tubules of insects. J Exp Biol 64:267–281. https://doi.org/10.1242/jeb.64.2.267
Mathew LG, Ponnuraj J, Mallappa B et al (2018) ABC transporter mis-splicing associated with resistance to Bt toxin Cry2Ab in laboratory and field-selected pink bollworm. Sci Rep 8:13531. https://doi.org/10.1038/s41598-018-31840-5
McCaffery AR, Head DJ, Jianguo T et al (1997) Nerve insensitivity resistance to pyrethroids in Heliothine Lepidoptera. Pestic Sci 51:315–320. https://doi.org/10.1002/(SICI)1096-9063(199711)51:3
McGaughey WH (1985) Insect resistance to the biological insecticide Bacillus thuringiensis. Science 229:193–195. https://doi.org/10.1126/science.229.4709.193
Meng X, Yang X, Wu Z et al (2020) Identification and transcriptional response of ATP-binding cassette transporters to chlorantraniliprole in the rice striped stem borer, Chilo suppressalis. Pest Manag Sci 76:3626–3635. https://doi.org/10.1002/ps.5897
Meng X, Wu Z, Jiang C et al (2022) Identification and characterization of glutathione S-transferases and their potential roles in detoxification of abamectin in the rice stem borer, Chilo Suppressalis. Pestic Biochem Physiol 182:105050. https://doi.org/10.1016/j.pestbp.2022.105050
Murray CL, Quaglia M, Arnason JT, Morris CE (1994) A putative nicotine pump at the metabolic Blood–Brain Barrier of the tobacco hornworm. J Neurobiol 25:23–34. https://doi.org/10.1002/neu.480250103
Ocelotl J, Sánchez J, Gómez I et al (2017) ABCC2 is associated with Bacillus thuringiensis Cry1Ac toxin oligomerization and membrane insertion in diamondback moth. Sci Rep 7:2386. https://doi.org/10.1038/s41598-017-02545-y
Pan Y, Xu P, Zeng X et al (2019) Characterization of UDP-glucuronosyltransferases and the potential contribution to nicotine tolerance in Myzus persicae. Int J Mol Sci 20:3637. https://doi.org/10.3390/ijms20153637
Petschenka G, Pick C, Wagschal V, Dobler S (2013) Functional evidence for physiological mechanisms to circumvent neurotoxicity of cardenolides in an adapted and a non-adapted hawk-moth species. Proc R Soc B Biol Sci 280:20123089. https://doi.org/10.1098/rspb.2012.3089
Qi W, Ma X, He W et al (2016) Characterization and expression profiling of ATP-binding cassette transporter genes in the diamondback moth, Plutella xylostella (L.). BMC Genom 17:760. https://doi.org/10.1186/s12864-016-3096-1
Ren XL, Jiang WL, Ma YJ et al (2016) The Spodoptera exigua (Lepidoptera: Noctuidae) ABCC2 mediates Cry1Ac cytotoxicity and in conjunction with cadherin, contributes to enhance Cry1Ca toxicity in Sf9 cells. J Econ Entomol 109:2281–2289. https://doi.org/10.1093/jee/tow193
Ricardo S, Lehmann R (2009) An ABC transporter controls export of a Drosophila germ cell attractant. Science 323:943–946. https://doi.org/10.1126/science.1166239
Rösner J, Merzendorfer H (2021) Identification of two ABCC transporters involved in malathion detoxification in the red flour beetle, Tribolium castaneum. Insect Sci. https://doi.org/10.1111/1744-7917.12981
Scott JG, Wen Z (2001) Cytochromes P450 of insects: the tip of the iceberg. Pest Manag Sci 57:958–967. https://doi.org/10.1002/ps.354
Sharma A, Kumar V, Shahzad B et al (2019) Worldwide pesticide usage and its impacts on ecosystem. SN Appl Sci 1:1446. https://doi.org/10.1007/s42452-019-1485-1
Shelton AM, Wyman JA, Cushing NL et al (1993) Insecticide resistance of diamondback moth (Lepidoptera: Plutellidae) in North America. J Econ Entomol 86:11–19. https://doi.org/10.1093/jee/86.1.11
Sorenson JS, Dearing MD (2006) Efflux transporters as a novel herbivore counter-mechanism to plant chemical defences. J Chem Ecol 32:1181–1196. https://doi.org/10.1007/s10886-006-9079-y
Srinivas R, Udikeri SS, Jayalakshmi SK, Sreeramulu K (2004) Identification of factors responsible for insecticide resistance in Helicoverpa armigera. Comp Biochem Physiol—C Toxicol Pharmacol 137:261–269. https://doi.org/10.1016/j.cca.2004.02.002
Sturm A, Cunningham P, Dean M (2009) The ABC transporter gene family of Daphnia pulex. BMC Genom 10:170. https://doi.org/10.1186/1471-2164-10-170
Tabashnik BE, Finson N, Johnson MW, Heckel DG (1994) Cross-Resistance to Bacillus Thuringiensis Toxin CryIF in the Diamondback Moth (Plutella Xylostella). Appl Environ Microbiol 60:4627–4629. https://doi.org/10.1128/aem.60.12.4627-4629.1994
Tanaka S, Endo H, Adegawa S et al (2016) Functional characterization of Bacillus thuringiensis Cry toxin receptors explains resistance in insects. FEBS J 283:4474–4490. https://doi.org/10.1111/febs.13952
Tay WT, Mahon RJ, Heckel DG et al (2015) Insect resistance to Bacillus thuringiensis toxin Cry2Ab is conferred by mutations in an ABC transporter subfamily A protein. PLoS Genet 11(11):e1005534. https://doi.org/10.1371/journal.pgen.1005534
Tian L, Song T, He R et al (2017) Genome-wide analysis of ATP-binding cassette (ABC) transporters in the sweet potato whitefly, Bemisia tabaci. BMC Genom 18:330. https://doi.org/10.1186/s12864-017-3706-6
Trifinopoulos J, Nguyen LT, von Haeseler A, Minh BQ (2016) W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res 44:W232–W235. https://doi.org/10.1093/nar/gkw256
Tyzack JK, Wang X, Belsham GJ, Proud CG (2000) ABC50 interacts with eukaryotic initiation factor 2 and associates with the ribosome in an ATP-dependent manner. J Biol Chem 275:34131–34139. https://doi.org/10.1074/jbc.M002868200
Vadlamudi RK, Weber E, Ji I, Ji TH, Bulla LA (1995) Cloning and expression of a receptor for an insecticidal toxin of Bacillus thuringiensis. J Biol Chem 270:5490–5494. https://doi.org/10.1074/jbc.270.10.5490
Vetter IR, Wittinghofer A (2001) The guanine nucleotide-binding switch in three dimensions. Science 294:1299–1304. https://doi.org/10.1126/science.1062023
Wang L, Kiuchi T, Fujii T, Daimon T, Li M, BannoY KS, Kikawada T, Katsuma S, Shimada T (2013) Mutation of a novel ABC transporter gene is responsible for the failure to incorporate uric acid in the epidermis of ok mutants of the silkworm, Bombyx mori. Insect Biochem Mol 43:562–571. https://doi.org/10.1016/j.ibmb.2013.03.011
Wang J, Wang H, Liu S et al (2017) CRISPR/Cas9 mediated genome editing of Helicoverpa armigera with mutations of an ABC transporter gene HaABCA2 confers resistance to Bacillus thuringiensis Cry2A toxins. Insect Biochem Mol Biol 87:147–153. https://doi.org/10.1016/j.ibmb.2017.07.002
Wang J, Ma H, Zhao S et al (2020) Functional redundancy of two ABC transporter proteins in mediating toxicity of Bacillus thuringiensis to cotton bollworm. PLoS Pathog 16(3):e1008427. https://doi.org/10.1371/journal.ppat.1008427
War AR, Paulraj MG, Ahmad T et al (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7:1306–1320. https://doi.org/10.4161/psb.21663
Waterhouse A, Bertoni M, Bienert S et al (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46(W1):W296–W303. https://doi.org/10.1093/nar/gky427
Wu C, Ding C, Chen S et al (2021) Exposure of Helicoverpa armigera larvae to plant volatile organic compounds induces cytochrome p450 monooxygenases and enhances larval tolerance to the insecticide methomyl. Insects 12(3):238. https://doi.org/10.3390/insects12030238
Xiong J, Feng J, Yuan D et al (2015) Tracing the structural evolution of eukaryotic ATP binding cassette transporter superfamily. Sci Rep 5:16724. https://doi.org/10.1038/srep16724
Xu J, Wang Z, Wang Y et al (2020) ABCC2 participates in the resistance of Plutella xylostella to chemical insecticides. Pestic Biochem Physiol 162:52–59. https://doi.org/10.1016/j.pestbp.2019.08.010
Yang X, Chen W, Song X et al (2019) Mutation of ABC transporter ABCA2 confers resistance to Bt toxin Cry2Ab in Trichoplusia ni. Insect Biochem Mol Biol 112:103209. https://doi.org/10.1016/j.ibmb.2019.103209
You M, Yue Z, He W et al (2013) A heterozygous moth genome provides insights into herbivory and detoxification. Nat Genet 45:220–225. https://doi.org/10.1038/ng.2524
Yu HZ, Xu JP, Wang XY et al (2017) Identification of four ATP-binding cassette transporter genes in Cnaphalocrocis medinalis and their in response to insecticide treatment. J Insect Sci 17(2):44. https://doi.org/10.1093/jisesa/iex017
Zhang T, Coates BS, Wang YQ et al (2017) Down-regulation of aminopeptidase N and ABC transporter subfamily G transcripts in Cry1Ab and Cry1Ac resistant Asian corn borer, Ostrinia furnacalis (Lepidoptera: Crambidae). Int J Biol Sci 13:835–851. https://doi.org/10.7150/ijbs.18868
Zhao S, Jiang D, Wang F et al (2021) Independent and synergistic effects of knocking out two ABC Transporter genes on resistance to Bacillus thuringiensis toxins Cry1Ac and Cry1Fa in diamondback moth. Toxins 13:9. https://doi.org/10.3390/toxins13010009
Zhu B, Sun X, Nie X et al (2020) MicroRNA-998–3p contributes to Cry1Ac-resistance by targeting ABCC2 in lepidopteran insects. Insect Biochem Mol Biol 117:103283. https://doi.org/10.1016/j.ibmb.2019.103283
Zuber R, Norum M, Wang Y et al (2018) The ABC transporter Snu and the extracellular protein Snsl cooperate in the formation of the lipid-based inward and outward barrier in the skin of Drosophila. Eur J Cell Biol 97:90–101. https://doi.org/10.1016/j.ejcb.2017.12.003
Zuo YY, Huang JL, Wang J, Feng Y, Han TT, Wu YD, Yan Y (2018) Knockout of a P-glycoprotein gene increases susceptibility to abamectin and emamectin benzoate in Spodoptera exigua. Insect Mol Biol 27:36–45. https://doi.org/10.1111/imb.12338
Acknowledgements
HMK is grateful to the Department of Science and Technology- Science and Engineering Board (DST-SERB; EEQ/2017/000691), Government of India for funding. PRB thanks Council of Scientific and Industrial Research (CSIR), New Delhi for the award of Junior and Senior Research Fellowship. MBT thanks Department of Science and Technology, New Delhi for the award of Women Scientist Fellowship. APG and RSJ acknowledge project funding from the CSIR under Agriculture, Nutrition and Biotechnology theme (MLP101526), and seed grant (MLP036626) to CSIR-National Chemical Laboratory. We are grateful to Dr. Prashant Mendki for his critical reading. We are extremely thankful to the anonymous reviewer for useful suggestions to improve our manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed toward writing different sections and editing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that they have no conflict of interest.
Additional information
Handling editor: Antonia Monteiro.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barve, P.R., Tellis, M.B., Barvkar, V.T. et al. Functional Diversity of the Lepidopteran ATP-Binding Cassette Transporters. J Mol Evol 90, 258–270 (2022). https://doi.org/10.1007/s00239-022-10056-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-022-10056-2