Abstract
In the chemoautotrophic theory for the origin of life, offered as an alternative to broth theory, the archaic reductive citric acid cycle operating without enzymes is in the center. The non-enzymatic (methyl)glyoxalase pathway has been suggested to be the anaplerotic route for the reductive citric acid cycle. In the recent years, much has been learned about methylglyoxal, but its importance in the metabolic machinery is still uncovered. If methylglyoxal had been essential participant of the early stage of evolution, then it is a legitimate question whether it might have played a role in the early oxido-reduction network, too. Therefore, an oxido-reduction network of methylglyoxal that might have functioned under ancient circumstances without enzymes was constructed and analyzed by virtue of group contribution method. Taking methylglyoxal as input material, it turned out that the evolutionary value of reactions and biomolecules were not similar. Glycerol, glycerate, and tartonate, the output components, were conserved to different degrees. Although the tartonate route was similarly favorable from energetic point of view, its intermediates are almost not present in extant biochemistry. The presence of two carboxyl or aldehyde groups, or their combination in tricarbons of the constructed network seemed disadvantageous for selection, and the inductive effect, resulting in an asymmetry in electron cloud of chemicals, might have been important. The evolutionary role for cysteine, H2S, and formaldehyde in the emergence of high-energy bonds in the form of thioesters and in Fe–S cluster formation as well as in imidazole synthesis was shown to bridge the gap between prebiotic chemistry and contemporary biochemistry. Overall, the ideas developed here represent an approach fitting to chemoautotrophic origin of life and implying to the role of methylglyoxal in triose formation. The proposed network is expected to have an impact upon how one may think of prebiological chemical processes on methylglyoxal, too. Finally, along the evolutionary time line, the network functioning without enzymes is situated between the formation of simple organic compounds and primeval cells, being closer to the former and well preceding the last common metabolic ancestor developed after primitive cells emerged.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nature uses the same techniques in a wide variety of contexts; therefore, biochemical mechanisms, at depth, are very similar but varied in today's living organisms, as a result of gradual modifications in the course of the evolutionary process. This implicit means an ancient common origin of living organisms had to exist.
A group of enzymes, named oxidoreductases, catalyzes the liberation of energy from biomolecules by moving electrons in the direction of an electron acceptor. And they are involved in synthetic processes, too. The most important final electron acceptor in living organisms today is oxygen (aerobic organisms), but other substances such as sulfur, sulfate, or proton can also fulfill this function (anaerobic organisms) (Thauer 1988).
Since oxido-reductions are common in existing organisms, it can be assumed that these reactions also played a role at the dawn of life. The major difference between early stage of evolution and current biochemical mechanisms is the appearance of enzyme catalysis. Almost all metabolic processes in a cell need enzyme catalysis. What the enzymes usually do is that they reduce the free energy level of the transient state, named activation energy (Ea), for already existing reactions, thus, increasing the reaction rate, and at the same time, the possibility of regulation appears and reactions become specific. On this basis, it seems plausible to suggest that much of the current pathways, at least the most basic routes, already existed in the early stages of evolution, but functioned without enzymes (Sousa and Martin 2014; Ralser 2018; Muchowska et al. 2019). Therefore, retrodiction as a method may be suitable for studying the phenomenon (Lipmann 1965).
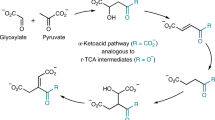
Prebiological evolution is the history of the origin of cells, and it is like a puzzle with too many pieces. The first comprehensive hypothesis in this regard was the primeval soup theory of Oparin (Oparin 1960; Haldene 1967). An alternative attractive suggestion is the chemoautotrophic scenario presented by Wächtershäuser (1988a). To remedy the shortcomings of the soup theory, this concept introduced a metabolic network anchored to a charged surface in volcanic hydrothermal vents and put reductive citric acid cycle (rTCA) in the center of the proposal based upon the self-sustaining feedback cycle, and pyrite formation from hydrogen sulfide (H2S) and ferrous ions provided driving force for the endergonic reactions (Yčas 1955; Morowitz 1966; Wächtershäuser 1988b, 1990). However, the fundamental question of anaplerotic reactions emerged. Although the original version of surface metabolism theory failed to give any suggestion to solve the above-mentioned problem, others raised the possible evolutionary role of (methyl)glyoxalase pathway as the anaplerotic route for the rTCA (Kalapos 1997).
The (methyl)glyoxalase system wide spread in extant metabolism consists of two cooperating enzymes, named glyoxalase I (GLO1, EC 4.4.1.5) and glyoxalase II (GLO2, EC 3.1.2.6), uses reduced glutathione (GSH) as a cofactor, and detoxifies the toxic glycolytic byproduct methylglyoxal (MGO) (Thornalley 1990; Kalapos 1999a). However, the formation of MGO in the extant metabolism is linked not only to glucose but also to lipid and amino acid metabolism (Thornalley 1990; Kalapos 2008). From the above, the ubiquitous nature of the (methyl)glyoxalase route and MGO itself are generally accepted (Thornalley 1990). By tracing back their existence to the era of prebiotic evolution (Kalapos 1997), the link between the early stage of evolution and the extant metabolism arises in a plausible manner, offering two advantages. On the one hand, the proposal provides the reason, why the glyoxalase route is ubiquitous among living organisms, thus proposing an answer to the glyoxalase “enigma,” the “long lasting” problem of biochemistry. On the other hand, the anaplerotic route put in the focus is fueled by the formose cycle using formaldehyde abundant under prebiotic environment (Breslow 1959; Quayle and Ferenczi 1978; Kalapos 1999b).
As seen, MGO became central in chemoautotrophic scenario participating in anaplerotic route for rTCA. If MGO had been an essential participant of the early stage of evolution, then the question is legitimate whether it would have played a role in the early oxido-reduction network, too.
In this article, the aim was to demonstrate how a MGO-initiated non-enzymatic oxido-reduction network could be systematically built up by stepwise changes. To reach the goal, tricarbon (C3) compounds were connected to MGO by reactions based on simple premises so that the theory effectively puts the network into the early evolutionary stage of metabolism. The thermodynamic calculations were made by using the group contribution method. It was also a question how non-enzymatic systems preceding enzymatic networks expanded their abilities to develop the ancient cell. Therefore, some of the factors affecting evolutionary modifications and leading to the development of enzymatic catalysis were examined in the case of MGO-initiated oxido-reduction network, too.
Methods of Compound Selection and Calculations
Rules for Network Building and Compound Selection
A network starting with MGO and functioning non-enzymatically was established (Fig. 1). The following rules were applied to changes. The aldehyde group was allowed either to be oxidized to carboxyl group or to be reduced to primary hydroxyl group. The keto group was only reduced to secondary hydroxyl group. The carboxyl group was allowed to be reduced to primary alcohol in two consecutive steps, with an aldehyde as intermediate. The oxidation of methyl group through three consecutive steps, with a primary hydroxyl group and an aldehyde group as intermediates, resulted in carboxyl group.
The oxido-reductive network of methylglyoxal with the free energies of formation of the compounds. For the easier tracking of changes, the molecules are shown in their non-ionized forms. Both the starting compound, methylglyoxal, and the final output materials of the network are shown framed in the figure. There are 24 positions, but only 18 compounds. The difference is due to the fact that there are compounds produced from two different ways in the network. Nevertheless, the question emerges whether in the case of glyceraldehyde, lactate, lactaldehyde, and 1,2-propanediol, known to exist as d- and l-isomer, which isomer has to be formed or a racemic mixture is produced. Calculations of free energies of formations refer to standard conditions, so carboxylate was used in the calculations instead of carboxyl. The typical error of the group contribution approach is less than 2 kcal/mol (8.36 kJ/mol), but it is worth mentioning that errors higher than 5 kcal/mol (20.90 kJ/mol) can also occur (Mavrovouniotis 1990, 1991). Thus, the method provides only an approximate value for the Gibbs free energy. Calculations were undertaken with the use of kcal/mol values, and the final results were only converted into kJ/mol. Rounding was performed to the second decimal. These values are shown in parentheses. The arrows show the downhill reactions
The changes could only be realized stepwise, i.e., only one reaction was allowed to occur between neighboring compounds.
Calculations
In the MGO-initiated oxido-reduction network, the corresponding molal Gibbs free energies of formation of given selected organic compounds were calculated on the basis of group contribution method (Mavrovouniotis 1990, 1991). Since calculations were performed under standard conditions supposing a value of pH 7, all carboxyl groups were taken in dissociated form. The Gibbs free energy of formation from elements for H2 and H+ originated from the work of Thauer and associates (Thauer et al. 1977). To minimize rounding errors, the calculations were performed using kcal/mol reported in the original article, and only final results were converted to kJ/mol. The unit of kcal/mol was converted into unit of kJ/mol by the use of the multiplication factor of 4.18. Data were given to the second decimal after correction. ΔGo′ values for the reactions between given compound pairs were calculated on the basis of the Hess principle.
Redox potential for So/SH2 couple was calculated from Go′ value given by Thauer and associates (Thauer et al. 1977). The basis of calculation was the following equation:
where n = number of electrons transferred in the reaction, F [Faraday constant] = 23 kcal/volt-gram equivalent (96.14 kJ/volt-equivalent), and Δεo′ = εo′(So/SH2) − εo′ (for standard hydrogen electrode).
Two-Electron Reactions
In Fig. 1, the network of two-electron oxido-reduction reactions of MGO is shown. Each transformation in the network can be described as a bimolecular reaction. For each one, ΔG is seen as being log proportional both to a concentration ratio and to a flux ratio (backward to forward flux) (Park et al. 2016).
It is well known that for the forward reaction of A ⇌ B bimolecular, dilute system, the Gibbs free energy change is expressed as follows:
where R is the universal gas constant, T is the temperature in grad Kelvin, and CB and CA are the concentrations for B and A, respectively (for further explanation see Appendix 1). While the temperature dependence of ΔG is apparent from Eq. (2), the pressure dependence, which may be important for certain prebiological considerations, particularly when hydrothermal vents are assumed to be the places for the advent of life, remains hidden (for details confer the Appendix 2).
The fluxes, the quantity of material converted per unit time for the forward and backward reactions is Ji+ and Ji−, respectively, are related to free energy change
The concentrations of both compounds are a function of fluxes and initial concentration of the given material. Given the time (t) of observation and supposed that the fluxes are time independent, the
equation describes the concentration for compound A in time, where CAo means the concentration of the compound at time point zero.
Define the ratio between the net flux through the reaction in comparison to the total flux as Flux-Force Efficacy (FFE) similarly to that already appeared in the literature (Beard and Qian 2007; Noor et al. 2014). FFE is also related to Gibbs free energy change, and by using Eq. (3), it can be described as follows:
When ΔG is about zero, i.e., Ji+ ≈ Ji−, then
which means an equilibrium. However, in the case of Ji+» Ji−, which means that ΔG « 0, Eq. (6) is described as follows:
Since exp (-ΔG/RT)» 1, Eq. (7) can be simplified to Eq. (8)
showing that the reaction is practically irreversible with a substantial dissipation of energy. The higher the decrease of ΔG, the higher the FFE.
Nevertheless, the time independence of conversion cannot be guaranteed as under evolutionary conditions, the parameters of the surroundings change. Hence, the flux should map these changes. Therefore, Eq. (4) has to be modified accordingly and expressed in the following form:
where Ji− (t) and Ji+ (t) are the functions describing the fluxes of backward and forward reactions in time, respectively.
The reactions displayed in Fig. 1, however, form a network of interconversions and a given compound participates in several reactions.
Suppose, there is a starting material A that can produce n different products, P1, P2 … Pn, in equilibrium reactions. Let Pn be a thermodynamically stable product with the lowest energy level, but P1 should be formed fastest. So kA→P1 > kA→Pn is seen, because Ea for the product P1 is lower than that for the product Pn. The other compounds are in the range appointed by P1 and Pn. If such a reaction is interrupted after a short time, then substance P1 is the main product as it is formed most rapidly from A and the other compounds’ concentrations follow it in an order of their k values, representing an inverse relation to their Eas (see also Appendix 3). However, after prolonged time, Pn can be isolated as the main product and the other compounds’ concentrations follow it in the order of their free energy change. For these reaction runs, P1 is the product mostly formed by the kinetic control, while Pn is mostly the result of the thermodynamic control. In considering the plausibility of these two controls, it is difficult to categorically state that under early evolutionary conditions presumably the former was superior to the latter or vice versa. Probably, both might have played a role, but in different settings with different weights, thus, influencing selecting out reaction pathways. Taking into account the timescale, too, according to which kinetic regulation is rather the short term, while thermodynamic regulation is rather the long-term actor in transformations, and additionally considering the fact that evolution is a continuous, but protracted process in time, the role of thermochemical regulation may have been crucial in the course of metabolic evolution. This view is supported by the constitution of extant biochemical networks, too. Nevertheless, in the lack of experimental data and simulations, the role of kinetic regulation cannot be excluded ab ovo, at least in part, as an important, short-term determinant in controlling reactions even under prebiotic conditions, while the reactions were mostly controlled by thermodynamics.
The change in the concentration of a given compound (A) in the network as a function of time can be described in the relation to its initial concentration and fluxes of reactions in which it participates
where n is the number of reactions in which compound A participates.
These rules are suitable for describing phenomena. By determining the direction of reactions, they probably gave the basis of network formation in the ancient stage of evolution in such a way that high FFE reactions were selected out. Nevertheless, the initial concentration of compounds as well as which substance(s) may have been the parent compound(s) of the network remain a question of debate. MGO may be one of the possible candidates as its role might have been essential in the anaplerotic pathway for rTCA, and its formation was clearly linked through formose cycle to formaldehyde, present under early evolutionary conditions (Kalapos 1997).
The corresponding Gibbs free energies of formation for given compounds are also shown in Fig. 1. Thoroughly examining the network, two striking properties of that can be recognized. On the one hand, MGO has the lowest energy of formation; therefore, it can be defined as input or raw molecule. On the other hand, the reactions pointing away from MGO are the same as the direction of free energy decrease and three endpoints can be recognized as output or products. These output components of the network are glycerol (GLY), glyceric acid (GLR), and tartronic acid (TRT) (Fig. 1). In this way, the complex network (©) can be simplified to a box depicted non-stoichometrically as follows:

It should be noted that GLR can be formed in two ways and lactic acid (LAC) is the last but one metabolite in one of the routes. A further interesting fact is that two of these three output compounds are inherent and important components of metabolic pathways existing today. It is also to be noted that the free energy change in the course of MGO → TRT path is more than three times higher and about one and half times higher than in the reactions resulting in GLY and GLR conversion, respectively.
The reactions in the oxido-reduction network need co-participants, too. It is suggested that SH2 is the hydrogen donor in hydrogenations, electron-accepting reactions, while pyrite formation is involved in dehydrogenations, electron-donating reactions. In general, the following types of reactions occur that are here shown from the aspect of MGO as starting molecule:
-
(i)
Reduction of the keto group resulting in the formation of α-hydroxy-carboxylic acid:
$${\text{ - CO - }}{\mkern 1mu} + {\text{ SH}}_{2} \Rightarrow {\text{ - HC}}\left( {{\text{OH}}} \right){\text{ - }}{\mkern 1mu} + {\text{ S}}^{{\text{o}}} .$$(12)
This kind of reactions is seen in other cases, too, e.g., in oxaloacetate/malate conversion (Kalapos 1997, 2002). Equation (12) can be subdivided into partial reactions:
where ΔGo′ for (14) is taken from the work of Thauer and associates (Thauer et al. 1977). It is noteworthy that SH2 exists in a 50:50 equilibrium with HS− + H+ at pH 7, and the εo’ values for So/HS− and H+/H2 redox couples are − 270 mV and − 414 mV, respectively (Thauer et al. 1977; Collmann et al. 2009). Although Wächtershäuser (Wächtershäuser 1990) suggested a pyrite-pulled reduction, it is unnecessary from an energy point of view as most of these reactions on their own go forward to hydroxy-acid formation (Kalapos 2002).
-
(ii)
Reduction of aldehyde group resulting in the formation of hydroxy group on the primary carbon:
$${\text{R}}-{\text{CHO }} + {\text{ SH}}_{{2}} \Rightarrow {\text{R}}-{\text{CH}}_{{2}} \left( {{\text{OH}}} \right) \, + {\text{ S}}^{{{\text{o}} }}\quad \Delta G_{{\text{o}}}^{\prime } = \, - { 12}.{\text{16 kJ}}/{\text{mol}}{.}$$(15) -
(iii)
Oxidation of methyl group resulting in the formation of hydroxy group:
$$-{\text{ CH}}_{{3}} + {\text{ H}}_{{2}} {\text{O}} \Rightarrow -{\text{ CH}}_{{2}} \left( {{\text{OH}}} \right) \, + {\text{ H}}_{{2}}^{ } \quad\Delta G_{{\text{o}}}^{\prime } = \, + { 21}.{\text{29 kJ}}/{\text{mol}}{.}$$(16)
The reaction displayed in Eq. (16) has two intriguing features. First, the reaction is endergonic on its own; therefore, hydrogen liberation has to be linked to a reaction that makes the whole process exergonic. In the literature, the pyrite formation has been offered taking advantage of electron transfer in the sulfur redox system (Wächtershäuser 1990):
Second, the oxidation reaction of the methyl group involves a hidden hydration, and practically, the water molecule is dehydrogenated. All in all, the whole reaction should be written as follows:
which is built up from the following partial reactions:
where (19) and (21) are identical to (16) and (17), respectively, and (20) is the reverse of (14).
The oxidation of methyl group in sequential steps results in hydroxyl, aldehyde, and carboxyl groups.
-
(iv)
Oxidation of aldehyde group resulting in the formation of carboxylic acid:
$${\text{R}}-{\text{CHO }} + {\text{ S}}^{{\text{o}}} + {\text{ H}}_{{2}} {\text{O }} + {\text{ FeS}} \Rightarrow {\text{R}}-{\text{COO}}^{-} + {\text{ H}}^{ + } + {\text{H}}_{{2}} + {\text{ FeS}}_{{2}} \;\;\Delta G_{{\text{o}}}^{\prime } = \, - { 96}.{\text{63 kJ}}/{\text{mol}}.$$(22)
Equation (22) is a combination of the half reactions of carbonyl → carboxyl transition with hydration and hydrogen liberation, and the reaction shown under (20) combined with pyrite formation featured under (21).
For the hydrogenation/dehydrogenation reactions shown in Fig. 1., the reaction heat can be calculated from the standard enthalpy values of the starting material(s) and the final product(s), taking their difference, according to the Hess law. These data are not presented, but the reactions are shown downwards from MGO, while the reverse reactions are not shown (Thauer et al. 1977; Mavrovouniotis 1990, 1991).
Enzyme-Catalyzed Reactions
Much of the reactions depicted in Fig. 1 can, however, be found in the extant metabolic machinery of several organisms as enzyme-catalyzed conversions. This note is particularly true to reactions between 3 and 6 columns (Fig. 1 and Table 1). It appears that the reactions of columns 3 to 6 are generally present in the metabolism as enzyme-catalyzed reactions, and these reactions are widespread among organisms, too. Therefore, it is likely that, from an evolutionary point of view, reactions within this range are more important and, for this reason, widely conserved.
To better understand the events, a simplified network is deduced from Fig. 1, in which the output components are LAC and GLR (Fig. 2). As it can be seen, the enzyme-catalyzed reactions in Table 1 show a very good agreement with the reactions of Fig. 2. These lines of reactions are thermodynamically favorable, giving support to the idea that the networks leading to either GLY or LAC formation were selected out by thermodynamic rather than kinetic at the early stage of evolution when enzymes were not available.
Reactions extracted from Fig. 1 on the basis of their significance in the extant metabolism. Bold lines show the interactions already presented in Fig. 1. Continuous thin lines represent those additional interactions that are functioning in extant metabolism, while dashed thin lines show those that are only suggested or not clearly explored. GSH = reduced glutathione. Aldose reductase (EC.1.1.1.21) functions in the presence of glutathione as a ketone reductase and lactaldehyde is the product, but when intracellular glutathione concentration is below normal, it catalyzes acetol formation (Vander Jagt et al. 2001). On this basis and on the data upon dihydroxyacetone and glyceraldehyde equilibrium (K = 5.25–5.98 and ΔG = − 4.07 to − 4.39 kJ/mol at pH 7.25)(Fedoronko and Königstein 1969), an acetol lactaldehyde transformation in an analogous way is suggested. Hydroxy-pyruvaldehyde and its phosphorylated form have been shown to be metabolized by glyoxalase I (Uotila and Koivusalo 1974; Cogoli-Greuter and Christen 1981), but the products of the reactions have not been identified yet
The enzymes and oxido-reductions catalyzed by them are summarized in Table 1. Of course, there are other types of reactions not belonging to the network constructed but present in contemporary biochemistry and in which these compounds participate, showing they are of importance. GLY is taken as an example, which can be phosphorylated by glycerol kinase (EC 2.7.1.30), and this way, it can be used for lipid synthesis or enters glycolytic pathway (Gull and Pasek 2021). And further, there are some of these chemicals that are not as well known as GLY but are starting materials for amino acid synthesis, e.g., hydroxy-pyruvaldehyde would mostly form serine (SER), while MGO would form alanine (ALA) (Pavlovskaya and Telegina 1974).
The Evolutionary Bridge Connecting Non-enzymatic Reactions with Enzyme-Catalyzed Reactions
It is, however, the question how evolution selected and preserved reactions that are finally found in extant metabolism. To find the clue, such a low molecular weight compound has to be sought that is sufficiently reactive, capable of metallic binding, and was available for primordial metabolism, and it is present in extant metabolism, too.
Cysteine (CYS) satisfies these requirements. While it is one of the least abundant amino acids in extant metabolism, it was formed under primitive conditions as the abundance of H2S in the prebiotic Earth and the reducing atmosphere favored the synthesis of organic compounds, including sulfur-containing small organic molecules (Quayle and Ferenczi 1978; Miller 1987; Olson and Straub 2016).
The guidance for restricting the domain of investigations to CYS comes from chemical as well as biochemical points of view since CYS offers several advantages.
Its abiotic, hydrothermal synthesis was reported on and two pathways for its generation from glycine were also proposed (Sagan and Khare 1971; Hennet et al. 1992; Parker et al. 2011). Besides the above, its synthesis from glycolaldehyde particularly well fits to the “from bottom up” approach, which could have primed by the use of formose cycle and H2S (Shalayel et al. 2020). Its molecular mass is 121.16 g/mol and exists in two forms. The thiol has low reactivity, while the thiolate is rendered a reactive nucleophile sensitive to interact with electrophiles, and it participates in redox reactions and creates disulfides (Fig. 3). Further, CYS has high-affinity metal binding (Zn finger, Fe–S cluster) (Poole 2015; Matsui et al. 2020).
Cysteine, cystine, and thiolate. The pKa for unperturbed CYS is about 8.25 (Matsui et al. 2020). It is obtained from the equilibrium constant Ka for the reaction CysSH ↔ CysS− + H+ with Ka = [H+][CysS−]/[CysSH] and pKa = − log Ka in which the equilibrium concentrations of participants are given in mol/l. The redox potential is gained for the reaction shown in the figure according to the following equation ε’ = ε’o − (RT/nF) ln([reduced form]/[oxidized form]), where T is the temperature measured in Kelvin (K) degrees, R is the universal gas constant (8.314 J K−1 mol −1), F is the Faraday constant, and n is the number of transferred electrons which is two for CYS reaction. The ε′o = − 220 mV (Jocelyn 1967). The ionization of neither the amine groups nor the carboxyl groups is shown in the figure
Indeed, in analogy with and a kind of generalization of Eq. (17), Fe–S cluster formation-fitting surface metabolism theory can be ascribed as follows:
In fact, the product of Eq. (23) in the next step can bind further CYSs resulting in a structure strongly resembling Fe–S clusters of extant metabolism:
Combining Eqs. (23) and (24), the overall reaction gained looks as follows:
The product of Eq. (25) (Fig. 4) can form a bridge to Fe–S proteins, since Fe–S clusters are formed under reducing conditions (Leal and Gomes 2010).
The simplest Fe–S cluster is the [Fe2S2] cluster constituting two irons bridged by two sulfide ions and coordinated by four amino acid ligands. Either two CYSs and two histidines (HIS) as in Rieske proteins (Conte and Zara 2011), or four CYSs as in ferredoxins (Campbell et al. 2019) are the coordinating amino acids ligating prosthetic group to the protein backbone. The iron is then either oxidized or reduced, thus participating in oxido-reductions as happens in extant metabolism. The ferredoxins offer the advantage that they can manage one-electron transfers and two-electron transfers, too (Gibson et al. 1966; Hall et al. 1971; Maiocco et al. 2019). And their further advantage is that unlike other proteins having a role in electron transfer, such as cytochromes or flavoproteins, they have only iron and inorganic sulfur in their active site (Hall et al. 1971).
It is noteworthy that HIS can easily enter the network since imidazole (IMI) can be synthetized from formaldehyde, MGO, and ammonia in the course of Debus-Radziszewski synthesis (Debus 1858; Radzisewski 1882). The possible role of IMI in MGO conversion in archaic network has been pointed out (Kalapos 1998). And reports are available on the participation of HIS residue in the conversion by glyoxalases of MGO in extant metabolism, too (Hall et al. 1978; Vander Jagt 1993; Ridderström and Mannervik 1996).
On the chemical basis, CYS thiol-moiety may react with aldehyde and carboxyl groups to form hemithioacetal (HT) and thioester (TE), respectively. The latter contains a high-energy bond having free energy of hydrolysis at pH 7 in the range of − 7 to − 14 kcal/mol (− 29.26 to − 58.52 kJ/mol) referred to as “energy-rich” bonding (Jencks et al. 1960). As Racker raised (Racker 1965), these had been the first high-energy bonds. In contrast to HT formation, it is, however, doubtful whether CYS thiols simply interact with carboxyl groups, because in contemporary biochemistry, such TEs are formed after an activation process like in the case of acetyl-coA formation. This comment is further supported by laboratory observations and thermochemical models mimicking hydrothermal vent conditions. The fact is that these reactions have low equilibrium constants (Chandru et al. 2018). On the other hand, according to in vitro experiments, TE formation was enhanced at neutral pH by ultraviolet irradiation or phosphate-imidazole catalysts (Weber 1981a, 1982a). Certainly, one selection pressure may have been how easy it was to produce high-energy molecules in the first place, but the clear mechanisms of thioester formation under ancient circumstances are yet uncovered.
The contemporary enzymes often use CYSs in their catalytic sites, and both HT and TE as intermediates frequently occur in enzymatic reactions as shown in the case for glyceraldehyde 3-phosphate dehydrogenase (EC 1.2.1.12)(Armstrong and Trentham 1976). These are seen in the rearrangement by GLO1 of the HT adduct of MGO and GSH into corresponding TE, S-D-lactoylglutathione, as well (Vander Jagt 1989; Ridderström and Mannervik 1996). Additionally, this rearrangement is effectively catalyzed by IMI without enzyme, too, and Mg2+ further accelerates this conversion (Hall et al. 1978). All these strongly suggest the early combination of CYS and IMI (HIS) in the course of the development of enzymatic catalysis, which is further supported by the existence of catalytic dyads frequently involving HIS and CYS couple (Leskovac and Pavkov-Peričin 1975; Richter et al. 2012). In a recent publication, experimental data have been provided according to which CYS is involved in prebiotic peptide synthesis as both precursor and catalyst, further stressing its evolutionary, but yet not fully uncovered role (Foden et al. 2020).
A notable fact is that thioacids and their carboxyl acid homologs were suggested to have existed in equilibrium:
and to have been central to the pyrite-pulled model for the origin of life (Wächtershäuser 1990). In this way, TE bond might have formed between thioacids and the hydroxyl of SER, as well. Nevertheless, the determinants of the above-mentioned equilibrium need further research as studies with thioacetic acid (TAA) showed that TAA was unstable in acidic media, while under alkaline conditions, this is the preferred pH range for the origin of life models, it has a half-life of a few years at about 40 °C (Chandru et al. 2018). Nevertheless, present-day analysis of posttranslational modification of proteins has stressed the superiority of CYS over SER and assigned a pivotal role to CYS in transacylation reactions (James et al. 2017).
It has, however, remained a question how the compounds for preservation were selected out. As presented above, the lines of reactions resulting in either LAC or GLR formation are thermodynamically favorable, strongly suggesting that selection was performed on the basis of thermodynamics. Although the TRT route is similarly favorable from the energetic point of view, it is not, or not widely, present in contemporary biochemistry, stressing out the role of other selection forces, too (Figs. 1 and 2). Although this mechanism of selection is yet hidden, an important feature of compounds of LAC and GLR paths is that neither of those contains in its structure two carboxyl or two aldehyde groups, or their combination. The structure of the compound and the features of the intramolecular electron cloud are interrelated, and, perhaps, the explanation to the different behavior of intermediates in the course of selection is due to the inductive effect. When the C3 compound has carboxyl groups or aldehyde groups at each end, or an aldehyde group and a carboxyl group, which are electron-retaining groups located at the edges of the compound, a kind of symmetry is given to the compound in the terms of electron density, while when the C3 compound contains a methyl or hydroxyl group at the one end and an aldehyde or carboxyl group at the other end, the electron density in the molecule is distorted by condensating electrons toward the aldehyde or carboxyl group, thus, resulting in an asymmetry. And this asymmetry in electron cloud may be the factor of selection being a feature affecting how the given compound can participate in chemical interactions. This might have been an innovative step in the straightforward development of enzymatic catalysis and suggests that this might have been a property having functioned as an additional selection factor.
The participation of CYS or other similar sulfur-containing compounds in the network offers an additional advantage regarding network thermodynamics. Indeed, they are suitable for the conservation of energy which is of evolutionary importance because the prompt use of energy formed is not needed, but rather it can be stored. At the same time, functioning as energy currency those can be used in another locale or reaction different from the place or reaction where or in which they were generated. All this means selection advantage.
One-Electron Reactions in the Absence of Atmospheric Oxygen
A one-electron reduction achieves free radical formation via a stepwise transfer of one electron from a donor molecule to an organic substrate without the participation of enzymes. The radical anions formed are unstable and thus engaged in secondary reactions attacking organic molecules or causing damage to cells.
For MGO, these reactions are particularly important in the investigation of atmospheric chemistry as well as in human pathology (Kalapos 2008; Fu et al. 2008). At the first sight, these types of reactions seem less relevant from an evolutionary aspect as when life emerged, there was no free molecular oxygen in the Archaean atmosphere (Quayle and Ferenczi 1978; Catling and Zahnle 2020). However, not only the oxygen can be taken into account as a possible partner in such interactions since charge transfer between amines and MGO can be carried out by hydrogen-bonding serving as a tunnel to form free radical fragments (Szent-Györgyi 1967; Abdulnur 1979). Amino acids as small organic molecules were formed under evolutionary conditions (Miller 1987; Hafenbrandl et al. 1995); therefore, they might have been particularly suitable to participate in such reactions in the early phase of evolution and are also in extant metabolism.
Findings of experiments with ALA and MGO in the absence of oxygen support above described view as the cross-linked radical cation in an enediol form and MGO radical anion were produced (Yim et al. 1995, 2000). Since neither ferro cations, nor chelating agents exerted any effect upon cross-linked cation formation a transition metal ion-independent direct electron transfer can be suggested (Yim et al. 1995, 2000). The interaction between arginine (ARG) and MGO also led to free radical generation (Wittmann et al. 2001). Ferric and ferrous ions increased and decreased free radical formation in a concentration-dependent manner, respectively, while the ferric ion-chelating agent, deferoxamine mesylate, prevented the ferric ion provoked increase of signal intensity, but the Fe2+-chelating agent, ferrozine, intensified that (Wittmann et al. 2001). The ARG MGO interaction without any addition of metal ions showed a saturation-like curve, most emphasized when ferrous ion was present (Wittmann et al. 2001). It was, therefore, suggested that ferric ion received an electron from MGO in the course of reactions and this redox reaction contributed to free radical generation detected (Wittmann et al. 2001). As to the mechanism, the produced ferrous ion would have been complexed by MGO and this complex would have been the agent attacking ARG.
To sum up, the direct one-electron transfer does not make obligatory the participation of either transition metal ions or oxygen in the reaction. The interaction between the amino group of amino acids and MGO under anaerobic conditions demonstrates that these reactions may also have played a role in the early chemical evolution.
Conclusions
One of the phenomenological characters of life is that without the expenditure of energy, it is unsustainable. For this reason, bioenergetics is central to any theory on the emergence of life. In the extant metabolism, oxygen is the universal electron acceptor (Babcock 1999). However, when life originated, about three and a half billion years ago, there was no free atmospheric molecular oxygen (Quayle and Ferenczi 1978; Tashiro et al. 2017; Catling and Zahnle 2020). Even the last common metabolic ancestor (LUCA), sometimes named progenote, the single ancestral form from which all today living organisms are deduced, is believed to have operated unaerobically (Weiss et al. 2018). Therefore, other ways were needed for the liberation of energy from biomolecules. An important alternative was the oxido-reductions by moving electrons in the direction of an electron acceptor other than oxygen.
Previous studies suggested that the non-enzymatic (methyl)glyoxalase pathway might have served as the anaplerotic route for rTCA in surface metabolism and MGO as an offshoot of formose cycle could have provided the starting material for this pathway (Kalapos 1997, 1998, 1999b). This approach had an additional benefit of explaining the ubiquitous nature of the (methyl)glyoxalase pathway. Nevertheless, given the multifaceted connections of MGO in present-day metabolism, it seemed more than plausible to suppose that its role had not been limited to the above-mentioned anaplerotic pathway, but rather other roles in prebiotic chemistry might have been assigned to it. Therefore, it was a rational purpose of this study to map the non-enzymatic oxidation–reduction network of MGO, thus, extending the possible roles of this dicarbonyl.
If this studied non-enzymatic network is placed along the evolutionary time scale, its position is between the formation of simple organic compounds and primeval cells, being closer to the former and well preceding LUCA, developed after a string of primitive cells emerged (Fani and Fondi 2009). The proposed network worked without enzymes, but in its development, it had the capacity to integrate catalysts, thus, paving the way for the formation of later enzyme catalysis. Although the evolvability of chemical networks is still a subject of debate, yet the primitive cells already possessed the machinery for gene expression with rudimentary translation process, while LUCA inherently had the ability for evolution (Woese 1998; Vasas et al. 2010, 2012; Ralser 2018; Weiss et al. 2018). Since both the glyoxalases and MGO as well as a large part of the compounds in the network are ubiquitous in contemporary metabolism, therefore, it cannot be so unlikely that a glyoxalase ancestor, perhaps a β-lactamase-type enzyme for GLO2 (Pettinati et al. 2016) and an ancient, yet unknown glutathione-dependent enzyme for GLO1, was already present in LUCA. The non-enzymatic origin of gluconeogenesis is supported by recent investigations (Ralser 2018) and the majority of the compounds in MGO network is either a participant in or related to glucose metabolism in the contemporary biochemistry.
In the course of extending the pyrite-pulled theory, an attempt was already made to outline sugar pathways, but networks recommended there were a bit complicated (Wächtershäuser 1992). Therefore, in contrast to that proposal, although the present work also builds on the theses of pyrite-pulled theory, the central element is the connection of MGO to triose formation, thus, to the future sugar and lipid metabolisms. In this way, trioses are appointed to one of the central factors of ancient metabolism. This situation is very similar to that of present-day metabolism. Since enzymatic mechanisms only kinetically alter reactions by reducing Ea, it is reasonable to assume that non-enzymatic pathways of MGO network had been already present earlier, well before the appearance of enzymes. This comment roots in the method of retrodiction that provided a methodological tool to investigations (Lipmann 1965; Szent-Györgyi 1968). The paradigm on which the approach is built up is well described by the opinion of De Duve, who pointed out that the early chemistry through which life was first produced already prefigured some of the key processes by which life constructs itself in present-day organisms (De Duve 2003).
Using MGO as an example a complex non-enzymatic network of oxido-reduction reactions was possible to build (Fig. 1). Since MGO itself and the oxido-reductions in which it is involved are generally preserved, all these point to the importance of this dicarbonyl. When these reactions were examined in the enzymatic era, it became evident that some reactions were generally retained in the living beings, while others became insignificant (see Table 1. and references therein). There are only few species in which these neglected reactions occur. All these indicate that during evolution some reactions were superior to others.
Although it is not yet possible to fully uncover the selection mechanism(s) in the absence of data, the already available results and calculations as well as a comparison with the present biochemistry allow a modest speculation and make possible to build up an acceptable sequence of events. Data collectively show (Figs. 1 and 2) that the thermodynamically favorable reactions of the MGO hydrogenation/dehydrogenation network led to the formation of GLY and GLR production present in contemporary biochemistry, suggesting that thermodynamics rather than kinetics was the acting factor in selection. Although the TRT route is similarly favorable from energetic point of view, these materials are rarely found in extant metabolic networks, and thus, additional factors have to be sought. These may be as follows.
An important feature of compounds participating in LAC-GLR and GLY paths is that none of the pathways contains a compound having two carboxyl groups or two aldehyde groups, or their combination (Fig. 1). The presence of two such groups in these C3s is obviously disadvantageous for some yet not proven reasons, while it does not mean a barrier for others, not belonging to the network such as malonate or succinate. Perhaps, the explanation can be given by the inductive effect as detailed above. In brief, the asymmetry and symmetry in electron cloud might have been the factor of selection for this network.
And further, the majority of selected compounds may in fact interact with thiol functional group forming either HT or TE. In the extant biochemistry, some of them (MGO, hydroxyl-pyruvaldehyde) by interacting with GSH are substrates for GLO1, while others forming TE (S-glyceryl-GSH, S-pyruvyl-GSH, S-lactoyl-GSH) are substrates for GLO2 or S-formylglutathione hydrolase (EC.3.1.2.12) (Uotila and Koivusalo 1974; Talesa et al. 1988; Vander Jagt 1989; Gonzalez et al. 2006). Intriguing feature of the mechanism of enzymatic catalysis is that HIS is a critical element of catalytic process in the case of both GLO1 and GLO2 (Hall et al. 1976, 1978; Mannervik and Ridderström 1993; Ridderström and Mannervik 1996; Vander Jagt 1993). Interestingly, CYS functioned as catalyst in such enzyme-free systems, too that mimicked some of the tricarboxylic acid cycle reactions and in which either oxidation or hydration took place (Shimizu 2007). These two types of reactions frequently occur in the constructed MGO network, as well (Fig. 1.), giving a further basis to the assumption that these interactions are of ancient origin and have a role in energy-producing processes.
The interaction with thiols might have been an innovation step in the straightforward development to enzymatic catalysis and energy production. This suggests that there might have been a property or perhaps more, having functioned as selection factor. Both the simplest thiol SH2 and CYS were already present in the early stage of evolution having the ability to react with and to bind to minerals, forming the parent molecules of Fe–S clusters of contemporary biochemistry (Fig. 4.). Iron-sulfur proteins are common in all known forms of cells and linked to energy-producing processes (Hall et al. 1971; Castresana and Saraste 1995; Beinert et al. 1997; Leal and Gomes 2010). Their use in such reactions is a characteristic way how life operates. In addition, a further property that might have been useful is that sulfur has the ability to adopt a wide variety of oxidation states ranging from − 2 to + 6, making thiols suitable to widely participate in a variety of oxido-reductions (Fig. 3) (Paulsen and Carroll 2013; Fra et al. 2017).
TEs, formed from carboxyl and thiol groups, are referred to as high-energy bond and assumed evolutionary ancestors of ATP (Racker 1965; Hartman 1975; de Duve 1995). Several TEs, the mechanism and conditions of their production, and their ability to enhance P ~ P bond formation were investigated. It was reported on that TEs were generated in the course of oxidation of HTs, too, and phosphate anions and IMI proved enhancers of generation and hydrolysis of TEs (Hall et al. 1978; Weber 1982a). Their photochemical syntheses were also documented (Weber 1981a). Whatever way TEs were formed, their formation opened the way for energy conservation. Their diffusion from the locale of formation and their acting as energy currency at the expenditure of the free energy released during their hydrolysis allowed an otherwise endergonic reaction to become exergonic. In a further step, TEs promoted P ~ P bond formation by virtue of IMI, hydroxyapatite, and phosphates (Weber 1981b, 1982b). Thus, instead of simultaneity, the possibility for spatially and temporally shifted reactions was created. It is a textbook knowledge that the initial oxidation of an aldehyde bound to a thiol leads to TE formation in relevant enzymatic reactions of substrate level oxidative phosphorylation, and those are commonly encountered in extant biochemistry. Finally, TEs possibly provided an opportunity to participate in biosynthetic processes due to their good acylating ability, too (Kulkarni et al. 2017).
According to a recently published analysis, it was possible to construct a phosphorus-free metabolic network, which was highly dependent on Fe–S enzymes and TE intermediates (Goldford et al. 2017). Although, this hypothetical network does not give evidence to decide in the regard of the primacy concerning TEs and ATP because enzymatic network was modeled, but strongly suggests that a phosphorus-free system can operate. All in all, ubiquity of TEs in biology is a strong argument in favor their early inclusion in the processes at the advent of life.
Nevertheless, the driving force(s) behind the selection of compounds mentioned is (are) still not fully understood, but, given the current biochemical systems, it is possible to risk that this pressure may have come from high-energy bonds and phosphorylation. A significant proportion of the molecules shown in MGO-centered non-enzymatic network is also present in phosphorylated forms in the extant biochemical networks and, on the other hand, at the early stage of evolution, as suggested, phosphate concentrations were relatively constant with the exception of Precambian oceans where it was elevated (Planavsky et al. 2010). Maybe, this was the time when phosphorylation as a mechanism became common. From the molecular side, one of the reasons for the selection of these molecules might have been the possibility for the interaction phosphorus with not fully oxidized functional groups, mainly for molecules in columns 3–6 featured by asymmetry in electron cloud. All these might have opened the way for the formation of high-energy phosphate bonds as seen in the second stage of glycolysis. Since the theory of the bottom-up evolution of enzymatic glycolysis is generally accepted (Fotherhill-Gilmore 1987; Potter and Fotherhill-Gilmore 1993), these steps fit with the theory.
Beside two-electron reactions, one-electron reactions were also possible in the prebiotic era and involved in secondary reactions attacking small organic molecules like amino acids under anaerobic conditions. The latter ones have high pathological impact in the present-day medicine, too.
It has long been known in the literature that the presence of oxygen in the atmosphere is not obligatory to the toxic effects of MGO to develop, but the presence of oxygen enhances that (Kalapos et al. 1993). Some of the one-electron reactions, e.g., reaction between MGO and amino acids, do not require the presence of oxygen, either, but it is required for the majority of reactions and for autoxidation. So, due to the oxygen dependence, it may be thought that these reactions have rather a pathological role in extant metabolism leading to advanced glycation or advanced lipoxidation end-product formation (Yim et al. 2000; Thornalley 2008; Vistoli et al. 2013; Wetzels et al. 2017). Their evolutionary role must have been limited, whereas important in the synthesis of small biomolecules in prebiotic era. This is also true to reactions in which free radicals did not participate, e.g., IMI synthesis, a compound that preceded the emergence of thiols as catalyst in the (methyl)glyoxalase path (Kalapos 1998; Aiona et al. 2017).
Limitations and Highlights
Most important limitation of this study that warrants discussion is the sparse repertoire of experimental data. Therefore, it was not possible to reasonably take into consideration the effect of such variables like pressure and temperature. In the terms of these environmental parameters, the proposed values either change in a wide range as for the temperature ranging 10–100 °C or are not given as in the majority of cases for pressure (Baross and Hoffman 1985; Russell and Hall 2002; Guzman and Martin 2009; Camprubi et al. 2017). The lack of data also constrained the discussion of kinetics, too.
Two approaches can be expected to help in the reduction of data gaps. On the one hand, more intensive collection of environmental data (present-day hydrothermal vents, rock fossils) and, on the other hand, more extensive experimental investigations under hydrothermal vents conditions are needed. In the latter case, there is, however, an inherent barrier to examinations. Namely, the only consensus upon the hydrothermal vents is that those might have been the cradles for the advent for life with alkaline pH (Martin et al. 2008; Kitadai and Maruyama 2018). Present-day vents are used as model systems, which immediately raises the question whether “deep-sea water” (located at depth > 200 m) or “swallow-water” (located at depth < 200 m) should be considered, with different fauna and environmental circumstances (Tarasov et al. 2005).
As mentioned above, the effects of the changes of environmental factors have not been and could not be considered, whereas their influences on chemical reactions and Gibbs free energy changes are obvious. Applying ΔGo′ for a reaction introduces only a minimal error for the same reaction, when the temperature is in the narrow segment of 15–37 °C (Amend and Shock 2001) (see Appendix 2). However, this range does not cover the assumed whole range of temperature in hydrothermal vents. In spite of that, our concern is with relative, rather than absolute. And, although the exact conditions cannot be specified, the framework in which the pyrite-pulled network may have operated can be delineated on thermodynamic considerations.
The reductive power of the FeS–H2S/FeS2 redox couple, Eq. (17), depends on H2S activity and is less reducing at elevated temperatures (> 250 °C), while its pressure dependence in the range of 0.1–1 kbar, 1 bar is equal about to the pressure of 10 m of water, is moderate (Schoonen et al. 1999). For the gaseous components, H2 and SH2, the gas solubility is more than twice at 200 °C than at 50 °C, and eight times higher at 0 °C than 200 °C, respectively (Amend and Shock 2001). These parameters cover the assumed hydrothermal vent environmental factors for rTCA (Guzman and Martin 2009). Finally, calculations showed that the production of both carboxylic acids and carbonyls are thermodynamically possible in the temperature range of 50–250 °C in vents (Shock and Schulte 1998).
The majority of calculations is based on the group contribution method that though it helps to give a picture of the processes, it has limitations of its own (Mavrovouniotis 1990, 1991). At the same time, it is an internally consistent database that is useful in bioenergetic calculations (Amend et al. 2013), thus, eliminating inconsistencies among various sets of thermodynamic data.
Furthermore, there are still uncertainties regarding the exact point of chemical evolution, at which IMI was incorporated into the system.
Despite all these, the highlights of the study that had the focus only on a selected part of the whole metabolic network are as follows.
The possible essential role for MGO in prebiotic chemical evolution received a further theoretical support that may explain why this dicarbonyl and the (methyl)glyoxalase route are ubiquitous in contemporary biochemistry. Since the enduring “enigma” of glyoxalases is still challenging and currently there is no consensus for their role in metabolism, such a chemistry depicted here could bridge the gap by showing the pathway as a prebiotic fossil with limited role in contemporary metabolism.
The reactions, shown in Fig. 2, are almost ubiquitously found as enzyme-catalyzed reactions in the present-day metabolism regardless of species, and the compounds are starting materials for the synthesis of other organic molecules showing that these reactions were superior to others from an evolutionary point of view and for this reason were generally conserved.
Linking to the pyrite-pulled system made possible to integrate reactions into one system and the anchoring to surface is an innovative feature. At the first glance, the latter is more of a limit. However, a closer look at the present biochemical processes shows that many reactions, including important ones such as terminal oxidation, are membrane bound or cytoskeleton bound. Thus, bonding to the pyrite surface may have been a precursor to these intracellular anchors.
CYS and H2S were shown essential to bridge the gap between prebiotic and contemporary metabolism explaining the emergence of high-energy bonds in the form of TEs and Fe–S cluster formation, thus, answering to the fundamental problem of any metabolism that is not other than energy production.
The article does not categorically answer the question of energy conservation but gives a strong theoretical support to the role of TE bonding that might have been the ancestor of energy-rich intermediates. Nor does this article rule out that CO2 assimilating routes other than rTCA that might have existed in the prebiotic era. The most recommended path in this regard is the Wood-Ljungdahl pathway (WLP) (Camprubi et al. 2017; Sousa and Martin 2014; Muchowska et al. 2019). However, it has to be seen, if a CO2 fixation mechanism is chosen, at the same time, an energy-producing process is also chosen. For rTCA and WLP, the sources for related energy-producing processes were assumed pyrite and H2, respectively (Martin 2011). Since both rTCA and WLP are present in extant metabolism, the question is open, what their relation to each other in the course of evolution might have been. Their ab initio parallel existence as hypothesis may be raised, particularly reminding the fact that H2 is a part of pyrite system (see Eq. 17). Such a relationship has very recently been stressed in the enzymatic world (Muchowska et al. 2020). Notably is that while contemporary research offers two alternatives: the replication-first and metabolism-first theories (Anet 2004), the scenario presented here is based on the metabolism-first approach.
For the sake of fidelity, it is to be noted that according to another scenario, rooted in the information-first view (Anet 2004), life would likely only have been feasible in a cold environment, since polymers, required for some type of genetic information system, would have been deteriorated at high temperatures (Bada and Lazcano 2002).
It looks that MGO was already bound to energy production in the early stages of evolution through oxido-reductions (this study) and through the (methyl)glyoxalase path (Kalapos 1997). The question, however, arises as to how its role has changed over the millions of years. In the course of evolution subtle, stepwise changes may occur in the metabolic context of biochemical networks and some characteristics, even though still suitable, may no longer serve their original function(s). The initial reactivity of MGO seemed to be advantageous at the advent of life, but this has turned into a disadvantage in cellular systems. This might explain why cells have developed enzymatic and non-enzymatic elements to protect themselves against reactive MGO.
All in all, the representation of this MGO network has then the capability of explaining how a network might have been organized in a consistent way. The consequences of these processes are inferred. Hence, it seems reasonable that the ideas developed here will become important for experimental studies in systems chemistry.
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- ALA:
-
Alanine
- ARG:
-
Arginine
- C3:
-
Tricarbon compound
- CYS:
-
Cysteine
- Ea :
-
Activation energy
- FFE:
-
Flux-Force Efficacy
- G:
-
Gibbs free energy
- GLO1:
-
Glyoxalase I
- GLO2:
-
Glyoxalase II
- GLR:
-
Glyceric acid
- GLY:
-
Glycerol
- GSH:
-
Reduced glutathione
- HIS:
-
Histidine
- H2S:
-
Hydrogen sulfide
- HT:
-
Hemithioacetal
- IMI:
-
Imidazole
- LAC:
-
Lactic acid
- LUCA:
-
Last universal common ancestor
- MGO:
-
Methylglyoxal
- rTCA:
-
Reductive citric acid cycle
- SER:
-
Serine
- TAA:
-
Thioacetic acid
- TE:
-
Thioester
- TRT:
-
Tartronic acid
- WLP:
-
Wood-Ljungdahl pathway
References
Abdulnur SF (1979) Interactions of methylglyoxal with methylamine. Ciba Found Symp 67:195–209
Aiona PK, Lee HJ, Leslie R, Lin P, Laskin A, Laskin J, Nizgorodov SA (2017) Photochemistry of products of the aqueous reaction of methylglyoxal with ammonium sulfate. ACS Earth Space Chem 1:522–532
Amend JP, Shock EL (2001) Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol Rev 25:175–245
Amend JP, LaRowe DE, McCollom TM, Shock EL (2013) The energetic of organic synthesis inside and outside the cell. Phil Trans R Soc B 368:20120255
Anet FAL (2004) The place of metabolism in the origin of life. Curr Opin Chem Biol 8:654–659
Argiles JM (1986) Has acetone a role in the conversion of fat to carbohydrate? Trends Biochem Sci 11:61–63
Armstrong JM, Trentham DR (1976) The reactions of D-glyceraldehyde 3-phosphate with thiols and the holoenzyme of D-glyceraldehyde 3-phosphate dehydrogenase and of inorganic phosphate with the acyl-holoenzyme. Biochem J 159:513–527
Babcock GT (1999) How oxygen is activated and reduced in respiration. Proc Natl Acad Sci USA 96:12971–12973
Bada JL, Lazcano A (2002) Some like it hot, but not the first biomolecules. Science (Washington DC) 296:1982–1983
Baross JA, Hoffman SE (1985) Submarine hydrothermal vents and associated gradient environments as sites for the origin and evolution of life. Orig Life Evol Biosph 15:327–345
Beard DA, Qian H (2007) Relationship between thermodynamic driving force and one-way fluxes in reversible processes. PLoS ONE 2:e0144
Beinert H, Holm RH, Münck E (1997) Iron-sulfur clusters: nature’s modular, multipurpose structures. Science (Washington DC) 277:653–659
Braun W, Kaltwasser H (1979) Untersuchungen zum Glyoxylsäurestoffwechsel von Bacillus fastidiosus. Arch Microbiol 121:129–134
Breslow R (1959) On the mechanism of the formose reaction. Tetrahedron Lett 21:22–26
Campbell IJ, Bennett GN (2019) Silberg JJ (2019) Evolutionary relationships between low potential ferredoxin and flavodoxin electron carriers. Front Energy Res 7:79
Camprubi E, Jordan SF, Vasiliadou R, Lane N (2017) Iron catalysis at the origin of life. IUBMB Life 69:373–381
Casazza JP, Felver ME, Veech RL (1984) The metabolism of acetone in rats. J Biol Chem 259:231–236
Castresana J, Saraste M (1995) Evolution of energetic metabolism: the respiration-early hypothesis. Trends Biochem Sci 20:443–448
Catling DC, Zahnle KJ (2020) The Archean atmosphere. Sci Adv 6:1420. https://doi.org/10.1126/sciadv.aax1420
Chandru K, Gilbert A, Butch C, Aono M, Cleaves HJ (2018) The abiotic chemistry of thiolated acetate derivatives and the origin of life. Sci Rep 6:29882
Christen P, Gasser A (1976) Oxidation of the carbanion intermediate of transaldolase by hexacyanoferrate (III). J Biol Chem 251:4220–4223
Cogoli-Greuter M, Christen P (1981) Formation of hydroxypyruvaldehyde phosphate in human erythrocytes. J Biol Chem 256:5708–5711
Collmann JP, Ghosh S, Dey A, Decreau RA (2009) Using functional enzyme model to understand the chemistry behind hydrogen sulfide induced hibernation. Proc Natl Acad Sci USA 106:22090–22095
Conte L, Zara V (2011) The Rieske iron-sulfur protein: import and assembly into cytochrome bc1 complex of yeast mitochondria. Bioorg Chem Appl. https://doi.org/10.1155/2011/363941
Cramer SD, Ferree PM, Lin K, Milliner DS, Holmes RP (1999) The gene encoding hydroxypyruvate reductae (GRHPR) is mutated in patients with primary hyperoxaluria type II. Hum Mol Genet 8:2063–2069
Debus H (1858) Ueber die Einwirkung des Ammoniaks auf Glyoxal. Justus Liebigs Ann Chem 107:199–208
De Duve C (1995) The beginnings of life on Earth. Am Sci 83:428–437
De Duve C (2003) The facts of life. Scripta Varia 105:71–100
De Groot MJL, van Berlo RJP, van Winden WA, Verheijen PJT, Reinders MJT, de Ridder D (2009) Metabolite and reaction inference based on enzyme specificities. Bioinformatics 25:2975–2982
De Windt FE, Van der Drift C (1980) Purification and some properties of hydroxypyruvate isomerase of Bacillus fastidiosus. Biochim Biophys Acta 613:556–562
Edenberg HJ (2007) The genetics of alcohol metabolism/role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health 30:5–13
Engqvist M, Drinkovich MF, Flügge U-I, Maurino VG (2009) Two D-2-hydroxyacid dehydrogenases in Arabidopsis thaliana with catalytic capacities to participate in the last reactions of the methylglyoxal and β-oxidation pathways. J Biol Chem 284:25026–25037
Fani R, Fondi M (2009) Origin and evolution of metabolic pathways. Phys Life Rev 6:23–52
Fedoronko M, Königstein J (1969) Kinetics of mutual isomerization of trioses and their dehydration to methylglyoxal. Collect Czechoslov Chem Commun 34:3881–3894
Foden CS, Islam S, Fernández-Garcia C, Maugeri L, Sheppard TD, Powner MW (2020) Prebiotic synthesis of cysteine peptides that catalyze peptide ligation in neutral water. Science (Washington DC) 370:865–869
Fotherhill-Gilmore LA (1987) Evolution in glycolysis. Biochem Soc Trans 15:993–995
Fra A, Yoboue ED, Sitia R (2017) Cysteines as redox molecular switches and targets of disease. Front Mol Neurosci 10:167. https://doi.org/10.3389/fnmol2017.00167
Fu T-Z, Jacob DJ, Wittrock F, Burrows JP, Vrekoussis M, Henze DK (2008) Global budgets of atmospheric glyoxal and methylglyoxal, and implications for formation of secondary organic aerosols. J Geophys Res 113:D15303
Gibson JF, Hall DO, Thornley JMH, Whatley FR (1966) The iron complex in spinach ferredoxin. Proc Natl Acad Sci USA 56:985–990
Goldford JE, Hartman H, Smith TF, Segrè D (2017) Remnants of an ancient metabolism without phosphate. Cell 168:1126–1134
Gotto AM, Kornberg HL (1961) The metabolism of C2 compounds in microorganisms. 7. Preparation and properties of crystalline tartronic semialdehyde reductase. Biochem J 81:273–284
Gonzalez CF, Proudfoot M, Brown G, Korniyenko Y, Mori H, Savchenko AV, Yakunin AF (2006) Molecular basis of formaldehyde detoxification/characterization of two S-formylglutathione hydrolases from Escherichia coli, FrmB and YeiG. J Biol Chem 281:14514–14522
Grazi E, Thrombetta G (1978) A new intermediate of the aldolase reaction, pyruvaldehyde-aldolase-orthophosphate complex. Biochem J 175:361–368
Gull M, Pasek MA (2021) The role of glycerol and its derivatives in the biochemistry of living organisms, and their prebiological origin and significance in the evolution of life. Catalysts 11:86
Guzman MI, Martin ST (2009) Prebiotic metabolism: production by mineral photochemistry of α-ketocarboxylic acids in the reductive tricarboxylic acid cycle. Astrobiology 9:833–842
Hafenbrandl D, Keller M, Wächtershäuser G, Stetter O (1995) Primordial amino acids by reductive amination of α-oxo acids in conjunction with the oxidative formation of pyrite. Tetrahedron Lett 36:5179–5182
Haldene JBS (1967) The origin of life. In: Bernal JD (ed) The origin of life. William Clowes and Sons Ltd, London, pp 242–249
Hall AN, Kulka D, Walker TK (1955) Formation of arabinose, ribulose and tartronic acid from 2-keto-D-gluconic acid. Biochem J 60:271–274
Hall DO, Cammack R, Rao KK (1971) Role for ferredoxins in the origin of life and biological evolution. Nature 233:136–138
Hall SS, Doweyko AM, Jordan F (1976) Glyoxalase I enzyme studies. 2. Nuclear magnetic resonance evidence for an enediol-proton transfer mechanism. J Am Chem Soc 98:7460–7461
Hall SS, Doweyko AM, Jordan F (1978) Glyoxalase I enzyme studies. 4. General base catalyzed enediol proton transfer rearrangement of methyl- and phenylglyoxalglutathionylhemithiol acetal to S-Lactoyl- and S-Mandeloylglutathione followed by hydrolysis. J Am Chem Soc 100:5934–5939
Hartman H (1975) Speculations on the origin and evolution of metabolism. J Mol Evol 4:359–370
Hennet RJ-C, Holm NG, Engel MH (1992) Abiotic synthesis of amino acids under hydrothermal conditions and the origin of life: a perceptual phenomenon? Naturwissenschaft 79:361–365
James AM, Hoogwijs K, Logan A, Hall AR, Ding S-j, Fearnley IM, Murphy MP (2017) Non-enzymatic N-acetylation of lysine residues by Acetyl-CoA often occurs via proximal S-acetylated thiol intermediate sensitive to glyoxalase II. Cell Rep 18:2015–2112
Jeffery J, Jörnvall H (1983) Enzyme relationships in a sorbitol pathway that bypasses glycolysis and pentose phosphates in glucose metabolism. Proc Natl Acad Sci USA 80:901–905
Jencks WP, Cordes J, Carriuolo J (1960) The free energy of thiol ester hydrolysis. J Biol Chem 235:3608–3614
Jocelyn PC (1967) The standard redox potential of cysteine-cystine from the thiol-disulphide exchange reaction with glutathione and lipoic acid. Eur J Biochem 2:327–331
Kalapos MP (1997) Possible evolutionary role of methylglyoxalase pathway: anaplerotic route for reductive citric acid cycle of surface metabolists. J Theor Biol 188:201–206
Kalapos MP (1998) From mineral support to enzymatic catalysis/further assumptions for the evolutionary history of glyoxalase system. J Theor Biol 193:91–98
Kalapos MP (1999a) Methylglyoxal in living organisms/chemistry, biochemistry, toxicology and clinical implications. Toxicol Lett 110:145–175
Kalapos MP (1999b) A possible evolutionary role of formaldehyde. Exp Mol Med 31:1–4
Kalapos MP (2002) A theoretical approach to the link between oxido-reductions and pyrite formation in the early stage of evolution. Biochim Biophys Acta—Bioenergetics 1553:218–222
Kalapos MP (2008) The tandem of free radicals and methylglyoxal. Chem Biol Interact 171:251–271
Kalapos MP, Littauer A, de Groot H (1993) Has reactive oxygen a role in methylglyoxal toxicity? A study on cultured rat hepatocytes. Arch Toxicol 67:369–372
Kitadai N, Maruyama S (2018) Origins of building blocks of life: a review. Geosci Front 9:1117–1153
Kleczkowski LA, Randall DD (1988) Purification and characterization of a novel NADPH(NADH)-dependent hydroxypyruvate reductase from spinach leaves. Biochem J 250:145–152
Kohn LD (1968) Tartaric acid metabolism. VIII. Crystallise tartronic semialdehyde reductase. J Biol Chem 242:4426–4433
Kornberg HL, Gotto AM (1966) Tartronic semialdehyde reductase (crystalline). Methods Enzymol 9:240–247
Kulkarni RA, Worth AY, Zengeya TT, Shrimp JH, Garlick JM, Roberts AM, Momtgomery DC, Sourbier C, Gibbs BK, Mesaros C, Tsai YC, Das S, Chan KC, Zhou M, Andresson T, Weissman AM, Martson Linehan W, Blair IA, Snyder NW, Meier JL (2017) Discovering targets of non-enzymic acylation by thioester reactivity profiling. Cell Chem Biol 24:231–242
Landau BR, Brunengraber H (1987) The role of acetone in the conversion of fat to carbohydrate. Trends Biochem Sci 12:113–114
Leal SS, Gomes CM (2010) Iron-sulfur clusters, protein folds, and ferredoxin stability. In: Gomes CM, Stafshede PW (eds) Protein folding and metal ions: mechanisms, biology and disease, 1st edn. CRC Press, Boca Raton, pp 81–96
Leskovac V, Pavkov-Peričin D (1975) Evidence for a histidine and a cysteine residue in the substrate-binding site of yeast alcohol dehydrogenase. Biochem J 145:581–590
Lipmann F (1965) Projecting backward from the present stage of evolution of biosynthesis. In: Fox WS (ed) The origins of prebiological systems and of their molecular matrices. Academic Press, New York, pp 259–280
Liu Y, Koh CMJ, Sun L, Ji L (2011) Tartronate semialdehyde reductase defines a novel rate-limiting step in assimilation and bioconversion of glycerol in Ustilago maydis. PLoS ONE 6:e16438
Maiocco SJ, Arcinas AJ, Booker SJ, Elliott SJ (2019) Parsing redox potentials of five ferrodoxins found within Thermogata maritima. Protein Sci 28:257–266
Mannervik B, Ridderström M (1993) Catalytic and molecular properties of glyoxalase I. Biochem Soc Trans 21:513–517
Marsden SR, Mestrom L, McMillan DGG, Hanefeld U (2020) Thermodynamically and kinetically controlled reactions in biocatalysis—from concepts to perspectives. ChemCatChem 12:426–437
Martin WF (2011) Hydrogen, metals, bifurcating electrons, and proton gradients: the early evolution of biological energy conservation. FEBS Lett 586:485–493
Martin WF, Baross J, Kelley D, Russell MJ (2008) Hydrothermal vents and the origin of life. Nat Rev Microbiol 6:805–814
Matsui R, Ferran B, Oh A, Croteau D, Shao D, Han J-g, Pimentel DR, Bachschmid MM (2020) Redox regulation via glutaredoxin-1 and protein S-glutathionylation. Antioxid Red Signal 32:677–700
Mavrovouniotis ML (1990) Group contributions for estimating standard Gibbs energies of formation of biochemical compounds in aqueous solution. Biotechnol Bioeng 36:1070–1082 [Errata in Biotechnol Bioeng 38:803, 1991]
Mavrovouniotis ML (1991) Estimation of standard Gibbs energy changes of biotransformations. J Biol Chem 266:14440–14445
Merino N, Aronson HS, Bojanova DP, Feyhl-Nuska J, Wong ML, Zhang S, Giovanelli D (2019) Living at the extremes: extremophiles and the limits of life in a planetary context. Front Microbiol 10:780
Miller SL (1987) Which organic compounds could have occurred on the prebiotic earth? Cold Spring Harb Symp Quant Biol 52:17–26
Morowitz HJ (1966) Physical background of cycles in biological systems. J Theor Biol 13:60–62
Muchowska KB, Chevallot-Beroux E, Moran J (2019) Recreating ancient metabolic pathways before enzymes. Bioorg Med Chem 27:2292–2297
Muchowska KB, Varma SJ, Moran J (2020) Nonenzymatic metabolic reactions and life’s origin. Chem Rev 120:7708–7744
Noor E, Bar-Even A, Flamholz A, Reznik E, Liebermeister W, Milo R (2014) Pathway thermodynamics highlights kinetic obstacles in central metabolism. PLOS Comput Biol 10:1003483
Olson KR, Straub KD (2016) The role of hydrogen sulfide in the evolution and in the evolution of sulfide in metabolism and signaling. Physiology 31:60–72
Oparin AJ (1960) Az élet keletkezése a földön. [The origin of the life on the Earth] Gondolat, Budapest (in Hungarian)
Park JO, Rubin SA, Xu Y-F, Amador-Noguez D, Fan J, Shiomi T, Rabinowitz JD (2016) Metabolite concentrations, fluxes, and free energies imply efficient enzyme usage. Nature Chem Biol 12:482–489
Parker ET, Cleaves HJ, Callahan MP, Dworkin JP, Glavin DP, Lazcano A, Bada JL (2011) Prebiotic synthesis of methionine and other sulfur-containing organic compounds on the primitive Earth: A contemporary reassessment based on an unpublished 1958 Stanley Miller experiment. Orig Life Evol Biosph 41:201–212
Paulsen CE, Carroll KS (2013) Cysteine-mediated redox signaling: chemistry, biology, and tools for discovery. Chem Rev 113:4633–4679
Pavlovskaya TA, Telegina TA (1974) Photochemical conversions of lower aldehydes in aqueous solutions and in the fog. In: Oró J, Miller SL, Ponnamperuma C, Young RS (eds) Cosmochemical evolution and the origins of lied. Springer, Dordrecht, pp 303–309
Pettinati I, Brem J, Lee SY, McHugh PJ, Schofield CJ (2016) The chemical biology of human metallo-β-lactamase fold proteins. Trends Biochem Sci 41:338–355
Planavsky NJ, Rouxel OJ, Bekker A, Lalonde SV, Konhauser KO, Reinhard CT, Lyons TW (2010) The evolution of the marine phosphate reservoir. Nature 467:1088–1090
Poole LB (2015) The basics of thiols and cysteins in redox biology and chemistry. Free Rad Biol Med 80:148–157
Potter S, Fotherhill-Gilmore LA (1993) Molecular evolution: the origin of glycolysis. Biochem Educ 21:45–48
Quayle JR, Ferenczi T (1978) Evolutionary aspects of autotrophy. Microbiol Rev 42:251–273
Racker E (1965) Mechanism of bioenergetics. Academic Press, New York, pp 6–7
Radzisewski B (1882) Ueber glyoxalin und seine homologe. Ber Dsch Chem Ges 15:2706–2708
Ralser M (2018) An appeal to magic? The discovery of a non-enzymatic metabolism and its role in the origins of life. Biochem J 475:2577–2592
Richter F, Blomberg R, Khare SD, Kiss G, Kutzin AP, Smith AJT, Gallaher J, Pianowski Z, Helgeson RC, Grjasnow A, Xiao R, Seetharaman J, Su M, Vorobiev S, Lew S, Forouhar F, Kornhaber GJ, Hunt JF, Montelione GT, Tong L, Houk KN, Hilvert D, Baker D (2012) Computational design of catalytic dyads and oxyanion holes for ester hydrolysis. J Am Chem Soc 134:16197–16206
Ridderström M, Mannervik B (1996) Optimized heterologous expression of human zinc enzyme glyoxalase I. Biochem J 314:463–467
Rumsby G, Cregeen DP (1999) Identification and expression of a cDNA for human hydroxypyruvate/glyoxylate reductase. Biochim Biophys Acta 1446:383–388
Russell MJ, Hall AJ (2002) From geochemistry to biochemistry/chemiosmotic coupling and transition element clusters in the onset of life and photosynthesis. Geochem News 113:6–12
Sagan C, Khare BN (1971) Long-wavelength ultraviolet photoproduction of amino acids on the primitive earth. Science (Washington DC) 173:417–420
Schoonen MA, Xu Y, Bebie J (1999) Energetics and kinetics of the prebiotic synthesis of simple organic acids and amino acids with the FeS-H2S/FeS2 redox couple as reductant. Orig Life Evol Biosph 29:5–32
Shalayel I, Youssef-Saliba S, Vazart F, Ceccarelli C, Bridoux M, Vallée Y (2020) Cysteine chemistry in connection with abiogenesis. Eur J Org Chem 2020:3019–3023
Shimizu M (2007) Histidine and cysteine can enhance the metabolic reaction rates in the TCA cycle and some autotrophic processes: genetic code world. Viva Orig 35:73–81
Shock EL, Schulte MD (1998) Organic synthesis during fluid mixing in hydrothermal systems. J Geophys Res 103:28513–28527
Siebert D, Wendisch VF (2015) Metabolic pathway engineering for production of 1,2-propanediol and 1-propnol by Corynebacterium glutamicum. Biotechnol Biofuels 8:91
Sophos NA, Vasiliou V (2003) Aldehyde dehydrogenase gene superfamily: the 2002 update. Chem Biol Interact 143–144:5–22
Sousa FL, Martin WF (2014) Biochemical fossils of the ancient transition from geoenergetics to bioenergetics in prokaryotic one carbon compound metabolism. Biochim Biophys Acta - Bioenerget 1837:964–981
Stafford HA (1956) Dehydrogenase activity of hydroxymalonate and related acids in higher plants. Plant Physiol 31:135–141
Szent-Györgyi A (1967) Charge transfer and electronic mobility. Proc Natl Acad Sci USA 58:2012–2014
Szent-Györgyi A (1968) Bioelectronics. Academic Press, Cambridge
Talesa V, Uotila L, Koivusalo M, Principato G, Giovannini E, Rosi G (1988) Demonstration of glyoxalase II in rat liver mitochondria. partial purification and occurrence in multiple forms. Biochim Biophys Acta 955:103–110
Tarasov VG, Gebruk AV, Mironov AN, Moskalev LI (2005) Deep-sea and swallow water hydrothermal vent communities: two different phenomena? Chem Geol 224:5–39
Tashiro T, Ishida A, Hori M, Igisu M, Koike M, Méjean P, Takahata N, Sano Y, Komiya T (2017) Early trace of life from 3.95 Ga sedimentary rocks in Labrador, Canada. Nature 549:516–518
Thauer RK (1988) Citric-acid cycle, 50 years on. Modification and an alternative pathway in anaerobic bacteria. Eur J Biochem 176:497–508
Thauer RK, Jungerman K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Thornalley PJ (1990) The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J 269:1–11
Thornalley PJ (2008) Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems—role in ageing and disease. Drug Metabol Drug Interact 23:125–150
Thornalley PJ, Stern A (1985) Red blood cell oxidative metabolism induced by hydroxypyruvaldehyde. Biochem Pharmacol 34:1157–1164
Timm S, Florian A, Jahnke K, Nunes-Nesi A, Fernie AR, Bauwe H (2011) The hydroxypyruvate-reducing system in Arabidopsis: multiple enzymes for the same end. Plant Physiol 155:694–705
Uotila L, Koivusalo M (1975) Purification and properties of glyoxalase I from sheep liver. Eur J Biochem 52:493–503
Vander Jagt DL (1989) The glyoxalase system. In: Dolphin D, Poulson R, Avramovic O (eds) Glutathione: chemical, biochemical and medical aspests—part A. Wiley, Hoboken, pp 597–641
Vander Jagt DL (1993) Glyoxalase II. Molecular characteristics, kinetics and mechanism. Biochem Soc Trans 21:522–526
Vander Jagt DL, Davison LM (1977) Purification and characterization of 2-oxoaldehyde dehydrogenase from rat liver. Biochim Biophys Acta 484:260–267
Vander Jagt DL, Hassenbrook RK, Hunsaker LA, Brown WM, Royer RE (2001) Metabolism of the 2-oxoaldehyde methylglyoxal by aldose reductase and by glyoxalase-I: roles for glutathione in both enzymes and implications for diabetic complications. Chem Biol Interact 130–132:549–562
Vasas V, Szathmáry E, Santos M (2010) Lack of evolvability in self-sustaining autocatalytic networks constraints metabolism first scenarios for the origin of life. Proc Natl Acad Sci USA 107:1470–1475
Vasas V, Fernando C, Santos M, Kauffman S, Szathmáry E (2012) Evolution before genes. Biol Direct 7:1
Vistoli G, De Maddis D, Cipak A, Zarkovic N, Carini M, Aldini G (2013) Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview on their mechanisms of formation. Free Radic Res 47(suppl1):3–27
Wächtershäuser G (1988a) Pyrite formation, the first energy source for life: a hypothesis. Syst Appl Microbiol 10:207–210
Wächtershäuser G (1988b) Before enzymes and templates: theory of surface metabolism. Microbiol Rev 52:452–484
Wächtershäuser G (1990) Evolution of the first metabolic cycles. Proc Natl Acad Sci USA 87:200–204
Wächtershäuser G (1992) Ground works for an evolutionary biochemistry: the iron-sulphur world. Prog Biophys Mol Biol 58:85–201
Weber AL (1981a) Formation of thioester, N, S-diacetylcysteine, from acetaldehyde and N, N’diacetylcystine in aqueous solution with ultraviolet light. J Mol Evol 17:103–107
Weber AL (1981b) Formation of pyrophosphate, tripolyphosphate, and phosphoylimidazole with the thioester, N, S-diacetylcysteamine, as the condensing agent. J Mol Evol 18:24–29
Weber AL (1982a) Formation of the thioester, N-acetyl, S-lactoylcysteine, by reaction of N-acetylcysteine with pyruvaldehyde in aqueous solution. J Mol Evol 18:354–359
Weber AL (1982b) Formation of pyrophosphate on hydroxyapatite with thioesters as condensing agent. BioSystems 185:183–189
Weiss MC, Preiner M, Xavier JC, Zimorski V, Martin WF (2018) The last universal common ancestor between ancient Earth chemistry and the onset of genetics. PLoS Genet 14:e1007518
Wetzels S, Wouters K, Schalkwijk CG, Vanmierlo T, Hendriks JJA (2017) Methylglyoxal-derived advanced glycation endproducts in multiple sclerosis. Int J Mol Sci 18:421
Wittmann I, Mazák I, Pótó L, Wagner Z, Wagner L, Vas T, Kovács T, Belágyi J, Nagy J (2001) Role of iron in the interaction or red blood cells with methylglyoxal. Modification of L-arginine by methylglyoxal is catalyzed by iron redox cycling. Chem Biol Interact 138:171–187
Woese C (1998) The universal ancestor. Proc Natl Acad Sci USA 95:6854–6859
Yannai S (ed) (2008) Dictionary of food compounds: [additives, flavors, and ingredients]. Chapman & Hall/CRC, Boca Raton
Yčas M (1955) A note on the origin of life. Proc Natl Acad Sci USA 41:714–716
Yim HS, Kang SO, Hah YC, Chock PB, Yim MB (1995) Free radicals generated during the glycation reaction of amino acids by methylglyoxal. J Biol Chem 270:28228–28233
Yim MB, Kang SA, Chock PB (2000) Enzyme-like activity of glycated cross-linked proteins in free radical generation. Ann N Y Acad Sci 899:168–181
Zhou I, Xu X, Wang I, Gong H, Yu B, Ma Y (2015) NADP+-preferring D-lactate dehydrogenase from Sporolactobacillus inulinus. Appl Environ Microbiol 81:6294–6301
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, and not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
There is only one author.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Handling editor: Michelle Meyer.
Appendices
Appendix 1
Like in the chemosynthetic organisms, the oxido-reductions are slow to equilibrate on their own (Amend and Shock 2001). Therefore, the Gibbs free energy for the real reaction has to be calculated from the following expression:
For the Eq. (27), the Qr is the activity (ai) of compounds and can be deduced according to Eq. (28)
where n and υ represent the number of participating compounds and the stoichometric reaction coefficient in the given reaction, respectively. And the ΔGo,r is the standard partial molal Gibbs free energy of reaction, and it reads
where T is the temperature measured on the absolute scale, and R is the gas constant.
From Eqs. (27) and (29), it follows that at equilibrium Q = K (ΔGr = 0), and if Q < K (ΔGr < 0), then the reaction proceeds spontaneously.
It should, however, be kept in mind that the evolutionary systems are open systems, capable of exchanging matter with their surroundings and the concentrations of reactants affect reaction rates.
Appendix 2
The basic laws of thermodynamics combine energy functions and so-called intensive properties. As for the Gibbs potential, it is also the function of these properties
where T is the temperature measured on the absolute scale, p is the pressure, and n is the amount of material. Chemical equilibrium can, in general, be expressed by the extreme values of these potential functions. In the perhaps most biologically relevant case (constant T and p), the criterion for the equilibrium is the minimum for the Eq. (30), and the equation of the condition is
in which the μi is the chemical potential for the compound i and the partial derivative of the energy function (U) according to material quantities as seen in Eq. (32)
S and V denote entropy and volume, respectively. Although the relationships are obvious, the question is what temperature, pressure, and other conditions (e.g., ionic strength, pH, radiation) can be expected to have operated under prebiological conditions. In the extant ecosystem, even though a wide range for environmental parameters is known, their limits exist (Merino et al. 2019).
Appendix 3
The original Arrhenius equation describes a simple relationship and reads
in which k is the reaction rate constant, T represents the temperature measured in Kelvin, R is the gas constant, Ea is the minimum energy needed for the reaction to occur, and A is the pre-exponential factor that is considered temperature dependent. As seen, k goes up at higher temperatures regardless of kinetically or thermodynamically favored products are considered (Marsden et al. 2020).
Reactions essentially are on a continuum between entirely kinetic and entirely thermodynamic controls. If a reaction is under thermodynamic control at a given temperature, it remains under thermodynamic control at any higher temperature. In contrast, when a reaction is under kinetic control at a given temperature, it remains under kinetic control at any lower temperature. For sufficiently long time scale, every reaction approaches, at least in principle, thermodynamic control. As suggested by de Duve, the requisite time for reactions was from centuries to millennia, perhaps tens of millennia or even more, but certainly not tens or hundreds of millions of years (De Duve 2003), thus, tilting the balance in the direction of thermodynamic control.
Rights and permissions
About this article
Cite this article
Kalapos, M.P. Evolutionary Aspects of the Oxido-Reductive Network of Methylglyoxal. J Mol Evol 89, 618–638 (2021). https://doi.org/10.1007/s00239-021-10031-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-021-10031-3