Abstract
During the past 50 years, the molecular clock has become one of the main tools for providing a time scale for the history of life. In the era of robust molecular evolutionary analysis, clock calibration is still one of the most basic steps needing attention. When fossil records are limited, well-dated geological events are the main resource for calibration. However, biogeographic calibrations have often been used in a simplistic manner, for example assuming simultaneous vicariant divergence of multiple sister lineages. Here, we propose a novel iterative calibration approach to define the most appropriate calibration date by seeking congruence between the dates assigned to multiple allopatric divergences and the geological history. Exploring patterns of molecular divergence in 16 trans-Bering sister clades of echinoderms, we demonstrate that the iterative calibration is predominantly advantageous when using complex geological or climatological events—such as the opening/reclosure of the Bering Strait—providing a powerful tool for clock dating that can be applied to other biogeographic calibration systems and further taxa. Using Bayesian analysis, we observed that evolutionary rate variability in the COI-5P gene is generally distributed in a clock-like fashion for Northern echinoderms. The results reveal a large range of genetic divergences, consistent with multiple pulses of trans-Bering migrations. A resulting rate of 2.8% pairwise Kimura-2-parameter sequence divergence per million years is suggested for the COI-5P gene in Northern echinoderms. Given that molecular rates may vary across latitudes and taxa, this study provides a new context for dating the evolutionary history of Arctic marine life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The molecular clock is an important and valuable tool in evolutionary biology, although it has remained controversial since it was first proposed 50 years ago (Zuckerkandl and Pauling 1965). In addition to concerns due to rate heterogeneity across taxa (e.g. Martin et al. 1992; Martin and Palumbi 1993; Mooers and Harvey 1994; Bleiweiss 1998a; Bromham 2002), calibrating the clock is also a challenge, especially when fossil evidence is poor or absent. For taxa without a fossil record, geological or climatological events represent the only calibration points to estimate rates of molecular divergence and to provide a time scale for the history of life. Yet, biogeographic calibrations are frequently used with simplistic methods and without considering the complexity of some geological events, as discussed below.
Well-dated geological events, such as island formation (e.g. Fleischer et al. 1998; Weir and Schluter 2008), paleotectonics (Landis 2016), or vicariance events—for example the closure of the Tethys Sea or the formation of the Isthmus of Panama (e.g. Held 2001; Williams and Reid 2004)—have been extensively used for clock calibrations. For tropical marine taxa, the most-used event has been the formation of the Isthmus of Panama (e.g. Knowlton and Weigt 1998; Lessios 2008; Hurt et al. 2009), with evolutionary rates calibrated using genetic divergences between sister species separated by the closure of the Isthmus, which are commonly known as “geminate” species (Jordan 1908).
Molecular clocks for Northern marine taxa have been largely calibrated using fossils associated with the major trans-Arctic interchange 3.5 million years ago (Ma) (e.g. Palumbi and Kessing 1991; Collins et al. 1996b; Kober and Bernardi 2013). In addition, for Arctic taxa lacking a good fossil record, divergence times have been estimated using calibrations generated using tropical taxa (e.g. Luttikhuizen et al. 2003; Boissin et al. 2011). However, this approach has limitations. First, the formation of the Isthmus was a slow process (Coates and Obando 1996; Bacon et al. 2015; O’Dea et al. 2016), making divergence dating problematic. Additionally, the widely used calibration from Panama ultimately relied on a single data point (Knowlton and Weigt 1998) and thus did not address possible rate heterogeneity among lineages. Moreover, using clock calibrations from other geographical regions could be problematic, especially as there is evidence that molecular evolutionary rates vary systematically in association with the environment (e.g. Bleiweiss 1998b; Gillooly et al. 2005; Wright et al. 2006). Therefore, further research is needed to investigate appropriate calibrations for Northern lineages.
Using the Bering Strait for Clock Calibration in Northern Marine Taxa
The geological history of the Bering Strait (Fig. 1) provides a unique, though complex, opportunity for calibrating molecular divergence rates for Northern taxa. North Pacific and Arctic-Atlantic marine faunas evolved separately since the middle Cretaceous (80–100 Ma) (Dunton 1992; Marincovich 1993) until the first opening of the Bering Strait. This opportunity for dispersal into a novel marine biogeographic region, followed by divergence in allopatry, is the first of a series of events that provide a strong opportunity for molecular clock calibration for Northern marine organisms.
Geological history and map of the Bering Strait (modified from Marincovich 2000; Gladenkov and Gladenkov 2004). a Shows the chronostratigraphic scale (Ogg et al. 2016) in relation to eustatic sea-level changes (Haq et al. 1987) and major Bering Strait geological events during the last 10 MY in the Northern Hemisphere; the black dot represents the first opening of the Bering Strait at the end of the Miocene as inferred from diatom and bivalve fossils (Gladenkov et al. 2002; Gladenkov and Gladenkov 2004). b Map showing the Bering Strait
Early fossil evidence of diatoms and of the mollusc Astarte suggest the first opening of the Bering Strait occurred between the Late Miocene and Early Pliocene approximately 4.8–7.4 Ma (Marincovich and Gladenkov 1999). However, with more recent fossil evidence of both diatom and bivalve fossils, the time of the first opening has been narrowed to 5.4–5.5 Ma, near the end of the Miocene (Gladenkov et al. 2002; Gladenkov and Gladenkov 2004). However, according to diatom fossils from Northern Eurasia, temporary connections between the Pacific and Arctic Oceans might have existed across Eastern Siberia since the Early Miocene; thus, temporary openings of the Strait have been proposed to have occurred since approximately 17 Ma (Polyakova 2001). However, the inference of an open Strait since the Early Miocene is not fully accepted in the literature; instead, it has been strongly questioned using the argument that it is based on weak evidence and poorly preserved diatom fossils (see details in Marincovich and Gladenkov 2001; Gladenkov and Gladenkov 2004), and thus should be considered only with great caution (Marincovich and Gladenkov 2001; Gladenkov and Gladenkov 2004). It is currently well accepted that the Bering Strait first opened 5.4–5.5 Ma (Gladenkov et al. 2002; Gladenkov and Gladenkov 2004).
The first opening of the Bering Strait allowed marine migrations between the Pacific and Arctic-Atlantic Oceans and is one of the most important oceanographic (Shaffer and Bendtsen 1994; Hu et al. 2007) and biogeographical events shaping the Northern marine biota (Durham and MacNeil 1967; Vermeij 1991). While the Strait opened 5.4–5.5 Ma (Gladenkov et al. 2002; Gladenkov and Gladenkov 2004), the major fossil evidence of the main trans-Arctic marine migration is dated at 3.5 Ma (Briggs 1970; Vermeij 1989, 1991; Marincovich 2000; Dodson et al. 2007). The major trans-Arctic marine migration might have occurred in response to a major change in ocean circulation in the Northern Hemisphere associated with the formation of the Isthmus of Panama (Marincovich 2000; Matthiessen et al. 2009). Shortly after, approximately 3.4 Ma, a cooling transition period began in the Arctic (Shackleton and Opdyke 1977; Herman and Hopkins 1980; Horikawa et al. 2015), culminating with extensive glaciation of the Northern hemisphere from 3 to 2.4 Ma (Einarsson et al. 1967; Schrader et al. 1976; Herman and Hopkins 1980; Maslin et al. 1996; Haug et al. 1999; Harris 2005; Horikawa et al. 2015). Glacial formations and the associated drop in sea level, of at least 50 m, triggered the closure of the Bering Strait (Hopkins 1967), resulting in the re-isolation of the Pacific and Arctic-Atlantic marine biotas.
Glacial events were more frequent during the Quaternary period (Dunton 1992; Maslin et al. 1996; Haug et al. 1999), with ca. nine successive glacial–interglacial cycles (Einarsson et al. 1967; Maslin et al. 1996; Haug et al. 1999; Harris 2005) causing the Bering Strait to open and close on several occasions. During interglacial periods, the sea level rose at least 20 m and as much as 100 m, suggesting an open Strait (Hopkins 1967). During glacial intervals, the reduction in sea level would have resulted in a continuous land connection between Alaska and Siberia, commonly known as the Bering Land Bridge (Hopkins 1967). Glacial–interglacial cycles during the Quaternary strongly influenced sea levels, habitat conditions, and the current distribution of Northern marine organisms. The most recent opening of the Bering Strait is dated to 15,000 years ago (Dodson et al. 2007; Hardy et al. 2011), allowing the contemporary exchange between Pacific and Arctic-Atlantic marine biotas, particularly among species that can tolerate the polar thermal conditions.
The Bering Strait has been previously used for clock calibration in sea stars (Leptasterias) (Foltz et al. 2008) and polychaetes (Carr 2010). In polychaetes, divergence times for the barcode region (Hebert et al. 2003a) of the mitochondrial cytochrome c oxidase subunit I (COI-5P) gene were estimated in 20 trans-Arctic species pairs using Kimura-2-parameter (K2P; Kimura 1980) distances and assuming that the first wave of dispersal across the Bering Strait took place 3.5 Ma during the major marine migrations, resulting in a rate estimate of 4.4% pairwise sequence divergence per million years (MY). The results also suggested that sister lineages did not diverge simultaneously, but rather exhibited multiple migration episodes (Carr 2010). In sea stars, a rate was calibrated for a nuclear gene region (Elongation factor 1α subunit intron 4) and two mitochondrial regions (control region and COI-5P); a single trans-Arctic sister pair was used for calibration assuming divergence at 3.5 Ma, resulting in a rate estimate of 1.8% pairwise divergence per MY for COI-5P in Leptasterias (Foltz et al. 2008).
Here, we argue that the key to using the Bering Strait for calibration is to consider multiple potential episodes of migration followed by divergence rather than enforcing one single calibration point, which requires the tenuous assumption that all species migrated at the same time during the major trans-Arctic marine interchange. In this study, we propose a novel iterative calibration approach for clock dating and demonstrate its advantage when using complex geological events, such as the glacial history of the Bering Strait. We also explore patterns of molecular divergence in the COI-5P barcode region of trans-Bering sister clades of echinoderms inhabiting the North Pacific vs. Arctic-Atlantic ocean regions and generate a new molecular rate estimate for Northern echinoderms.
Methods
We used publicly available DNA sequences of the COI-5P barcode region (Hebert et al. 2003b) from echinoderms, a taxonomic group that has been extensively used in tropical marine calibration studies (e.g. Lessios 1979, 2008; Hart et al. 1997; McCartney et al. 2000; Coppard et al. 2013). We explored the evolutionary rate of the COI-5P gene and focused explicitly on finding proper calibration dates. It is beyond the scope of this study to reconstruct phylogenies as species tree hypotheses. Despite the limitations of using only one gene, we suggest that COI represents the best choice across mitochondrial genes to answer the research questions based on several factors, including its greater range of phylogenetic signal, compared with other mitochondrial genes (Hebert et al. 2003a). Also, this marker was favoured due to its patterns of divergence within and between species, rapid enough to allow the discrimination of phylogeographic groups (e.g. Wares and Cunningham 2001; Hebert et al. 2003b; Bastrop and Blank 2006; Bleidorn et al. 2006; Radulovici et al. 2010). In addition, introgression events are rarely reported in echinoderms (Lessios and Pearse 1996; Harper and Hart 2007; Harper et al. 2007; Addison and Pogson 2009) and are not known to occur in our studied taxa. Thus, introgression might not be a concern when using the COI gene in this study. Moreover, the extensive and fast-growing availability of public sequences and usage of this standardized marker across animal groups provide the ability to explore substitution patterns in the same genomic region for an extensive range of taxonomic groups, which represents a great opportunity for large-scale and cross-taxon research such as this study.
Finding Potential Trans-Bering Sister Clades and Data Collection
Using the phenogram-building tool available in the Barcode of Life Data Systems v3 (BOLD; http://www.boldsystems.org/; last accessed July 2015) (Ratnasingham and Hebert 2007), preliminary neighbour-joining (NJ) trees (Saitou and Nei 1987) were constructed for each of the five classes within the phylum Echinodermata using K2P distances and publicly accessible data, excluding sequences with stop codons, those flagged within BOLD as containing possible misidentifications or contaminants, and sequences shorter than 200 base pairs (bp). The trees were colour coded by Barcode Index Number (BIN) (Ratnasingham and Hebert 2013), and information about Country/Province of collection and sequence length were included in the tip label. BINs were used as taxonomic units since it has been demonstrated that barcodes perform well to discriminate and delineate echinoderm species (e.g. Ward et al. 2008; Bribiesca-Contreras et al. 2013; Layton et al. 2016). We pinpointed potential trans-Bering sister clades, i.e. allopatric groups having one BIN distributed only in the North Pacific and the closest related BIN distributed in the Arctic-Atlantic. Allopatric sister clades where one or both sisters consisted of multiple BINs were also considered. If one of the potential sister taxa was not yet identified to genus level, we still considered this as a target taxon to be included for further analysis. On the other hand, we also considered allopatric genetic clusters within a single Linnaean species name as target taxa. Distribution of the potential trans-Bering clades was verified by searching for the species name, when available, in the Encyclopedia of Life (EOL; http://www.eol.org last accessed August 2015) and the World Register of Marine Species (WoRMS; http://www.marinespecies.org last accessed August 2015). Trans-Bering pairs of echinoderms will be referred to as sister clades. The NJ trees for each class were used as preliminary trees to select the target genera for sequence data retrieval and further analysis, and to select the outgroup for each of the data sets based upon the next closest related lineage to each genus containing candidate sister clades.
Trans-Bering Sister Clades Confirmation Through Maximum Likelihood Phylogenetic Analysis
When possible, previously published molecular phylogenies from the taxa of interest were used to confirm or refute sister clade relationships (Arndt et al. 1996; Lee 2003; Foltz et al. 2008; Mah and Foltz 2011; Kober and Bernardi 2013), giving preference to mitochondrial trees as we are most interested in accurate gene trees rather than species trees for calibration purposes (Mendes and Hahn 2016). In the absence of published multi-gene trees, phylogenetic relationships were then reconstructed using a maximum likelihood (ML) tree for each genus containing a potential pair.
From April 18th to July 22nd, 2015, publicly available COI sequences from each genus containing potential trans-Bering sister clades were retrieved from BOLD v3, including all sequences within BINs that fell in the candidate sister clades regardless of the presence of taxonomic identifications, but excluding sequences with stop codons, those flagged as containing possible misidentifications, and sequences shorter than 200 bp. We then created genus-level datasets to be used in the following analysis. Each genus dataset, including its assigned outgroup sequence, belonging to a different genus, was aligned in MEGA 6 (Tamura et al. 2013); then the alignment was visually checked for gaps and translated to amino acids to verify the reading frame and absence of stop codons. Even though the COI region used for DNA barcoding is standardized, COI sequences retrieved for echinoderms did not overlap completely due to different sequence lengths and primers used among source studies. As a result, each genus dataset was trimmed to a different alignment length (615–923 bp) (Supplementary Material 1.1). After inspecting each alignment, we eliminated problematic sequences when > 1% of unknown bases were present, when potential misidentification cases were noticeable on the NJ tree, or when a base-calling error was suspected and trace files were not available (Supplementary Material 1.2). In addition, identical sequences were eliminated using the tool Duplicate Finder (http://bioinfotutlets.blogspot.ca/2009/09/duplicates-finder-java-standalone.html; last accessed September 2015). For each genus dataset, the best-fit molecular evolutionary substitution model was obtained according to the Bayesian information criterion (BIC) using MEGA 6. Phylogenetic relationships were then reconstructed in MEGA 6 using ML with 1000 bootstrap replicates. Trans-Bering sister clade relationships were supported for 16 pairs, displaying reciprocal monophyly on both the NJ and ML trees. BOLD process IDs and GenBank accession numbers for trans-Bering sister clades sequences can be found in Supplementary Material 1.3.
Rate Constancy Test
A Tajima’s relative rate test (Tajima 1993) was used to test for rate constancy within each of the 16 sister clades using MEGA 6. This test involved comparing relative rates between sequences of sister taxa using a third taxon as a reference point or outgroup. We selected the longest sequence per species; when multiple sequences with the same length were available, we randomly selected one sequence per species for the comparisons. The outgroups were designated based on the ML trees, selecting the closest related taxon to the sister clade of interest (Robinson et al. 1998).
A separate relative rate test was performed for each sister clade. The relative test was repeated for the two sister clades with poor bootstrap support (< 70%) using a more distantly related outgroup to ensure that the outgroup is phylogenetically outside the test ingroup (further details in Supplementary Material 1.4).
Kimura 2-Parameter Distances Between Trans-Bering Sister Clades
Using the full available sequence length for each genus-level dataset, average K2P genetic distances between each trans-Bering sister clade were calculated in MEGA 6, with pairwise deletion of missing nucleotides. In addition to enabling comparison with prior studies that used K2P distances (e.g. Lessios 2008; Carr 2010; Miura et al. 2010; Carr et al. 2011), a simple distance metric is expected to perform well at estimating evolutionary distances for shallow divergences (Hebert et al. 2003a). However, a drawback of this approach is that it would overlook rate variability across the phylogeny, and taxa with anomalous evolutionary rates may have undue influence upon the final calibration. Another drawback is that evolutionary divergences for deeply divergent sister pairs might be underestimated due to saturation. To address these limitations, we have complemented the use of K2P distances with a whole-tree approach for clock calibration (see below).
For clock calibration, we first mimic a traditional and simplistic practice where an average divergence of sister species pairs is calibrated using the most accepted single date for the vicariant event of interest (e.g. Lessios 2008; Carr 2010; Hoareau et al. 2013). For the traditional clock calibration, only strict sister species pairs were used, and we calculated an average of divergences from the cluster of points with the most similar distances, which was visually selected. Using the average sequence divergence from the cluster of the most similar distances consisting of four sister species pairs, the divergence rate (r) was estimated as r = D/T, where D is the average percentage of K2P distance and T is the time of divergence assuming that the cluster of sister pairs with the most similar divergences migrated during the major marine trans-Arctic interchange 3.5 Ma (further details in Supplementary Material 1.5). K2P distances were also used for clock calibration using the iterative approach (see below).
Generation of Relative Node Ages Through Bayesian Analysis
Relative node ages were also estimated using an ultrametric tree generated using a Bayesian approach (Drummond et al. 2012), applying the best-fit model of sequence evolution and smoothing rate heterogeneity across the tree. For computational efficiency, only unique sequences of each member of the 16 trans-Bering sister pairs were included. The dataset including 247 sequences was aligned and trimmed to a length of 459 bp (Supplementary Material 2). The best-fit substitution model for this dataset was GTR + G + I (Generalised Time Reversible with gamma-distributed rate heterogeneity among sites and a proportion of invariant sites parameter) according to the lowest BIC score obtained in MEGA 6.
An ultrametricized Bayesian inference tree was constructed in BEAST v1.8.2 (Drummond et al. 2012) employing a Birth–Death speciation model (Gernhard 2008) tree prior and an uncorrelated relaxed lognormal clock model. The BEAUTi v1.8.2 (Drummond et al. 2012) interface was used to generate the input XML files using the analysis settings that are detailed in the Supplementary Material 3. The only trans-Bering sister pair representing the echinoderm class that is most distantly related from all others (Crinoidea) (Littlewood et al. 1997; Perseke et al. 2010; Telford et al. 2014) was used as the outgroup; the remaining 15 pairs were constrained as the ingroup. Reciprocal monophyly of the outgroup, ingroup, and each of the 16 sister clades, 15 of which represented different genera, was assumed a priori for computational efficiency. Six independent Markov chain Monte Carlo (MCMC) analyses were each run with a MCMC length of 100,000,000 generations, sampling every 10,000 with a final sample size of 10,000 trees. Adequate sampling (ESS > 100) for all parameters and convergence were assessed in Tracer v1.6 (Rambaut et al. 2014). Due to low support in deeper nodes, as indicated by the posterior probability values, and independent MCMC analysis showing slightly different tree topologies in two nodes, it was not possible to combine runs and their trees as suggested by the software authors, due to negative resulting branch lengths (Drummond et al. 2012). Within the phylum Echinodermata, relationships among classes, orders, and families remain controversial (e.g. Littlewood et al. 1997; Kamarudin et al. 2010; Perseke et al. 2010; Mah and Foltz 2011; Telford et al. 2014; Feuda and Smith 2015); thus, it was not feasible to constrain the full tree topology. Therefore, six independent runs and trees were analysed as a way to test for replicability of the calibrations; i.e. the iterative calibration approach (see below) was applied to the relative node heights resulting from each of the six runs. As the resulting rate calibrations were similar across runs, we selected the run with the highest effective sample size (ESS) values for detailed reporting of results. The final tree was visualized in FigTree v1.4.2 (Rambaut et al. 2014), and all the branch tips were collapsed by allopatric taxa for visualization purposes. Branch lengths were given as relative time, and the posterior molecular evolutionary rate was mapped onto the tree. Relative node ages were then used for clock calibration using the iterative approach (see below).
Iterative Calibration and Divergence Time Estimations
The iterative calibration approach involves a sequential process of clock calibration with the aim of finding congruence between the times assigned to allopatric divergences in relation to the geological/climatological event being used for calibration (Fig. 2); this study uses the example of the Bering Strait to demonstrate this approach. First, we applied the iterative approach for clock calibration using K2P distances and next using the relative node ages from the ultrametricized gene tree generated via Bayesian analysis.
Flowchart describing the iterative calibration approach. The iterative calibration approach is a falsifiable and consecutive process of clock calibration that involves comparing node dates with the geological time scale and any other available information, such as the fossil record, in order to reach congruence between molecular divergences and biogeographic evidence. For the purpose of this study, the oldest possible date of dispersal through the Bering Strait was assigned to the most divergent node as the starting calibration date. 1All reasonable calibrations according with the geological event of interest. 2If the iterative calibration approach results in calibration failure, alternative sources of calibration should be used (e.g. fossil calibration, different geological events). In extreme cases, clock calibration could be unsuccessful
To begin, a starting calibration date was assigned to the node that joins the most divergent pair of sister lineages (reference node), representing the most recent common ancestor preceding the dispersal event. We started with the earliest possible opening of the Bering Strait that has been suggested and well supported to date in the literature according to stratigraphic evidence and fossils from diatoms and molluscs (7 Ma; Marincovich and Gladenkov 1999) despite more recent evidence that the first opening has been narrowed to a date of 5.4–5.5 Ma (Gladenkov et al. 2002; Gladenkov and Gladenkov 2004). Subsequently, we manually assigned the time of divergence for the remaining 15 pairs in relation to the most divergent sister node. Next, we graphed and compared the divergence time estimates from all 16 trans-Bering sister clades against the geological history of the Bering Strait. Geological evidence and sea-level data available from the literature (Fig. 1) (e.g. Vermeij 1991; Dunton 1992; Haug et al. 1999; Gladenkov et al. 2002; Gladenkov and Gladenkov 2004; Harris 2005; Horikawa et al. 2015) were used to elucidate the timeframes when marine trans-Bering migrations were feasible vs. improbable. We gave special attention to the two periods of time when trans-Bering migrations were extremely unlikely: preceding the first opening of the Strait dated at 5.5 Ma and during the maximum glacial period (2.4–3 Ma). Calibration attempts that generated trans-Bering divergences within these time periods are considered highly improbable, as trans-Bering migration (followed by allopatric divergence) could not occur when the Strait was closed. Evidence from the major trans-Arctic marine interchange and the repeated glacial–interglacial cycles during the Quaternary provided additional information when looking for the most plausible calibration. For example, according to the fossil evidence from the trans-Arctic interchange, several migratory events are expected around this period of time (3.5 MY). If the genetic divergences and geological history of the Bering Strait were not concordant, then we proceeded to re-assign a new slightly younger date (stepping by 0.1 MY) to the most divergent sister clade and all other nodes proportionally; again, we compared the resulting date estimates with the geological history. The process was repeated until finding concordance between node divergences and the Bering Strait geological time scale. Using the same principles, 47 calibration dates were tested in the range between 2 and 7 Ma, using K2P distances and relative node ages from the ultrametricized gene tree independently. When two or more calibrations were concordant with the geological events, independent evidence (e.g. fossil evidence from the major trans-Arctic interchange) was used to select the best possible scenario and therefore the most probable calibration date. In cases where ties cannot be resolved, a range of calibrations should be presented rather than a single calibration date. Lastly, the K2P divergence rate was estimated using the most probable calibration date. The divergence rate (r) for the most divergent trans-Bering sister clade was estimated as r = D/T, where D is the percentage of K2P distance and T is the time of divergence according to the best calibration from the iterative calibration approach considering all pairs.
The results from the iterative calibration approach, considering relative node heights of all sister pairs, allowed the assignment of a calibration date to the most divergent sister pair using a Bayesian analysis framework. In a subsequent step, absolute divergence times of nodes as well as uncertainty in node ages were estimated using a relaxed molecular clock implemented in BEAST v1.8.2 with a MCMC length of 100,000,000 generations, sampling every 10,000 with a final sample size of 10,000 trees, and with the same parameters and tree topology constraints used to generate relative node ages (see above). However, a normal distribution was used to calibrate the divergence of Ophiopholis japonica and Ophiopholis aculeata. Two calibration scenarios were tested. The first scenario dated the divergence of Ophiopholis at 5 Ma [mean = 5, SD = 0.5], due to support for this date from the iterative calibration using relative node heights derived from the first Bayesian analysis, and in the second scenario the divergence of Ophiopholis was dated at 5.25 Ma [mean = 5.25, SD = 0.25], based upon refining the calibration date after examining the results of the first absolute dating analysis. The second chosen date also reflects the average favoured calibration date obtained through the iterative calibration applied to K2P divergences and Bayesian-derived relative node heights (see “Results”). The analysis using absolute dating, rather than relative, allows us to further inform estimates of rate heterogeneity and to present posterior intervals for both divergence times and rate estimates.
Additionally, biological traits from all trans-Bering sisters were investigated when available. Deep (> 50 m) vs. shallow distribution (≤ 50 m), habitat zone during adult stage (benthic vs. pelagic), thermal affinity (polar, temperate, or tropical), and developmental model (planktonic larvae vs. direct development) were the target of attention. Specific traits might have allowed alternative dispersal routes, and the phylogenetic events may not be truly associated with the geological history of the Bering Strait. For example, taxa inhabiting deep waters are less likely to disperse during a shallow open Strait than taxa from the intertidal, especially as the Bering Strait is approximately 50 m deep (Hopkins 1967). Likewise, taxa with a wide range of temperature tolerance are more likely to have alternative migration routes through tropical pathways (e.g. before the closure of the Isthmus of Panama) than taxa that exclusively inhabit polar waters. After integrating all information sources (e.g. biological traits), the resulting date estimates were compared to the geological history, with the traits graphically visualized. Biological information was used to aid in selecting the most probable calibration date and to break ties if more than one calibration was favourable. Information about biological traits was primarily obtained from online databases such as WoRMS and SeaLifeBase (http://www.sealifebase.org; last accessed May 4th, 2017).
Results
Trans-Bering Sister Clades
After examining the preliminary NJ COI gene trees generated in BOLD for each of the five classes of echinoderms, 16 potential trans-Bering sister clades were identified. Previous molecular phylogenies were not available in most cases; therefore, sister clade relationships were confirmed based on the genus-level ML phylogenetic trees. Fourteen of the candidate sister clades were supported with high bootstrap support (> 76%), while support for one pair (Asterias) was only 47%, although this relationship was also supported in previous studies (Mah and Foltz 2011). Support for the other sister clade (Ophiopholis) was 69%. The sixteen sister clades were phylogenetically diverse, falling within 15 genera from four classes of echinoderms: seven sister clades from class Asteroidea, four sister clades from each of Holothuroidea and Ophiuroidea, and one more pair from Crinoidea. However, trans-Bering pairs from the class Echinoidea were not detected in the available data. Thirteen trans-Bering sister clades were comprised of genetically divergent clusters and are likely to be separate species according to the BIN system (Ratnasingham and Hebert 2013). Sequence divergence between the remaining three sister clades was low (< 2% average pairwise K2P distance), and they are likely to be populations within species. Detailed information about genus-level datasets and sequence lengths can be found in Supplementary Material 1.1.
Relative Rate Test on Trans-Bering Lineages
The molecular clock hypothesis was not rejected for 15 of the 16 pairs (Supplementary Material 1.4). Although relative rates significantly differed for Pseudostichopus tuberosus and Pseudostichopus aemulatus (χ2 = 8.53; p ≤ 0.005), this pair was first included in the following analysis after accounting for rate variability. The calibration was then repeated after omitting this one pair.
Kimura 2-Parameter Distance Analysis and Clock Calibration
Mean COI K2P distances between trans-Bering sister clades ranged from 0.5 to 15.5% divergence (Fig. 3). Both small and large K2P distances were observed within multiple echinoderm classes. A cluster of four sister pairs with the most similar divergences (8.4–11.4% K2P) was observed. The remaining pairs showed a much lower or higher genetic divergence or consisted of a sister lineage made up of two or more BINs (Supplementary Material 1.5).
Mean COI K2P % divergences between 16 trans-Bering sister clades of echinoderms. The 16 trans-Bering comparisons are assigned to one of three categories: sister clades (light grey), sister pairs within BINs (dark grey), and strict sister species pairs (white). Two points with the same divergence are offset vertically. Only strict sister species pairs (white) were considered for clock calibration with the traditional methodology using the average sequence divergence of the visually determined cluster of points marked with arrows and assuming a divergence time of 3.5 Ma (see “Methods” section for further details). The triangle represents the divergence of the trans-Bering sister clade of sea stars in the genus Leptasterias. A trans-Bering sister species pair of Leptasterias was previously used in the literature (Foltz et al. 2008) for clock calibration, assuming divergence during the major trans-Arctic interchange 3.5 Ma
After considering only strict sister species pairs (nine pairs; Fig. 3, Supplementary Material 1.5) and using a traditional clock calibration approach assuming that the cluster of four similar divergences (8.4–11.4% K2P) migrated during the major marine trans-Arctic interchange 3.5 Ma, an average sequence divergence of 9.7% K2P (10% after removing Pseudostichopus) indicates an average COI divergence rate of 2.8%/MY (2.9%/MY after removing Pseudostichopus).
Additionally, given the complexity of the Bering Strait glacial history, we used the new iterative calibration approach instead of assuming simultaneous divergence during the major trans-Arctic interchange. The most divergent trans-Bering sister pair was O. aculeata and O. japonica, with 15.5% K2P divergence and representing the earliest migration event through the Bering Strait; this genetic distance was then assigned to the 47 candidate calibration dates in sequence and with divergence ages for all other pairs assigned relative to this (for complete details see Supplementary Material 1.6). After comparing the distribution of divergence ages using all calibrations against the geological events, calibrations in the range of 6.7–5.5 Ma for the reference node were concordant with the possible glacial history of the Bering Strait. However, calibrations in this range are not equally likely when considering previous independent fossil and geological evidence (Vermeij 1991; Marincovich 2000) and the fact that the date for the first opening of the Bering Strait has been narrowed to 5.4–5.5 Ma according to fossil evidence from diatoms and molluscs and stratigraphic evidence (Gladenkov et al. 2002; Gladenkov and Gladenkov 2004). After considering the best possible scenario, it was found that dating the most divergent sister clade at 5.5 Ma is likely to be the most feasible calibration (Fig. 4). Dating the most divergent trans-Bering sister clade at 5.5 Ma would lead to divergence estimations for five sister clades in the range of 3–4 Ma, consistent with the well-documented major marine migration through the Bering Strait (Briggs 1970; Vermeij 1991; Marincovich 2000; Dodson et al. 2007). In addition, stratigraphic evidence suggests that at around 5 Ma the sea-level rose by > 75 m around the Bering Strait (Haq et al. 1987; Marincovich 2000), enabling migration through the strait and supporting the best calibration date. According to the best calibration (5.5 Ma), the divergence time for the 16 trans-Bering sister clades ranged from 0.2 to 5.5 Ma (Fig. 4), while the inferred divergence rate was 2.8% pairwise K2P sequence divergence per million years for the COI barcode region in echinoderms.
Divergence time estimation of 16 trans-Bering sister clades of echinoderms using K2P divergences and the iterative calibration approach. Dots represent divergence date estimations in Ma (X axis). Each horizontal row of points represents a different calibration attempt (Y axis, right) using a different calibration date and the divergence rate generated in K2P%/MY (Y axis, left). For visualization purposes, 8 of the total 47 calibration attempts are presented in this figure including the first calibration date (7 Ma; calibration attempt ine) and the last calibration date (2 Ma; calibration attempt eight) tested (for further details see Supplementary Material 1.6). Overlapping points representing two identical sister divergences are highlighted with a thicker outline. All calibration attempts were compared with the geological history of the Bering Strait to find congruence between the genetic and geologic evidence. Shaded areas on the timescale indicate times when trans-Bering migration was extremely unlikely. Calibrations generating divergence times that fall in these regions are considered improbable, whereas calibrations where these areas contain no points are favoured. Calibration attempt 3 (star), to which a divergence time of 5.5 Ma was assigned to the most divergent clade, represents the most feasible calibration that is concordant with the geological evidence
Bayesian Results and Clock Calibration
Similar results were found across all six independent runs (Supplementary Material 1.7), one of which is presented in detail. The following results correspond to the run with the highest ESS for all the parameters. The estimated coefficient of variation (CV) across the whole tree was 0.407, indicating that the COI-5P gene evolves nearly in a clock-like fashion in Northern echinoderms. Rate heterogeneity among lineages can be determined by the CV, where values closer to zero (< 0.1) are consistent with a strict clock, larger values (> 0.1) suggest the use of a relaxed molecular clock would be appropriate, and values > 1 suggest substantial rate heterogeneity among lineages such that those data should not be used for divergence time estimations (Drummond et al. 2006; Drummond and Bouckaert 2015). The results suggest that sequences of echinoderms in this analysis typically varied by 40.7% of the absolute clock rate. Therefore, the choice to impose a relaxed clock is largely supported.
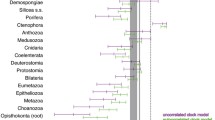
Before clock calibration, results from Bayesian analysis are only relative estimates as neither a lineage rate nor a calibration date were pre-assigned. The relative mean rate of evolution was 6.87 [95% highest posterior density intervals (HPD): 2.05–10.91]. The estimated relative rate for the 16 trans-Bering sister clade nodes ranged from 4.99 to 12.59, with the fastest branch 2.5 times faster than the slowest branch (Fig. 5). It is interesting that the second-slowest branch corresponds to P. tuberosus, while the second-fastest branch is from P. aemulatus (Fig. 5), comprising the sole sister pair for which the clock hypothesis was rejected via the relative rate test. Aside from Pseudostichopus, rate variability was distributed along the entire tree without major deviations.
Maximum credibility ultrametricized tree for the COI-5P gene in 16 trans-Bering sister clades of echinoderms generated in BEAST v1.8.2. The 16 trans-Bering sister clade nodes are marked by stars. Rates are colour mapped for each branch where the brightest red branch represents the fastest rate, while the lightest blue represents the lowest rate. The scale bar represents branch length in nucleotide substitutions per site. Tip nodes were collapsed by allopatric sister taxa. (Color figure online)
The Bayesian analysis was repeated without sequences from the sister clade with the highest rate variability (Pseudostichopus). The best substitution model describing this dataset was GTR + G + I. In this case, the relative mean rate of evolution was 6.73 (95% HPD: 1.89–10.25). The rate of the fastest branch (7.89) was now just 1.3 times faster than that of the slowest-evolving branch (5.98) (Supplementary Material 1.8). The CV was 0.185, supporting the use of a relaxed molecular clock but approaching a strict clock and indicating generally constrained rate variability across the tree.
The relative node ages were used to calibrate the molecular clock using the iterative calibration approach applied to an ultrametricized gene tree (Fig. 5), meaning that terminal nodes are aligned with each other and internal branch lengths are scaled to display the divergence among sister clades rather than among individual species (Gregory 2008). Imitating the iterative calibration using K2P distances, we used the relative node age from the most divergent trans-Bering sister clade (O. aculeata vs. O. japonica) assigned to the 47 calibration dates; relative to this node, we then assigned node dates to the remaining sister clades (Fig. 6; for complete details see Supplementary Material 1.9). After comparing the distribution of node ages using all calibrations against the geological events, a single calibration was supported, as with the K2P analysis above. Dating the most divergent trans-Bering sister clade at 5–4.7 Ma yielded the preferred calibration according to the narrowed date for the first opening of the Bering Strait and the earliest possible migration from the Arctic to the Pacific Ocean at 5.4–5.5 Ma (Gladenkov et al. 2002). According to the preferred calibration, the divergence time of trans-Bering sister clades ranged from 0.2 to 5 Ma (Fig. 6). Very similar results were found when repeating the calibration process using the relative node ages from the analysis without the Pseudostichopus pair. However, the range of dates for the best calibration is narrower (5–4.9 Ma instead of 5–4.7 Ma) (Supplementary Material 1.10).
Divergence time estimation of 16 trans-Bering sister clades of echinoderms using relative node ages from the ultrametricized gene tree generated in BEAST v1.8.2 and the iterative calibration approach. Dots represent divergence date estimations in MY (X axis). Each horizontal row of points represents a different calibration attempt (Y axis) using a different calibration date. For visualization purposes, 8 of the total 47 calibrations attempts are presented in this figure including the first calibration date (7 Ma; calibration attempt one) and the last calibration date (2 Ma; calibration attempt eight) tested (for further details see Supplementary Material 1.9). All calibrations were compared with the geological history of the Bering Strait to find congruence between the genetic and geologic evidence. Shaded areas on the timescale indicate times when trans-Bering migration was extremely unlikely. Calibrations generating node dates that fall in these regions are considered improbable, whereas calibrations where these areas contain no points are favoured. Calibration attempt 4 (star), to which a divergence time of 5 Ma was assigned to the most divergent node, represents the most feasible calibration that is concordant with the geological evidence. Divergence rates are not presented because results from Bayesian analysis are only relative estimates since neither a lineage rate nor a calibration date were pre-assigned during the Bayesian analysis (see further details in “Results”)
In addition, results from the iterative calibration approach allowed for the placement of a calibration date in a Bayesian analysis framework. Two calibration scenarios were tested. Divergence time estimations ranged from 0.14 to 4.85 Ma in scenario 1 (mean = 5, SD = 0.5), and from 0.15 to 5.23 Ma in scenario 2 (mean = 5.25, SD = 0.25). However, results from scenario 1 suggest the divergence of one trans-Bering pair at 2.9 Ma during the maximum glacial when trans-Bering migrations were extremely unlikely. As previously mentioned, calibrations generating divergence times that fall in these regions are considered improbable, whereas calibrations where these areas contain no points are favoured. Thus, scenario 2 was preferred. According with the results from scenario 2, the substitution rate estimated for the COI barcode region in echinoderms using the GTR + G + I model is 0.035 substitutions per site per MY (95% HPD: 0.018–0.055), equating to a pairwise divergence rate of 6.94%/MY (Supplementary Material 1.11).
Considering Biological Traits of the Trans-Bering Sister Clades
Biological traits from trans-Bering sister clades were not always accessible (See Supplementary Material 1.12). From those trans-Bering pairs with available information, it was noticed that the majority of pairs have planktonic larval development, and only two pairs have benthic direct development (Supplementary Material 1.12). Similarly, the majority of pairs have shallow distribution, and only three sisters are distributed in deep waters (Fig. 7 and Supplementary Material 1.12). Interestingly, the results suggest that trans-Bering pairs of echinoderms with deep distributions (> 50 m) have divergences between 3 and 5.5 Ma, placing their dispersal across the Bering Strait and subsequent allopatric divergence after the first opening of the Strait and before the subsequent closure of the Strait due to the maximum glacial (Fig. 7). Moreover, all trans-Bering sister pairs of echinoderms are benthic as adults and have affinity for temperate to polar waters (Supplementary Material 1.12).
Divergence time estimates for trans-Bering echinoderms using K2P divergences based on the best calibration and mapping depth distribution. The trans-Bering sister clades that were used for calibration and the sister clade that was omitted during the calibration process after rejecting the clock hypothesis are shown. The shaded boxes show the two periods of time when trans-Bering migrations were extremely unlikely, during the maximum glacial (2.4–3 Ma) and before the earliest possible opening of the Bering Strait at 5.5 Ma
Discussion
Strengths of the Iterative Calibration Method
An important element in molecular-dating research is the choice of calibrations. It is recognized that uncertainty in divergence estimates is mainly driven by the calibrations (e.g. Yang and Rannala 2006; Ho and Duchêne 2014). In the absence of fossil evidence, biogeographical events are the key for clock dating. However, accurate biogeographic calibrations are determined by the availability of reliable geological dates (Ho and Phillips 2009). This study revealed that the iterative calibration approach can be effectively used for clock dating when using complex geological events such as the repeated opening and reclosing of the Bering Strait.
A reason for the successful use of the Bering Strait is the constrained periods of time associated with the geological event. While migration through the Strait could have occurred at any time while it was open, there are strong constraints about time periods when migrations did not occur. The level of uncertainty as to the timing of the closures generally ranges from tens to hundreds of thousands of years, rather than millions. By contrast, the closure of the Isthmus of Panama was a process spanning approximately 12 MY (Duque-Caro 1990; Coates and Obando 1996; Collins et al. 1996a; Lessios 2008; Leigh et al. 2014).
Additional strengths of the iterative calibration method are that it not only recognizes the complexity of the geological history, it also incorporates further sources of evidence such as a comprehensive geological signal. Additionally, the iterative calibration approach searches for the most accurate calibration date, guarding against using a single or few sister pairs, which may possess anomalous rates of molecular evolution within their larger taxonomic group. The usage of many sister lineages is possible because it is not necessary to assume that all pairs are perfect sister species, as was required in previous calibration attempts using the Isthmus of Panama and Bering Strait. Instead, all geographically separated sister lineages are considered, recognizing that trans-Bering migration may have occurred at varying times among them. Subsequent diversification within one or both sister lineages is compatible with the iterative calibration approach. Moreover, allopatric phylogroups below the species level are also suitable for contributing to the total evidence. The iterative calibration would benefit from but does not require globally comprehensive sampling of species within genera. One important assumption of this methodology is that organisms dispersed through the water, rather than aerially or via any other means. Therefore, this approach is best for taxa for which this assumption is likely to be true as well as for aquatic taxa represented by many allopatric sister lineage pairs, such that the total signal is apparent despite anomalous data points. In that regard, echinoderms are an exceptional taxonomic group for study due to their ecology (i.e. exclusively marine dispersal; there are no terrestrial or freshwater members of the phylum). On the other hand, the fact that the Bayesian analysis allows for rate variability across lineages, through using a relaxed clock, should minimize the potential impact of errors associated with molecular rate variation. Despite the uncertainty associated with the use of the molecular clock for divergence dating, here we used more data and investigated the influence of anomalous data points to integrate all available sources of evidence. Consequently, the times of trans-Bering divergence in echinoderms estimated during this study are all in agreement with the glacial history of the Bering Strait.
Divergence Times for Northern Echinoderms
The initial finding of a wide range of K2P distances between trans-Bering sister clades (0.5–15.5%) could have been due to rate heterogeneity across taxonomic groups or to non-simultaneous divergence of the sister clades. However, we found that rate heterogeneity was not the dominant explanation since the molecular clock hypothesis was rejected for only one trans-Bering pair. In addition, the Bayesian results suggested that the COI-5P gene evolves in a nearly clock-like fashion for Northern echinoderms, although significant rate variation is observed for a single taxon (Pseudostichopus) (Fig. 5). Thus, the wide range of K2P divergences observed in this study (Fig. 3), with a similar pattern shown by the relative node ages from the Bayesian gene tree (Fig. 5), strongly suggests multiple pulses of trans-Bering migrations for Northern echinoderms. Furthermore, our final Bayesian analysis based upon imposing an absolute date calibration and using a relaxed clock to smooth rate heterogeneity also supported multiple pulses of trans-Bering migrations, with some nodes having non-overlapping 95% posterior densities (Supplementary Material 1.11). Multiple trans-Bering migrations are concordant with previously suggested scenarios. It has been proposed that the marine trans-Arctic interchange occurred in two stages. The first marine interchange occurred from the Atlantic to the Pacific Ocean, immediately after the initial opening of the Bering Strait (5.5 Ma) (Marincovich and Gladenkov 1999; Marincovich 2000). Then, a second interchange took place around 3.5 Ma from Pacific to Atlantic, co-occurring with a reorganization of Northern Hemisphere ocean circulation after the formation of the Isthmus of Panama (Marincovich and Gladenkov 1999; Marincovich 2000). The Pacific–Arctic/Atlantic marine interchanges during the Pleistocene are also consistent with ca. nine cycles of glaciation in the last 2.4 MY (Harris 2005). Multiple marine migrations through the Bering Strait have also been suggested in polychaetes according to molecular evidence (Carr 2010). In addition to migratory events during the major trans-Arctic interchange 3.5 Ma, several polychaete migrations also occurred between 1.9 and 0.4 Ma and even during the last 120,000 years (Carr 2010).
Rate Heterogeneity in the Pseudostichopus Sister Pair
The only trans-Bering pair for which the rate constancy hypothesis was rejected was Pseudostichopus. Further examination of Pseudostichopus sequences and alignment allowed us to detect high sequence divergence and probable base-calling errors in this taxon (Supplementary Material 1.2). After eliminating the sequence with possible editing errors, high rate variability in this pair was still observed, particularly in P. aemulatus in relation to other rates across the tree (Fig. 5). The observed high COI-5P rate in P. aemulatus could be explained by the expected rate heterogeneity across taxa (e.g. Martin and Palumbi 1993). However, further, more subtle, sequence editing errors are still a possibility, and investigation of the sequence chromatograms, which are not presently available, would be needed. Having access to the original trace files, which is feasible for many barcode sequences in BOLD database, is highly valued and would have permitted in this case a re-examination of the original sequences to discard or correct errors. Despite the rate heterogeneity in Pseudostichopus, the inclusion of this pair is still valuable to allow comparison with the rest of the trans-Bering sister clades. Moreover, after accounting for rate variability, it was possible to assign the time of divergence (3.1 Ma) for the Pseudostichopus pair.
Rate Estimation for Northern Echinoderms
Despite that clock calibration using K2P divergences was very similar when using a simplistic and traditional approach (COI divergence rate of 2.77%/MY) versus using the iterative calibration (2.81%/MY), the second method provides a more reliable outcome by including further sources of information such as a comprehensive geological history and the use of more sister lineages. A problem when using an average of divergences for clock dating is that if one or more sister species pairs were missing, the results would greatly fluctuate. For example, the calibrated rate estimate here varies between 2.6 and 2.9% per MY upon dropping each of the four data points in sequence and recalculating the rate based upon three points; the rate varies from 2.5 to 3.0% per MY when systematically dropping the largest two divergences, followed by the lowest two divergences. Moreover, the results would vary even more if more data points were included, and the “cluster” used for calibration is sometimes visually inferred, which is a subjective process. Finally, assuming simultaneous divergence of trans-Bering pairs through the trans-Arctic interchange can produce misleading estimates of divergence times (see further discussion below).
A single preferred calibration date assigned to the most divergent sister pair is suggested by the K2P results using the iterative approach (5.5 Ma), and a similar date is supported by the Bayesian results (5–4.7 Ma). It is unlikely that echinoderms from this study would have migrated before the first opening of the Bering Strait at 5.5 Ma (Gladenkov et al. 2002; Gladenkov and Gladenkov 2004). Although this scenario cannot be completely ruled out, it is less probable; therefore, it was not used for clock calibration. It is unlikely that trans-Bering taxa would have colonized both Pacific and Arctic-Atlantic Oceans well before the first opening of the Bering Strait as it is well established that North Pacific and Arctic-Atlantic marine faunas evolved separately since the mid-Cretaceous (80–100 Ma) until the formation of the Bering Strait marine corridor (Dunton 1992; Marincovich 1993). Although migration of the trans-Bering pairs through the Panama seaway could have been possible before the first opening of the Bering Strait, that is also unlikely given that all trans-Bering sisters included in this study have affinity for temperate to polar waters (Supplementary Material 1.12) and also because echinoderms tend to exhibit limited ranges in their thermal tolerance, and entire genera and even families tend to be endemic to the Arctic or tropics (e.g. Lessios et al. 2012; Mah and Blake 2012). Another implausible scenario is if the most divergent pair would have migrated between 3 and 3.4 Ma. This would imply that only one of the 16 pairs migrated through the Bering Strait shortly after the major trans-Arctic interchange (3.5 Ma; Vermeij 1991). Dating the beginning of the second group of invasions right after the maximum glacial, around 1.9–2.4 Ma, would suggest ongoing trans-Arctic migrations within the last 2.4 MY.
The difference in the best calibration date proposed by the iterative approach when using K2P distances (5.5 Ma) vs. Bayesian results (5–4.7 Ma) can be understood as an effect of rate variability across taxa and due to differences in sequence length between K2P distances and Bayesian analysis (> 651 vs. 459 bp). It has been demonstrated that low-frequency variants, indicative of sequencing or base-calling errors, are most frequently located near the ends in the COI-5 barcode region in the first and last 50 bp of the sequences (Athey 2013). Thus, trimming the alignment to 459 bp for the Bayesian analysis could have reduced miscalculations due to low-frequency variants.
Rate Estimation in Northern Echinoderms vs. Other Rates in the Literature
Our results suggest a divergence rate of 2.8% pairwise K2P sequence divergence per million years for a > 651-bp section of the COI barcode region in trans-Bering echinoderms. Moreover, results from the clock calibration using a Bayesian framework and the GTR + G + I model (Supplementary Material 1.11) suggest a substitution rate of 0.0347 per MY (equating to a pairwise divergence rate of 6.94%/MY pairwise GTR + G + I sequence divergence). Here, we report a faster COI divergence rate for Arctic echinoderms than a prior report for Leptasterias sea stars (0.009 nucleotide substitutions per site per MY, equating to a pairwise divergence rate of approximately 1.8%/MY pairwise GTR + G + I sequence divergence; Foltz et al. 2008). Substitution rates in Leptasterias were estimated using one trans-Bering sister pair (L. muelleri and L. stolacantha) assuming divergence at 3.5 ± 0.25 Ma during the major trans-Arctic interchange (Foltz et al. 2008). Contrary to the previous Leptasterias study, the results from this study suggest that Leptasterias migrated through the Bering Strait more recently, approximately 2 Ma (Figs. 4, 6). Assigning a time of divergence of 3.5 Ma to the Leptasterias trans-Bering sister clade in this study would imply a pairwise divergence of approximately 1.6%/MY and that the most divergent pair migrated as early as 9.7 Ma, prior to the first opening of the Bering Strait, which is very unlikely. We suggest that this discrepancy highlights the value of using more data and the iterative calibration approach.
The divergence rate reported here for Arctic echinoderms is faster than a previous report for the tropical Diadema sea urchin, but slower than previously stated for other tropical echinoderms. However, comparison is challenging because the taxonomic range, sequence lengths, and the included region of the COI gene are not identical among studies. Low genetic divergences for the COI-3P region were observed between a geminate pair of Diadema (4.57% K2P), which the authors interpreted as being consistent with isolation at the last point of the closure of the Isthmus of Panama (Lessios et al. 2001); therefore, the molecular clock for Diadema was calibrated using a date of 1.8 Ma, resulting in a K2P divergence rate of 2.6%/MY (Lessios et al. 2001). By contrast, higher rates of K2P divergence in COI-5P have been reported for one pair of sister species in the genus Oreaster (5%/MY; Hart et al. 1997) and for the COI-3P region for the sea urchins Echinometra (3.49%/MY; McCartney et al. 2000). Furthermore, a per-lineage substitution rate of 3.12%/MY, equating to a pairwise divergence rate of 6.23%/MY, was reported for the COI 5P and 3P regions in the sand dollar Mellita (Coppard et al. 2013). Previous studies based their calibration on the assumption that geminate species diverged at the completion of the Panamanian Isthmus (Hart et al. 1997; McCartney et al. 2000; Coppard et al. 2013). Additionally, a range of 4.2–13.5% K2P divergences has been reported for eight geminate species of tropical sea urchins (Lessios 2008). The author selected which genetic distances were “close enough to each other to be considered as a cluster” (Lessios 2008, p. 79), suggesting similar times of isolation at the time of the Isthmus of Panama closure (Lessios 2008). The resulting average divergence rate of 3.7%/MY (calculated by later authors from geminate species divergence values presented in Lessios 2008) has been then used for dating in other echinoderms (Vogler et al. 2008; Boissin et al. 2011; Hoareau et al. 2013). The results presented here revealed that nine of the 16 trans-Bering clades exhibited K2P divergences (5.6–13.7%, Fig. 3) similar to those reported for tropical sea urchins (4.2–13.5%; Lessios 2008). However, after discounting rate heterogeneity for Northern echinoderms as the primary cause of this large range, the results suggested different times of divergence instead of simultaneous isolation, contrary to what has been generally anticipated or assumed for tropical sea urchins. It is possible that a temporal spread of divergence times has occurred among tropical echinoderm sister pairs, as noted as likely in the case of shrimps inhabiting various habitats (Knowlton and Weigt 1998).
Interestingly, the above studies on tropical echinoderms have used different dates for the closure of the Panamanian Isthmus in their respective clock calibrations: 3.5 Ma (Hart et al. 1997), 3.1 Ma (McCartney et al. 2000), 3 Ma (Lessios 2008; Coppard et al. 2013), and as recently as 1.8 Ma (Lessios et al. 2001). The use of different calibration dates for the same geological event has affected molecular rate estimates for tropical echinoderms. In addition, the long period of time involved in the Isthmus formation and a probable secondary oceanic connection through the land bridge are other factors needing further consideration when estimating molecular rates in tropical lineages. Further research for tropical taxa could benefit from using the iterative calibration approach in order to unify molecular and geological evidence and provide more consistent results.
In contrast with the well-cited divergence rate of 1.4%/MY in Alpheus shrimps estimated using the Isthmus of Panama (Knowlton and Weigt 1998), this study revealed a higher K2P divergence rate for Northern echinoderms (2.8%/MY). Results from trans-Bering echinoderms also differ from the high rate in the COI barcode region previously reported for Arctic polychaetes (4.4%/MY; Carr 2010) and tropical marine molluscs from the genus Arcopsis (5.1%/MY; Marko and Moran 2002). Further work is necessary to expand the novel calibration approach to other marine groups, in order to investigate rate estimates among taxa using consistent methods and genetic regions.
Phylogenetic Scope for the Substitution Rate Presented in This Study
Difference in evolutionary rates could be taxon specific and fluctuate across genes (Ho and Duchêne 2014); therefore, the substitution rate for the COI-5P gene presented in this study should not be generalized to all taxa, nor to all genes. It would be particularly important to consider rate variability when using genes with more severe clock deviations or genome-scale datasets. Furthermore, the resulting substitution rate might not be appropriate for dating recent demographic events, nor for dating extremely deep nodes in the tree of life. In the context of the time-dependency hypothesis, evolutionary rates are not constant over time, with an exponential decline as a function of the calibration age (Ho et al. 2005, 2011). Thus, the rate based on interspecific divergences between sister lineages would not be an accurate generalization for divergence estimations far outside of the calibration time frame (2–5 MY) from this study, especially if the transition from high, short-term (< 1–2 MY) mutation rates to lower, long-term substitution rates (Ho et al. 2005; Ho and Larson 2006) applies to echinoderms as previously observed in other lineages (Ho et al. 2015).
This study used a relaxed uncorrelated clock to account for rate variability across the phylum as suggested for the dataset, which slightly deviates from the strict clock model. The observed rate variability across the phylum was not excessively large (with the only exception of Pseudostichopus), indicating that the calibration for COI-5P presented here might be carefully used in molecular clock research for Arctic echinoderms.
It has been proposed that evolutionary rates and speciation processes are faster in warmer climate regions (e.g. Rohde 1992; Bargelloni et al. 1994; Bleiweiss 1998b; Gillooly et al. 2005; Wright et al. 2006). Therefore, our results are most appropriate for research involving Northern echinoderms and might only be used for comparative purposes when estimating rates and dates for tropical echinoderms, considering that rates might vary along the latitudinal gradient. While the resulting rate for Northern echinoderms is somewhat lower than prior estimates for tropical lineages, further research is needed to evaluate tropical vs. Arctic rates using phylogenetically paired taxa as well as consistent gene regions and analysis methods. Furthermore, it is important to consider that these results might not represent the appropriate evolutionary rate for Northern members of the class Echinoidea. Sea urchins were not included here as we were not able to detect any trans-Bering sister clade, given the limited COI-5P data available for that class. The absence of sea urchins in this analysis contrasts with the fact that most tropical research is based on the assessment of trans-Isthmus sea urchin divergences (e.g. Lessios 1979, 2008; McCartney et al. 2000; Lessios et al. 2001).
Sources of Error of This Method
Extremely high rate variability would make this method difficult to use for obtaining a single calibration for a large taxon. In those cases, evidence for more phylogenetically localized clocks may be explored. However, a strength of the iterative calibration approach is explicitly considering potential rate variability. One more source of error is related to currently partial phylogenies. For highly incomplete phylogenies, it is possible that the geographical shift might have been assigned to the incorrect node (Supplementary Material 1.13). Another source of error is that phylogenetic events may not be associated with the biogeographical event. For example, divergence could have occurred earlier, and then one lineage later migrated through the Bering Strait; as well, migrations may not be recorded due to extinctions. These uncertainties may be partially mitigated through seeking large sample sizes of sister pairs and generalities in the divergence trends. Moreover, uncertainties about the exact age of the geological events persist as a limitation when calibrating the clock. Whereas some major events like the closure of the Isthmus of Panama in Central America are subject to ongoing controversy, other events like the opening/closing of the Bering Strait are dated with more accuracy and with strongly constrained time periods; nevertheless, some error associated with the calibration itself likely remains. On the other hand, calibration failure using the iterative approach can occur when there is no resolution between geological and genetic divergence data. This issue could arise due to excessive molecular rate variability or due to different migratory routes (e.g. aerial/terrestrial dispersal); in those cases, highly variable and uncertain node age estimations might occur.
Future Directions
This research would benefit from estimating divergence rates using other substitution models in order to allow further comparisons across taxonomic groups and to provide a range of calibrations for future use. Complementing COI-5P with additional genes would be advantageous for assessing whether patterns in molecular rates are consistent beyond the mitochondrial genome. However, we would advocate for the increased usage of genes evolving in a clock-like fashion for molecular dating. When using genes with more severe clock deviations, rate variability must be considered even more carefully. Ideally, more trans-Bering sister clades should be used to estimate the most accurate rate of evolution for Arctic echinoderms. However, this improvement is associated with the general need to increase the taxonomic and geographic breadth of molecular data for marine fauna. Molecular clock research, including Bayesian molecular clock dating, can benefit from the iterative calibration approach by integrating all available sources of evidence when dating phylogenies. A promising future direction would be to explore further the iterative calibration method using other major marine taxonomic groups, including taxa with an excellent fossil record, such as many molluscs. Clock calibrations for terrestrial groups can also take advantage of the iterative calibration approach, for example considering that land migration events would have occurred during the existence of the Bering Land Bridge, when the Bering Strait was closed. Furthermore, the iterative calibration approach could be applied when using other geological events for clock dating. Well-dated phylogenies may, in turn, represent new opportunities for dating other taxonomic groups with neither fossil nor geological data associated with divergence events, always guarding against anomalous rates of evolution or an exceptional dispersal history. Additionally, future developments from this study could be to model probability densities for dispersal over time rather than a “Strait is open for dispersal” vs. “closed for dispersal” approach as we have used here, or implementing multi-epoch models similar to those mentioned in Landis (2016) for biogeographic dating.
Conclusions
Here, we have presented a novel approach for molecular clock calibration integrating phylogenetic, distributional, and geological/climatological history data. The iterative calibration approach is a more falsifiable method than earlier approaches, and, after incorporating several sources of evidence, it anticipates more accurate results than when using simplistic methods for clock dating (e.g. using a divergence average). Therefore, the iterative calibration represents the most accurate approach when using complex biogeographic events for clock dating. This study demonstrates how the opening/closing of the Bering Strait, together with the new iterative calibration approach, can successfully be used in clock dating research to shed light upon the evolutionary history of Northern taxa and the influence of major climatic upheaval upon biogeography and diversity. The results suggest that the COI-5P divergence rate in trans-Bering echinoderms is slower than rates previously reported for tropical sea urchins (Hart et al. 1997; McCartney et al. 2000; Lessios 2008; Coppard et al. 2013), but faster than earlier-reported rates for Arctic sea stars (Foltz et al. 2008). Additionally, the results strongly support several migrations across the Bering Strait as previously suggested for polychaetes (Carr 2010). This study represents a significant advantage over prior calibrations after using a substantial number of sister clades, together with the iterative calibration approach. Lastly, we recognize the potential to expand upon molecular clock research using the iterative calibration approach in other taxa and with additional biogeographical events.
References
Addison JA, Pogson GH (2009) Multiple gene genealogies reveal asymmetrical hybridization and introgression among strongylocentrotid sea urchins. Mol Ecol 18:1239–1251. https://doi.org/10.1111/j.1365-294X.2009.04094.x
Arndt A, Marquez C, Lambert P, Smith MJ (1996) Molecular phylogeny of Eastern Pacific sea cucumbers (Echinodermata: Holothuroidea) based on mitochondrial DNA sequence. Mol Phylogenet Evol 6:425–437. https://doi.org/10.1006/mpev.1996.0091
Athey T (2013) Assessing errors in DNA barcode sequence records. Master thesis, University of Guelph
Bacon CD, Silvestro D, Jaramillo C et al (2015) Biological evidence supports an early and complex emergence of the Isthmus of Panama. Proc Natl Acad Sci 112:E3153–E3153. https://doi.org/10.1073/pnas.1509107112
Bargelloni L, Ritchie PA, Patarnello T et al (1994) Molecular evolution at subzero temperatures: mitochondrial and nuclear phylogenies of fishes from Antarctica (suborder Notothenioidei), and the evolution of antifreeze glycopeptides. Mol Biol Evol 11:854–863
Bastrop R, Blank M (2006) Multiple invasions - a polychaete genus enters the Baltic Sea. Biol Invasions 8:1195–1200. https://doi.org/10.1007/s10530-005-6186-6
Bleidorn C, Kruse I, Albrecht S, Bartolomaeus T (2006) Mitochondrial sequence data expose the putative cosmopolitan polychaete Scoloplos armiger (Annelida, Orbiniidae) as a species complex. BMC Evol Biol 6:47. https://doi.org/10.1186/1471-2148-6-47
Bleiweiss R (1998a) Relative-rate tests and biological causes of molecular evolution in hummingbirds. Mol Biol Evol 15:481–491
Bleiweiss R (1998b) Slow rate of molecular evolution in high-elevation hummingbirds. Proc Natl Acad Sci USA 95:612–616. https://doi.org/10.1073/pnas.95.2.612
Boissin E, Stöhr S, Chenuil A (2011) Did vicariance and adaptation drive cryptic speciation and evolution of brooding in Ophioderma longicauda (Echinodermata: Ophiuroidea), a common Atlanto-Mediterranean ophiuroid? Mol Ecol 2:4737–4755. https://doi.org/10.1111/j.1365-294X.2011.05309.x
Bribiesca-Contreras G, Solís-Marín FA, Laguarda-Figueras A, Zaldívar-Riverón A (2013) Identification of echinoderms (Echinodermata) from an anchialine cave in Cozumel Island, Mexico, using DNA barcodes. Mol Ecol Resour 13:1137–1145. https://doi.org/10.1111/1755-0998.12098
Briggs JC (1970) A faunal history of the North Atlantic Ocean. Syst Zool 19:19–34. https://doi.org/10.2307/2412025
Bromham L (2002) Molecular clocks in reptiles: life history influences rate of molecular evolution. Mol Biol Evol 19:302–309. https://doi.org/10.1093/oxfordjournals.molbev.a004083
Carr CM (2010) The Polychaeta of Canada: exploring diversity and distribution patterns using DNA barcodes. Master thesis, University of Guelph
Carr CM, Hardy SM, Brown TM et al (2011) A tri-oceanic perspective: DNA barcoding reveals geographic structure and cryptic diversity in Canadian polychaetes. PLoS ONE 6:e22232. https://doi.org/10.1371/journal.pone.0022232
Coates AG, Obando JA (1996) The geologic evolution of the Central American Isthmus. In: Jackson JBC, Budd AF, Coates AG (eds) Evolution and environment in tropical America. The University of Chicago Press, Chicago, pp 21–56
Collins LS, Coates AG, Berggren WA et al (1996a) The late Miocene Panama isthmian strait. Geology 24:687–690
Collins TM, Frazer K, Brown WM (1996b) Evolutionary history of Northern Hemisphere Nucella (Gastropoda, Muricidae): molecular, morphological, ecological, and paleontological evidence. Evolution 50:2287–2304
Coppard SE, Zigler KS, Lessios H (2013) Phylogeography of the sand dollar genus Mellita: cryptic speciation along the coasts of the Americas. Mol Phylogenet Evol 69:1033–1042. https://doi.org/10.1016/j.ympev.2013.05.028
Dodson JJ, Tremblay S, Colombani F et al (2007) Trans-Arctic dispersals and the evolution of a circumpolar marine fish species complex, the capelin (Mallotus villosus). Mol Ecol 16:5030–5043. https://doi.org/10.1111/j.1365-294X.2007.03559.x
Drummond AJ, Bouckaert RR (2015) Bayesian evolutionary analysis with BEAST. Cambridge University Press, Cambridge
Drummond AJ, Ho SYW, Phillips MJ, Rambaut A (2006) Relaxed phylogenetics and dating with confidence. PLoS Biol 4:699–710. https://doi.org/10.1371/journal.pbio.0040088
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973. https://doi.org/10.1093/molbev/mss075
Dunton K (1992) Arctic biogeography: the paradox of the marine benthic fauna and flora. Trends Ecol Evol 7:183–189. https://doi.org/10.1016/0169-5347(92)90070-R
Duque-Caro H (1990) Neogene stratigraphy, paleoceanography and paleobiogeography in northwest South America and the evolution of the Panama seaway. Palaeogeogr Palaeoclimatol Palaeoecol 77:203–234. https://doi.org/10.1016/0031-0182(90)90178-A
Durham J, MacNeil F (1967) Cenozoic migrations of marine invertebrates through the Bering Strait region. In: Hopkins D (ed) The Bering land bridge. Stanford University Press, Stanford, pp 326–349
Einarsson T, Hopkins DM, Doell RR (1967) The stratigraphy of Tjornes, North Iceland and the history of the Bering land bridge. In: Hopkins D (ed) The Bering land bridge. Stanford University Press, Stanford California, pp 312–325
Feuda R, Smith AB (2015) Phylogenetic signal dissection identifies the root of starfishes. PLoS ONE 10:e0123331. https://doi.org/10.1371/journal.pone.0123331
Fleischer RC, McIntosh CE, Tarr CL (1998) Evolution on a volcanic conveyor belt: using phylogeographic reconstructions and K-Ar-based ages of the Hawaiian Islands to estimate molecular evolutionary rates. Mol Ecol 7:533–545. https://doi.org/10.1046/j.1365-294x.1998.00364.x
Foltz DW, Nguyen AT, Kiger JR, Mah CL (2008) Pleistocene speciation of sister taxa in a North Pacific clade of brooding sea stars (Leptasterias). Mar Biol 154:593–602. https://doi.org/10.1007/s00227-008-0952-9
Gernhard T (2008) The conditioned reconstructed process. J Theor Biol 253:769–778. https://doi.org/10.1016/j.jtbi.2008.04.005
Gillooly JF, Allen AP, West GB, Brown JH (2005) The rate of DNA evolution: effects of body size and temperature on the molecular clock. Proc Natl Acad Sci USA 102:140–145. https://doi.org/10.1073/pnas.0407735101
Gladenkov AY, Gladenkov YB (2004) Onset of connections between the Pacific and Arctic Oceans through the Bering Strait in the Neogene. Stratigr Geol Correl 12:175–187
Gladenkov A, Oleinik A, Marincovich LJ, Barinov K (2002) A refined age for the earlier opening of Bering Strait. Paleogeogr Paleclimatol Paleoecol 183:321–328
Gregory TR (2008) Understanding evolutionary trees. Evol Educ Outreach 1:121–137. https://doi.org/10.1007/s12052-008-0035-x
Haq B, Hardenbol J, Vail P (1987) Chronology of fluctuating sea levels since the Triassic. Science 235:1156–1167. https://doi.org/10.1126/science.235.4793.1156
Hardy SM, Carr CM, Hardman M et al (2011) Biodiversity and phylogeography of Arctic marine fauna: insights from molecular tools. Mar Biodivers 41:195–210. https://doi.org/10.1007/s12526-010-0056-x
Harper FM, Hart MW (2007) Morphological and phylogenetic evidence for hybridization and introgression in a sea star secondary contact zone. Invertebr Biol 126:373–384. https://doi.org/10.1111/j.1744-7410.2007.00107.x
Harper FM, Addison JA, Hart MW (2007) Introgression versus immigration in hybridizing high-dispersal echinoderms. Evolution 61:2410–2418. https://doi.org/10.1111/j.1558-5646.2007.00200.x
Harris SA (2005) Thermal history of the Arctic Ocean environs adjacent to North America during the last 3.5 Ma and a possible mechanism for the cause of the cold events (major glaciations and permafrost events). Prog Phys Geogr 29:218–238. https://doi.org/10.1191/0309133305pp444ra
Hart MW, Byrne M, Smith MJ (1997) Molecular phylogenetic analysis of life-history evolution in asterinid starfish. Evolution 51:1848–1861
Haug GH, Sigman DM, Tiedemann R et al (1999) Onset of permanent startification in the subarctic Pacific Ocean. Nature 401:779–782
Hebert PDN, Cywinska A, Ball SL, DeWaard JR (2003a) Biological identifications through DNA barcodes. Proc R Soc Lond B Biol Sci 270:313–321. https://doi.org/10.1098/rspb.2002.2218
Hebert PDN, Ratnasingham S, DeWaard JR (2003b) Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc R Soc Lond B Biol Sci 270 Suppl:S96–S99. https://doi.org/10.1098/rsbl.2003.0025
Held C (2001) No evidence for slow-down of molecular substitution rates at subzero temperatures in Antarctic serolid isopods (Crustacea, Isopoda, Serolidae). Polar Biol 24:497–501. https://doi.org/10.1007/s003000100245
Herman Y, Hopkins DM (1980) Arctic oceanic climate in Late Cenozoic time. Science 209:557–562
Ho SYW, Duchêne S (2014) Molecular-clock methods for estimating evolutionary rates and timescales. Mol Ecol 23:5947–5965. https://doi.org/10.1111/mec.12953
Ho SYW, Larson G (2006) Molecular clocks: when times are a-changin’. Trends Genet 22:79–83. https://doi.org/10.1016/j.tig.2005.11.006
Ho SYW, Phillips MJ (2009) Accounting for calibration uncertainty in phylogenetic estimation of evolutionary divergence times. Syst Biol 58:367–380. https://doi.org/10.1093/sysbio/syp035
Ho SYW, Phillips MJ, Cooper A, Drummond AJ (2005) Time dependency of molecular rate estimates and systematic overestimation of recent divergence times. Mol Biol Evol 22:1561–1568. https://doi.org/10.1093/molbev/msi145
Ho SYW, Lanfear R, Bromham L et al (2011) Time-dependent rates of molecular evolution. Mol Ecol 20:3087–3101. https://doi.org/10.1111/j.1365-294X.2011.05178.x
Ho SYW, Duchêne S, Molak M, Shapiro B (2015) Time-dependent estimates of molecular evolutionary rates: evidence and causes. Mol Ecol 24:6007–6012. https://doi.org/10.1111/mec.13450
Hoareau TB, Boissin E, Paulay G, Bruggemann JH (2013) The Southwestern Indian Ocean as a potential marine evolutionary hotspot: perspectives from comparative phylogeography of reef brittle-stars. J Biogeogr 40:2167–2179. https://doi.org/10.1111/jbi.12155
Hopkins D (1967) Quaternary marine trangressions in Alaska. In: Hopkins DM (ed) The Bering land bridge. Stanford University Press, Stanford, pp 47–90
Horikawa K, Martin EE, Basak C et al (2015) Pliocene cooling enhanced by flow of low-salinity Bering Sea water to the Arctic Ocean. Nat Commun 6:7587. https://doi.org/10.1038/ncomms8587
Hu A, Meehl GA, Han W (2007) Role of the Bering Strait in the thermohaline circulation and abrupt climate change. Geophys Res Lett 34:1–6. https://doi.org/10.1029/2006GL028906
Hurt C, Anker A, Knowlton N (2009) A multilocus test of simultaneous divergence across the Isthmus of Panama using snapping shrimp in the genus Alpheus. Evolution 63:514–530. https://doi.org/10.1111/j.1558-5646.2008.00566.x
Jordan DS (1908) The law of geminate species. Am Nat 42:73–80
Kamarudin KR, Hashim R, Usup G (2010) Phylogeny of sea cucumber (Echinodermata: Holothuroidea) as inferred from 16 s mitochondrial rRNA gene sequences. Sains Malays 39:209–218
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. https://doi.org/10.1007/BF01731581
Knowlton N, Weigt LA (1998) New dates and new rates for divergence across the Isthmus of Panama. Proc R Soc Lond B Biol Sci 265:2257–2263
Kober KM, Bernardi G (2013) Phylogenomics of strongylocentrotid sea urchins. BMC Evol Biol 13:88. https://doi.org/10.1186/1471-2148-13-88
Landis MJ (2016) Biogeographic dating of speciation times using paleogeographically informed processes. Syst Biol 66:128–144. https://doi.org/10.1093/sysbio/syw040
Layton KKS, Corstorphine EA, Hebert PDN (2016) Exploring Canadian echinoderm diversity through DNA barcodes. PLoS ONE 1–16. https://doi.org/10.1371/journal.pone.0166118
Lee YH (2003) Molecular phylogenies and divergence times of sea urchin species of Strongylocentrotidae, Echinoida. Mol Biol Evol 20:1211–1221. https://doi.org/10.1093/molbev/msg125
Leigh EG, O’Dea A, Vermeij GJ (2014) Historical biogeography of the Isthmus of Panama. Biol Rev 89:148–172. https://doi.org/10.1111/brv.12048
Lessios H (1979) Use of Panamanian sea urchins to test the molecular clock. Nature 280:599–601
Lessios H (2008) The great American schism: divergence of marine organisms after the rise of the central American Isthmus. Annu Rev Ecol Evol Syst 39:63–91. https://doi.org/10.1146/annurev.ecolsys.38.091206.095815
Lessios H, Pearse J (1996) Hybridization and introgression between Indo-Pacific species of Diadema. Mar Biol 126:715–723. https://doi.org/10.1007/BF00351338
Lessios H, Kessing BD, Pearse JS (2001) Population structure and speciation in tropical seas: global phylogeography of the sea urchin Diadema. Evolution 55:955–975. https://doi.org/10.1111/j.0014-3820.2001.tb00613.x
Lessios H, Lockhart S, Collin R et al (2012) Phylogeography and bindin evolution in Arbacia, a sea urchin genus with an unusual distribution. Mol Ecol 21:130–144. https://doi.org/10.1111/j.1365-294X.2011.05303.x
Littlewood DTJ, Smith AB, Clough KA, Emson RH (1997) The interrelationships of the echinoderm classes: morphological and molecular evidence. Biol J Linn Soc 61:409–438. https://doi.org/10.1111/j.1095-8312.1997.tb01799.x
Luttikhuizen PC, Drent J, Baker AJ (2003) Disjunct distribution of highly diverged mitochondrial lineage clade and population subdivision in a marine bivalve with pelagic larval dispersal. Mol Ecol 12:2215–2229. https://doi.org/10.1046/j.1365-294X.2003.01872.x
Mah CL, Blake DB (2012) Global diversity and phylogeny of the Asteroidea (Echinodermata). PLoS ONE 7:e35644. https://doi.org/10.1371/journal.pone.0035644
Mah C, Foltz D (2011) Molecular phylogeny of the Forcipulatacea (Asteroidea: Echinodermata): systematics and biogeography. Zool J Linn Soc 162:646–660. https://doi.org/10.1111/j.1096-3642.2010.00688.x
Marincovich LJ (1993) Danian mollusks from the Prince Creek formation Northern Alaska and implications for Arctic Ocean paleogeography. Paleontol Soc Mem 35:1–35. https://doi.org/10.2307/1315585
Marincovich LJ (2000) Central American paleogeography controlled Pliocene Arctic Ocean molluscan migrations. Geology 28:551–554
Marincovich LJ, Gladenkov A (1999) Evidence for an early opening of the Bering Strait. Nature 397:149–151. https://doi.org/10.1038/16446
Marincovich L, Gladenkov AY (2001) New evidence for the age of Bering Strait. Quat Sci Rev 20:329–335. https://doi.org/10.1016/S0277-3791(00)00113-X
Marko PB, Moran AL (2002) Correlated evolutionary divergence of egg size and a mitochondrial protein across the Isthmus of Panama. Evolution 56:1303–1309
Martin AP, Palumbi SR (1993) Body size, metabolic rate, generation time, and the molecular clock. Proc Natl Acad Sci 90:4087–4091. https://doi.org/10.1073/pnas.90.9.4087
Martin AP, Naylor GJ, Palumbi SR (1992) Rates of mitochondrial DNA evolution in sharks are slow compared with mammals. Nature 357:153–155. https://doi.org/10.1038/357153a0
Maslin MA, Haug G, Sarnthein M, Tiedemann R (1996) The progressive intensification of Northern Hemisphere glaciation as seen from the North Pacific. Geol Rundschau 85:452–465. https://doi.org/10.1007/BF02369002
Matthiessen J, Knies J, Vogt C, Stein R (2009) Pliocene palaeoceanography of the Arctic Ocean and subarctic seas. Philos Trans R Soc Lond A 367:21–48. https://doi.org/10.1098/rsta.2008.0203
McCartney M, Keller G, Lessios H (2000) Dispersal barriers in tropical oceans and speciation in Atlantic and eastern Pacific sea urchins of the genus Echinometra. Mol Ecol 9:1391–1400. https://doi.org/10.1046/j.1365-294X.2000.01022.x
Mendes FK, Hahn MW (2016) Gene tree discordance causes apparent substitution rate variation. Syst Biol 65:711–721. https://doi.org/10.1093/sysbio/syw018
Miura O, Torchin ME, Bermingham E (2010) Molecular phylogenetics reveals differential divergence of coastal snails separated by the Isthmus of Panama. Mol Phylogenet Evol 56:40–48. https://doi.org/10.1016/j.ympev.2010.04.012
Mooers AO, Harvey PH (1994) Metabolic rate, generation time, and the rate of molecular evolution in birds. Mol Phylogenet Evol 3:344–350. https://doi.org/10.1006/mpev.1994.1040
O’Dea A, Lessios HA, Coates AG et al (2016) Formation of the Isthmus of Panama. Sci Adv 2:1–12. https://doi.org/10.1126/sciadv.1600883
Ogg J, Ogg G, Gradstein F (2016) A concise geological time scale. Elsevier, Amsterdam
Palumbi SR, Kessing BD (1991) Population biology of the Trans-Arctic exchange: MtDNA sequence similarity between Pacific and Atlantic sea urchins. Evolution 45:1790–1805
Perseke M, Bernhard D, Fritzsch G et al (2010) Mitochondrial genome evolution in Ophiuroidea, Echinoidea, and Holothuroidea: insights in phylogenetic relationships of Echinodermata. Mol Phylogenet Evol 56:201–211. https://doi.org/10.1016/j.ympev.2010.01.035
Polyakova YI (2001) Late Cenozoic evolution of Northern Eurasian marginal seas based on the diatom record. Polarforschung 69:211–220
Radulovici AE, Archambault P, Dufresne F (2010) DNA barcodes for marine biodiversity. Moving fast forward? Diversity 2:450–472. https://doi.org/10.3390/d2040450
Rambaut A, Suchard MA, Xie D, Drummond AJ (2014) Tracer v1.6. Available from http://beast.bio.ed.ac.uk/Tracer
Ratnasingham S, Hebert PDN (2007) BOLD: the barcode of life data system: barcoding. Mol Ecol Notes 7:355–364. https://doi.org/10.1111/j.1471-8286.2007.01678.x
Ratnasingham S, Hebert PDN (2013) A DNA-based registry for all animal species: the Barcode Index Number (BIN) system. PLoS One 8:e66213. https://doi.org/10.1371/journal.pone.0066213
Robinson M, Gouy M, Gautier C, Mouchiroud D (1998) Sensitivity of the relative-rate test to taxonomic sampling. Mol Biol Evol 15:1091–1098
Rohde K (1992) Latitudinal gradients in species diversity: the search for the primary cause. Oikos 65:514–527. https://doi.org/10.2307/3545569
Saitou N, Nei M (1987) The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evo 4:406–425
Schrader H-J, Bjorklund K, Manum S et al (1976) Cenozoic biostratigraphy, physical stratigraphy and paleooceanography in the Norwegian-Greenland Sea, DSDP Leg 38 paleontological synthesis. In: Talwan M, Al E (eds) Initial reports deep sea drilling project. U.S. Goverment Printing Office, Washington DC, pp 1197–1211
Shackleton NJ, Opdyke ND (1977) Oxygen isotope and palaeomagnetic evidence for early Northern Hemisphere glaciation. Nature 270:216–219. https://doi.org/10.1038/270216a0
Shaffer G, Bendtsen J (1994) Role of the Bering Strait in controlling North Atlantic ocean circulation and climate. Nature 367:354–357. https://doi.org/10.1038/367354a0
Tajima F (1993) Simple methods for testing the molecular evolutionary clock hypothesis. Genetics 135:599–607
Tamura K, Stecher G, Peterson D et al (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. https://doi.org/10.1093/molbev/mst197
Telford MJ, Lowe CJ, Cameron CB et al (2014) Phylogenomic analysis of echinoderm class relationships supports Asterozoa. Proc R Soc B Biol Sci 281:20140479. https://doi.org/10.1098/rspb.2014.0479
Vermeij GJ (1989) Geographical restriction as a guide to the causes of extinction: the case of the cold Northern Oceans during the Neogene. Paleobiology 15:335–356
Vermeij GJ (1991) Anatomy of an invasion: the trans-Arctic interchange. Paleobiology 17:281–307
Vogler C, Benzie J, Lessios H et al (2008) A threat to coral reefs multiplied? Four species of crown-of-thorns starfish. Biol Lett 4:696–699. https://doi.org/10.1098/rsbl.2008.0454
Ward RD, Holmes BH, O’Hara TD (2008) DNA barcoding discriminates echinoderm species. Mol Ecol Resour 8:1202–1211. https://doi.org/10.1111/j.1755-0998.2008.02332.x
Wares JP, Cunningham CW (2001) Phylogeography and historical ecology of the North Atlantic intertidal. Evolution 55:2455–2469. https://doi.org/10.1111/j.0014-3820.2001.tb00760.x
Weir JT, Schluter D (2008) Calibrating the avian molecular clock. Mol Ecol 17:2321–2328. https://doi.org/10.1111/j.1365-294X.2008.03742.x
Williams ST, Reid DG (2004) Speciation and diversity on tropical rocky shores: a global phylogeny of snails of the genus Echinolittorina. Evolution 58:2227–2251. https://doi.org/10.1111/j.0014-3820.2004.tb01600.x
Wright S, Keeling J, Gillman L (2006) The road from Santa Rosalia: a faster tempo of evolution in tropical climates. Proc Natl Acad Sci USA 103:7718–7722. https://doi.org/10.1073/pnas.0510383103
Yang Z, Rannala B (2006) Bayesian estimation of species divergence times under a molecular clock using multiple fossil calibrations with soft bounds. Mol Biol Evol 23:212–226. https://doi.org/10.1093/molbev/msj024
Zuckerkandl E, Pauling L (1965) Evolutionary divergence and convergence in proteins. In: Bryson V, Vogel H (eds) Evolving genes and proteins. Academic Press, New York, pp 97–166
Acknowledgements
This work was supported by a graduate scholarship from Consejo Nacional de Ciencia y Tecnología (315757 to TLQ) and by the Natural Sciences and Engineering Research Council of Canada (Discovery Grant 2010-386591 and 2016-06199 to SJA). We also acknowledge the Ontario Ministry of Research Innovation for providing funding to Paul Hebert for the development of BOLD, which was the main resource of data for this project. Special thanks to Teresa J. Crease, Elizabeth G. Boulding, Jonathan D.S. Witt, and Mari Kekkonen for discussion and their valuable feedback on earlier versions of this manuscript. We sincerely thank two anonymous reviewers for their helpful and constructive comments. Finally, we want to acknowledge all the researchers who have made public COI barcode sequences from echinoderms.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Loeza-Quintana, T., Adamowicz, S.J. Iterative Calibration: A Novel Approach for Calibrating the Molecular Clock Using Complex Geological Events. J Mol Evol 86, 118–137 (2018). https://doi.org/10.1007/s00239-018-9831-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-018-9831-2