Abstract
One of the main open questions in origin of life research focuses on the formation, by self-organization, of primitive cells composed by macromolecular compounds enclosed within a semi-permeable membrane. A successful experimental strategy for studying the emergence and the properties of primitive cells relies on a synthetic biology approach, consisting in the laboratory assembly of cell models of minimal complexity (semi-synthetic minimal cells). Despite the recent advancements in the construction and characterization of synthetic cells, an important physical aspect related to their formation is still not well known, namely, the mechanism of solute entrapment inside liposomes (in particular, the entrapment of macromolecules). In the past years, we have investigated this phenomenon and here we shortly review our experimental results. We show how the detailed cryo-transmission electron microscopy analyses of liposome populations created in the presence of ferritin (taken as model protein) or ribosomes have revealed that a small fraction of liposomes contains a high number of solutes, against statistical expectations. The local (intra-liposomal) macromolecule concentration in these liposomes largely exceeds the bulk concentration. A similar behaviour is observed when multi-molecular reaction mixtures are used, whereby the reactions occur effectively only inside those liposomes that have entrapped high number of molecules. If similar mechanisms operated in early times, these intriguing results support a scenario whereby the formation of lipid compartments plays an important role in concentrating the components of proto-metabolic systems—in addition to their well-known functions of confinement and protection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cells are the basic unit of life. The intricate mesh of autopoietic reactions, kept by high local solute concentrations and confined within a semi-permeable barrier (the lipid/protein membrane), permits cells to self-maintain, grow and reproduce by the continuous biosynthesis of all its chemical compounds. How living cells originated from inanimate matter is still unknown, but in the last decades, the experimental research on the physical and chemical mechanisms leading to the emergence of primitive cells—carried out using lipid vesicles (liposomes) as models—has been growing considerably (Oberholzer et al. 1995; Luisi et al. 1999; Szostak et al. 2001; Monnard and Deamer 2002; Rasmussen et al. 2003, 2009; Walde 2010; Kurihara et al. 2011; Ichihashi et al. 2012; Torino et al. 2013;Blain and Szostak 2014).
In particular, semi-synthetic approaches (Oberholzer et al. 1995; Luisi et al. 2006; Stano et al. 2011) aim at constructing protocellular models of minimal complexity by combining liposomes and free molecules such as nucleic acids, ribosomes, proteins, and low-molecular weight compounds (amino acids, nucleotides, etc.).
One example of the undergoing research on “minimal cell” construction is given by the encapsulation of gene expression kits (i.e., transcription–translation or TX–TL kits) inside liposomes. Owing to intra-liposomal protein synthesis, it is possible—at least in principle—to generate desired functions in these “synthetic cells.” The goal is to reconstruct cellular models of minimal complexity that can perform operations like enzymatic synthesis of lipids (for membrane self-reproduction), DNA duplication, RNA synthesis, reconstruction of minimal metabolic networks and so on. This will help us to understand how these basic cellular processes work inside lipid micro-compartments, and how these chemical reactions cope with intrinsic physical constraints, providing insights into the primitive cellular system that they are supposed to mimic. Several groups are currently involved in this synthetic cells research, both from the viewpoint of origin of life and for biotechnological applications (Pohorille and Deamer 2002; Nomura et al. 2003; Noireaux and Libchaber 2004; Sunami et al. 2006; Kita et al. 2008; Mansy et al. 2008; Moritani et al. 2010; Caschera et al. 2011; Martini and Mansy 2011; Maeda et al. 2012; Stano et al. 2012; Nourian and Danelon 2013).
An intriguing aspect of semi-synthetic approach is that the procedures used for protocells/synthetic cell construction rely on self-assembly and self-organization phenomena, which involve not only the lipid molecules (which easily form bilayers and closed lipid vesicles, i.e., liposomes) but also the molecules that are present during the liposome formation. This aspect is relevant for origin of life studies if we assume—as it is generally done—that the construction of synthetic cells is based on the same physico-chemical phenomena that promoted the emergence of primordial cells from free primitive solutes and primitive lipids (e.g., fatty acids, isoprenoids and other simple amphiphilic compounds; see Walde (2006) for a review).
Liposome formation and solute encapsulation can occur simultaneously, as shown in Fig. 1, and this mini-review is devoted to annotate our recent experimental findings on this fundamental process at the roots of cells origin. In particular, we will describe that macromolecules like ferritin and ribosomes may self-concentrate inside lipid vesicles, generating solute-filled compartments whose internal solute concentration exceeds the external one. Similar phenomena occur also in the case of complex multi-molecular systems, such as the TX–TL machinery. Although the details of the mechanism underlying these intriguing observations are not known, we believe that the reported observations have a great relevance in origin of life scenarios and help explaining in a rational way the emergence of functional primitive cells, starting from an environment where solutes are present at low concentration.
The self-assembly competence of lipids (phospholipids or fatty acids) brings about the formation of lipid vesicles (liposomes) which can capture (entrap, encapsulate) the solutes present in the aqueous solution that is employed for their formation. A population of liposomes emerges from this process. Liposomes are heterogeneous in terms of size, morphology and solute content. The actual solute occupancy distribution can be compared with the ideal case of random solute entrapment, obtained under the hypothesis that liposomes blindly sample portions of the solution according to their volume (probability of entrapment = vesicle volume/overall sample volume). Reproduced from Souza et al. (2011) with the permission of Wiley
After a short introduction on the motivations of our research on solute encapsulation, we will first focus on the encapsulation of single molecular species, and then on multi-molecular mixtures. A more detailed discussion on the historical developments of our research has been recently presented by Luisi (2012).
Experimental Studies on the Encapsulation of Single Solute Species
Motivations and Historical Developments
Our interest on the mechanisms of solute encapsulation during liposome formation derives both from previous unpublished data on solute-filled vesicles obtained during studies on fatty acid vesicle self-reproduction (Berclaz et al. 2001a, b), and from theoretical considerations on the success of protein synthesis experiments reactions inside liposomes (commented in Luisi (2006)). It might be useful to shortly recapitulate the historical developments bringing about current research.
The first polypeptide synthesis inside liposomes (i.e., poly(Phe) synthesis) was demonstrated by Luisi et al. 1999 (Oberholzer et al. 1999), and soon after the first cases of functional protein synthesis (green fluorescent protein, GFP) were reported (Yu et al. 2001; Oberholzer and Luisi 2002). The success of protein synthesis inside lipid micro-compartments implies that all components of these complex TX–TL kits [which are bacterial extracts or synthetic reconstituted systems (Shimizu et al. 2001)] were simultaneously entrapped inside liposomes during their formation. Intuitively, this is an event difficult to conceive. Note that the vesicles used in these early studies were micrometer-sized in the case of Yu et al. (2001), but they were not characterized in the work presented by Oberholzer and Luisi (2002).
In the following years (Souza et al. 2009), we reported protein synthesis from cell extracts inside conventional vesicles—which are quite small (diameter <300 nm) compared with today’s cells, and we were intrigued by the fact that many different molecules could be encapsulated inside the same compartment, somehow beyond statistical expectations. It was evident that a kind of anomalous effect could drive an accumulation of solutes inside liposomes. In fact, calculations reveal that a Poisson distribution cannot explain the simultaneous presence of all components of the TX–TL reaction in a small individual lipid vesicle (Souza et al. 2009).
What is then the mechanism of macromolecules encapsulation inside lipid vesicles?
We realized that a detailed study on this topic is crucial to understanding the physics of vesicle formation and solute entrapment—two coupled mechanisms that are at the origin of cellular life.
Average Versus Individual Encapsulation (or Entrapment) Efficiency (EE) in Liposome Populations
Intrigued by these considerations, we started a focused investigation on the entrapment of macromolecular solutes inside lipid vesicles, intended as a process that mimics the formation of primitive cells. Thus, we looked for an experimental model capable of giving a direct and precise report of solute content and solute occupancy distribution inside liposomes.
In the field of liposomes, the study of the encapsulation efficiency is a well-known practice, but this is generally done by quantifying (via chemical analysis) the overall amount of the encapsulated substance. In these traditional studies, the encapsulation efficiency (EE) is given either as the fractional amount of encapsulated substance (e.g. 7 %) or as the amount of encapsulated substance divided by the amount of lipids in the preparation (e.g. 0.05 mg substance per mg lipid). After estimating (or measuring) the number of liposomes in the samples, one could evaluate from these EE values an average intra-liposome solute content. Clearly, these methods are limited as far as determining the individual liposome EE is desired.
In contrast, techniques like microscopy and flow cytometry allow the analysis of the solute content inside each (individual) liposome. Our approach is therefore, based on cryogenic-transmission electronmicroscopy (cryo-TEM) and optical (confocal fluorescence) microscopy, and aims at measuring of the solute occupancy distribution via direct counting the number of solutes in individual vesicles and making a comparison with the expectations.
In order to calculate the expected value—i.e. the expected numbers of solutes inside a particular vesicle—we need a theoretical model. The simplest one (our “null hypothesis,” against which data will be compared) simply states that in the absence of specific lipid/solute interactions, it is expected that the solution encapsulated inside liposome has the same composition of the external solution, and therefore, that the expected intra-liposome solute concentration (C in) is simply equal to C bulk, the solute concentration in bulk. If the liposome volume V is known, it is then possible to calculate the average number of molecules N in inside it, N in = N A V C in = N A V C bulk, where N A is the Avogadro constant. Basic statistical theory also predicts that not all vesicles will contain the same number of molecules, and that the number of solutes inside a population of vesicles follows a Poisson distribution, with average N in and standard deviation equal to √N in. Essentially, thus, this simple model considers the encapsulation of molecules inside vesicles as a random sampling process weighted by the vesicle volume. These being the expectations, let us see what was experimentally observed.
Ferritin Encapsulation
Ferritin is an almost spherical protein (diameter about 12.5 nm) composed of 24 subunits arranged in 4,3,2-symmetry around a 7.5 nm iron core, composed of thousands of iron atoms as hydrous ferric oxide phosphate, resembling the mineral ferrihydrite (Bell et al. 1984). Thanks to its high scattering capacity, ferritin is classically used as a probe for electronic microscopy; individual ferritin molecules can be traced as single spots in cryo-TEM.
We prepared ferritin solutions (from 4 to 32 μM) and formed liposomes by methods that simulate spontaneous lipid self assembly, namely the film hydration method (Bangham et al. 1965) and the ethanol injection method (Batzri and Korn 1973). Liposomes, which form instantaneously when the ferritin solutions is mixed with lipids, were made of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylcholine (POPC) or POPC-based mixtures containing 20 mol % oleate or cholesterol. During vesicle formation, a number of ferritin molecules are entrapped inside and cannot leak out because of their large size. The liposome preparation protocol, importantly, did not include freeze–thaw cycles in liquid nitrogen (Mayer et al. 1985), which is a procedure usually done in liposome technology for reducing liposome lamellarity and homogenizing the liposomes content. Clearly, we avoided such additional step because we were interested in the genuine solute encapsulation distribution as obtained just after liposome formation.
After removal of non-entrapped ferritin via size exclusion chromatography, the samples of ferritin-containing vesicles were analysed by cryo-TEM (Luisi et al. 2010).
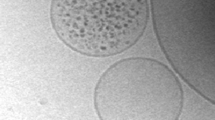
Figure 2a shows a cryo-TEM image reporting two liposomes of similar size which contain, respectively, no ferritin (C in = 0) and about 150 ferritin molecules (C in = 30 μM). The vesicles were prepared starting from the same ferritin solution (C bulk = 8 μM). The Poisson distribution predicts that the existence of both vesicles is highly improbable, because an average content of 40 ± 18 ferritin molecules (mean ± three standard deviations) is expected for vesicles of that size.
Cryo-TEM images of ferritin-containing liposomes. a Two vesicles of approximately similar size show completely different entrapment efficiency. The vesicles (diameter: ca. 260 nm) contain 0 and 150 ferritin molecules,instead of the expected 40 molecules. Observing both vesicles would be highly improbable if the entrapment follows a Poisson distribution. b The same vesicle sample before removing non-entrapped ferritin. It is evident that ferritin molecules do not aggregate with each other or with the lipid membrane. Reproduced from Luisi et al. (2010) with the permission of Wiley
It is evident that ferritin molecules self-concentrate inside these (few “special”) vesicles in a very surprising way and that such a sort of condensed system clearly resembles a successful case of self-organization, i.e., creation of order from a chaotic mixture.
Note that neither the encapsulated nor the free ferritin molecules aggregate under our experimental conditions (cf. Fig. 2b), suggesting that the self-concentration phenomenon is not a trivial solute aggregation process; moreover, strong and stable ferritin/bilayer interactions are also ruled out by directly observing the cryo-TEM images, where it is evident that ferritin molecules are not located near the lipid membrane.
By analysing the data of about 7,700 vesicles, an experimental solute occupancy distribution is achieved. It is characterized by: (i) a large number of empty vesicles (ca. 90 %); (ii) a few vesicles (ca. 9–10 %) whose internal content matches with the expectation; and (iii) very few vesicles (<1 %) containing a large number of ferritin inside, so that the internal ferritin concentration markedly exceeds the expectations (C in > C bulk). Looking at these data one could roughly say that the entrapment of ferritin in spontaneously formed vesicles follows an “all-or-nothing” mechanism.
The experimental distribution is plotted in Fig. 3a together with the expected bell-shaped Poisson distribution in a bilogarithmic plot. Being approximately linear, the experimental distribution can be described by a power law of the form p(N) ~ N −a.
a Comparison between expected Poisson distributions (continuous lines) and the observed occupation frequency (circles) of ferritin-containing liposomes plotted as theoretical or observed frequency versus the number of entrapped ferritin molecules per vesicle (bilogarithmic plot). The linear dependence of log p from log (N + 1), i.e. the power law (slope ca. −2.24), is shown as a dashed line. The concentrations of ferritin solutions are indicated by different colours. Poisson curves refer to vesicles 100 nm in diameter. Note that Poisson distributions converge quickly to zero for high N. b Size dependence of internal ferritin concentration, as obtained by directly measuring the number of ferritin molecules inside each vesicle. Only cases with N/N in >1 have been plotted. The dashed line represents an exponential function that fit the data. Reproduced from Luisi et al. (2010) with the permission of Wiley (Color figure online)
The difference between Poisson and power-law distributions is mostly evident for large N values (corresponding to vesicles containing a large number of solutes): the probability of finding a “super-filled” vesicle is essentially zero according to the Poisson distribution, which predicts only small deviations from the average value. On the contrary, according to the power law, the probability of observing a vesicle that has entrapped a very high number of solutes—an “extreme” in the distribution—is low but not impossible.
When analysed in terms of vesicle size, a clear inverse dependence is observed (Fig. 3b), with the highest local ferritin concentrations found in very small vesicles.
Finally, note that even if a very small fraction of 0.1 % represents the interesting part of the vesicle population, in typical laboratory conditions (1 mM lipid concentration, vesicles of diameter 200 nm) this corresponds to about one million of super-filled vesicles per microliter. Therefore, the phenomenon we are describing is interesting and remarkable even if the value of 0.1 % turned out to be an overestimate of the true super-filled vesicle fraction by one or two orders of magnitude.
In summary, ferritin experiments show that liposomes can concentrate solutes initially present in solution, so as to reach an intra-liposome concentration higher than the bulk value. The concentration factor can reach very high values inside small vesicles (e.g. even more than 30× in vesicles with a diameter of 50–60 nm) but it is typically <10 in 100–200 nm (diameter) vesicles. Intrigued by this initial observation, we then asked whether other solutes may behave similarly.
Ribosomes and Ribo-Peptidic Complexes Encapsulation
Ribosomes are key components of cellular life, as well as of the TX–TL machinery that is often inserted inside liposomes for constructing synthetic cells. Ribosomes can be also detected by cryo-TEM, although as faint particles when compared with ferritin. We, therefore, extended our investigation on spontaneous encapsulation by carrying out experiments with bulk ribosome concentration of 0.48 and 4 μM (Souza et al. 2011).
According to the Poisson distribution, when 100 nm (diameter) vesicles are prepared in these solutions, they should contain on average about 0.15 and 1.26 ribosomes, respectively. If we calculate the Poisson probability of entrapping more ribosomes, for example more than 10 ribosomes inside that same vesicle, this gives such a low value that is nil for all practical purposes.
In contrast with these expectations, the cryo-TEM analysis of ribosome-containing vesicles showed that few vesicles—again a fraction of about 0.1 %—were able to entrap tens of ribosomes (up to hundreds in some cases, especially for large vesicles), as it was observed in ferritin-containing samples. One can go further and calculate from the experimental results the fraction Φ of internal vesicle volume that is occupied by ribosomes, to estimate the degree of crowding inside this type of artificial cell model. By keeping in mind that the close packing limits correspond to Φ ≈ 70 %, we defined a “crowded” solution when 20 % ≤ Φ ≤ 70 %, and a “diluted” solution when Φ < 20 %. Surprisingly, more than one-third of ribosome-containing liposomes contained a “crowded” ribosome solution. In most cases, the internal ribosome concentration was 4–10 times higher than the expectations, with peaks of 25–30×.
A detailed statistical analysis (Souza et al. 2011) revealed that also in this case, the encapsulation follows a power law. Again, the formation of these ribosome-rich vesicles could not be predicted from a Poisson distribution.
Ferritin and ribosomes showed a quite interesting pattern of entrapment, but it would be interesting to check whether solutes that are more primitive would behave similarly. For example, simple macromolecular complexes—which can be taken as model of primitive complexes—would be the best candidates to explore this possibility.
We prepared ribo-peptidic complexes, consisting in 20 nucleotides-long RNA strands complexed with 7.5 kDa (ca. 40 residues long) poly-l-arginine through electrostatic interactions, vaguely resembling the possible structure of primitive ribosomes. The size of these complexes can be controlled by adjusting the proportion between negative and positive charges, as well as mixing rates. After optimisation, we chose 5 nm ribo-peptidic complexes for an entrapment test. Cryo-TEM images were analysed only qualitatively, but the observed pattern (few super-filled and several empty liposomes) matched with the well-detailed cases of ferritin and ribosome encapsulation (Souza et al. 2012).
Our findings suggest that a diluted solution of macromolecules can self-concentrate several times the bulk concentration, but this phenomenon occurs inside a small percentage of vesicles. This left us wondering whether this intriguing effect would give liposomes the capability to concentrate substances and allow intra-liposome reactions that otherwise would be not efficient. In other words, could this phenomenon help to assemble and sustain metabolic-like reactions inside compartments? If so, the role of lipid membranes in the origin of life would not simply be that of providing a confinement to cellular reaction, letting nutrients enter the cell and preventing macromolecules from being lost in the medium (semi-permeability), but they would also play a more active role, facilitating the reaction via a mechanism of solute concentration along with the protocell formation.
TX–TL Reactions as a Realistic Model of Cellular Metabolism
The most important and dramatic consequence of macromolecular self-concentration inside liposomes becomes evident in experiments where realistic mixtures containing metabolic pathway elements are entrapped inside the liposomal lumen, establishing a confined and concentrated reactive mixture. For example, a model reaction with sufficient complexity, namely the coupled TX–TL system, can be employed (actually, our early observations on anomalous entrapment (Souza et al. 2009) were indeed based on TX–TL systems).
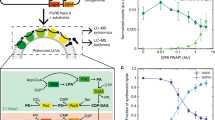
Ueda et al. developed a purified TX–TL systems (the PURE system) containing the minimal number of Escherichia coli enzymes, t-RNAs and ribosomes, as well as amino acids and nucleotides that are able to synthesize a functional protein starting from the corresponding DNA gene (Shimizu et al. 2001, 2005). The PURE system works with about 80 macromolecules (exactly: 83) and about two dozens of small molecules. The incorporation of the PURE system inside liposomes (Fig. 4) mimics the formation of early cells from lipids and a complex molecular set, so that it is a plausible test for investigating the “super-concentration” effect in the case of multi-molecular mixtures of non-trivial complexity. Moreover, the production of a protein is an easily detectable output, especially if the enhanced green fluorescent protein (eGFP) is chosen as the reporter protein (details given in Fischer et al. 2002).
Liposomes encapsulating the whole TX–TL kit are able to produce a protein in their lumen starting from the corresponding DNA. The PURE system (Shimizu et al. 2001, 2005) is a reconstructed TX–TL kit composed by the minimal number of compounds, namely 20 amino acyl-t-RNA synthetase, ribosomes, 10 translation factors, 5 additional enzymes, and 46 t-RNAs (Dong et al. 1996), which, together with the DNA, sum up to 83 different macromolecules, which react according to a modular scheme (transcription, translation, aminoacylation and energy regeneration). Reproduced from Stano et al. (2010) with the permission of Humana Press-Springer, and from Shimizu et al. 2005 with the permission of Elsevier
In order to evidence in the best way the liposome capacity of concentrating multi-molecular mixtures certain particular conditions should be met, namely conditions where the protein production would be somehow limited or made difficult, unless a spontaneous concentration of solutes inside liposomes occurs.
According to the formula N in = N A V C bulk, there are two ways for keeping N in at a low value (and therefore, meeting conditions for poor encapsulation and poor reaction): (1) lowering V (i.e. using small liposomes) and (2) lowering C bulk (i.e. forming liposomes in diluted solutions). In these “hampered” conditions, a successful intra-liposome reaction necessarily implies that the number of encapsulated molecules has exceeded the expected one. The two cases will be presented in “eGFP synthesis inside submicrometer-sized liposomes” and “eGFP synthesis in micrometer-sized vesicles starting from diluted PURE system” sections, respectively.
eGFP Synthesis Inside Submicrometer-Sized Liposomes
As commented in “Motivations and historical developments” section, protein synthesis in sub-micron conventional vesicles (Souza et al. 2009) was actually precedent to the ferritin/ribosome experiments, and it was one of our motivations to investigate the mechanism of solute encapsulation (Luisi et al. 2010; Souza et al. 2011, 2012).
Our group has been involved in pioneer studies on cell-free protein synthesis inside liposomes (Oberholzer et al. 1999; Oberholzer and Luisi 2002; Murtas et al. 2007), but the synthesis of a functional protein inside conventional sub-micron vesicles remained an elusive result. An experimental test based on small vesicles was indeed needed. It has an implication in origin of life scenarios, because some theoretical considerations have suggested that primitive organisms could have been very small in size (Boal et al. 1999). We applied optimised liposome formation protocols to spontaneously obtain liposomes with a diameter below 400 nm in the presence of the PURE system. The liposome size distribution was measured by dynamic light scattering (Fig. 5b) and the progress of eGFP synthesis inside the liposomes was monitored by fluorimetry (Fig. 5a). External protein synthesis was prevented by digesting mRNA or proteins, or by sequestering magnesium ions.
eGFP synthesis inside conventional vesicles. a Time profile of eGFP production; the negative control sample was obtained by co-entrapping EDTA together with the TX–TL kit inside liposomes. Error bars represent the standard deviation of three (eGFP synthesis) and two (negative control) independent samples. b Number-weighted dynamic light scattering size distribution of vesicles obtained by the injection method (recorded at the end of the incubation period). Reproduced from Souza et al. (2009) with the permission of Wiley
The data recorded show that eGFP synthesis occurs inside conventional vesicles with 300 nm average diameter, with an average yield of 50 ± 20 eGFP molecules produced in 1,000 vesicles. This clearly indicates that not all vesicles are able to produce eGFP; very probably only few vesicles were functional, whereas the majority were not. This is per se an intriguing observation, and confirmed the intuitive conclusions that not all vesicles were able to capture at least one copy of all PURE system solutes. However, a careful quantitative statistical analysis reveals that in principle eGFP synthesis would not take place, at any rate, in any vesicle.
Observing eGFP synthesis implies, in fact, that all PURE system macromolecules and the small molecules are successfully entrapped inside vesicles with an average volume of about 14 × 10−18 L (14 aL). This is not an easy task, especially considering that if just one of its components is missing the protein expression would not occur. What really matters here is the co-entrapment statistics. Details can be found in Souza et al. (2009), and the results can be summarized as it follows.Using a Poisson distribution for each of the PURE system components, it is possible to calculate a cumulative co-entrapment probability that estimates how many liposomes of a certain size would entrap at least one copy of each PURE system component (resulting, therefore, in a system capable of synthesizing eGFP). In the case of vesicles with diameter <300 nm, this cumulative probability is of the order of 10−26, i.e., nil for all practical purposes.
Calculations also show that the cumulative co-entrapment probability should reach values that are compatible with the experimental observations only if the concentration of all PURE system components were locally increased by a factor ca. 10–20.
This study revealed that eGFP synthesis inside small vesicles is possible only if multi-component mixtures can be concentrated inside vesicles.
A posteriori, we have realized that these initial observations actually match with the encapsulation following the power-law, as demonstrated in the case of ferritin or ribosomes.
eGFP Synthesis in Micrometer-Sized Vesicles Starting from Diluted PURE System
The second approach for assessing the possibility of concentrating multi-component mixtures within liposomes is based on the formation of liposomes in a diluted PURE system solution (Stano et al. 2013). Micrometer-sized liposomes were used, given their facile detection via fluorescence confocal microscopy. In addition, this experimental design has a strong connection with the origin of life scenarios. In fact, it simulates a quite realistic condition where diluted macromolecular compounds, formed by prebiotic chemical routes, are dissolved in primitive aqueous pools, like fresh water lagoons, but their extreme dilution prevents any efficient reaction.
The rate of protein production rapidly drops off when the PURE system is even moderately diluted (e.g. with one volume of buffer), due to the highly non-linear characteristic of its reaction rate. When liposomes are formed in a diluted PURE system mixture, it is expected (according to our “null hypothesis”) that the intra-liposome concentrations of all PURE system species are equal to the bulk ones, and therefore, that eGFP synthesis inside liposome would fail.
We actually carried out this experiment simply by forming micrometer-sized vesicles in a diluted PURE system mixture, without any other treatment. Surprisingly, as evident in Fig. 6, just a few vesicles resulted to be fluorescent, demonstrating a successful micro-compartmentalized protein synthesis. The number of functional micrometer-sized liposomes was estimated to be around 1 % by a membrane staining procedure (Nile-Red staining).
Confocal microscopy images of POPC liposomes (3.3 mM) prepared by the ethanol injection method in the presence of a diluted PURE system solution (×0.65). In the left panel, bright vesicles are visible against a dark background, which indicates intra-liposomal eGFP synthesis. Central panel displays bright field image. Nile-Red staining (right panel) of eGFP-producing liposomes reveals that the large majority of liposomes do not synthesize the protein. Reproduced from Stano et al. (2013) with the permission of Wiley
In contrast, the absence of significant reactivity outside vesicle (evident by the dark background of Fig. 6), is indeed expected because the PURE system in the extra-liposome environment remains diluted. Finally, a micro-spectrofluorimetric study on the eGFP emission band suggested that the lumen of eGFP-producing vesicles is a crowded-like microenvironment.
The presence of eGFP-synthesizing vesicles, again, is not compatible with the Poissonian expectations. Due to the relatively large vesicle size (diameter ca. 1 μm), the expected average number of encapsulated solute molecules (for each of the PURE system species) is also high, and the stochastic fluctuations around such high value are small. For example, the ribosome concentration in the PURE system is 1.2 μM, which corresponds to about 380 ± 60 molecules (mean ± three standard deviations) inside liposomes of diameter 1 μm. In 1:1 diluted PURE system, the expected number of ribosomes in the same liposome drops to 190 ± 40 (mean ± three standard deviations) and, therefore, Poisson fluctuations would “never” be able to double the number of entrapped ribosomes. Similar conclusions can be obtained for almost all other PURE system species (except for the less concentrated ones), and the final cumulative probability is the product of all these individual very low values. Instead, the long-tailed power law distribution could justify the observations (Mavelli and Stano, submitted manuscript).
Note that previous work (Nomura et al. 2003) has already shown that protein synthesis in giant vesicles (diameter ca. 4–5 μm) can occur with enhanced rate also when a non-diluted TX–TL mixture is used; differences were detected in the first 3 h. The spontaneous concentration of macromolecules, here advocated by us, could possibly explain also these previous observations.
Discussion
Relevance
Both the simple encapsulation experiments with ferritin, ribosomes and ribo-peptidic complex, and the more complex experiments with TX–TL machinery show in a clear way what could have been the active role of self-assembling lipid micro-compartments in promoting primitive chemical reactions.
This is probably the most important message for origin of life research. Under some particular circumstances, macromolecules can be encapsulated efficiently inside liposomes. The resulting structures, in contrast to empty liposomes and to free macromolecules in solution, are capable of effectively developing a proto-metabolism due to the presence of several different types of macromolecules (i.e. with potential catalytic activity), possibly all in high enough concentration and in a confined space. This scenario (Fig. 2a) would explain the origin of a number of functional cells in environmental conditions where—according to standard statistics—this would appear essentially impossible.
Not much is known about these solute-rich structures. They can represent local minima in an energy landscape shaped by local favourable conditions, or they can emerge due to kinetic reasons (kinetic traps). We believe that water-related entropic effects similar to those operating in the “hydrophobic effect” (Tanford 1973) could drive the formation of such structures.
One can envisage two situations where self-concentration effects can be important: the first refers to the facilitation of reactions after intra-liposome concentration. We have shown it by the two examples of TX–TL reactions incorporated inside liposomes. But even without referring to such complex system, simple enzymatic reactions or the formation of molecular complexes can be made possible by concentration effects. For example, diluted enzyme/substrate react sluggishly when [substrate] ≪ K M (the Michaelis–Menten constant), and complex formation proceeds poorly when [ligand] ≪ K d (the complex dissociation constant). Thanks to the described spontaneous concentration of molecules in the vesicle lumen, these reactions would work more efficiently because the concentration of reactants increases locally (inside liposomes). For example, the formation of large non-covalent complexes like proto-ribosomes can be attributed to this effect. The interbreeding reactions between proteins and nucleic acids, as those are preliminary to the origin of the genetic code could also benefit from it. These results leave also room for speculations like fusion events of differently crowded vesicles or breaking and recomposing of vesicles as answer to environmental changes in these prebiotic conditions. These hypothetical considerations may also climax in the question, if a steady growing vesicle or if fusion events of vesicles may have lead to the first cells.
On the Importance of Measuring Individual Entrapment Efficiency (EE) Rather than the Overall EE in Lipid Vesicle Populations
Very few previous studies reported a detailed solute occupancy distribution as derived by microscopy observations. Sun and Chiu (2005) presented a method for the determination of individual vesicle entrapment yields based on single-vesicle photolysis and confocal single-molecule detection, applied to carboxyfluorescein-containing giant vesicles (diameter 3–9 μm). Stamou et al. (Lohse et al. 2008) reported that the EE of a low-molecular weight fluorescent dye (CoroNa Green, 586 Da) in immobilized vesicles with diameter from 0.1 to 0.9 μm is not size independent (i.e. local concentration independent from vesicle size), but follows a 1/diameter trend similar to the ferritin/ribosome findings (cf. Fig. 3b). Analyses were carried out by fluorescent microscopy and a membrane dye was employed to independently measure the vesicle size. Keating, on the other hand, investigated the individual encapsulation efficiency of giant vesicles when prepared—by natural swelling methods—in the presence of carboxyfluorescein, fluorescent dextran derivatives (from 4 to 2,000 kDa) and PEGs (from 5 to 20 kDa), showing that the vesicle population was heterogeneous in terms of solute content (Dominak and Keating 2007). Together with a non-negligible number of solute-poor vesicles, the majority of GVs had internal concentrations of polymer or small molecules equal to or slightly greater than the external concentration. Additional studies focused on “vesicle diversity” (Stano et al. 2014) based on microscopy observations have been carried out by Yoshikawa et al. (Yamaji et al. 2009; Saito et al. 2009; Kato et al. 2012); and by the Danelon group (van Nies et al. 2013). The use of flow cytometry for analysing individual vesicles has also been reported in recent years. In particular, Yomo et al. have exploited the potential of this technique for reporting about the individual encapsulation efficiency and intra-vesicle TX–TL reactivity (Hosoda et al. 2008; Nishimura et al. 2012; Sakakura et al. 2012).
Our approach underlines the importance of looking at the vesicle sample as it emerges from molecular self-organization and without pre-treatment. For example, we avoided submitting solute-filled vesicles to freeze–thaw cycles (as it is usually done when solute-filled vesicles are prepared), because it is known that the resulting vesicles become more homogeneous in terms of solute content (Mayer et al. 1985).
Another important point to be mentioned deals with reproducibility. Although we consistently observed a small number of liposomes containing a high number of encapsulated solutes (ferritin, ribosomes, ribo-peptidic complexed, TX–TL kits), one has to be aware of the fact that these events are essentially stochastic and refer to small number of liposomes. This means that the number of “super-filled” liposomes strongly varies between experiments. On the other hand, the well-documented existence of these structures demonstrates that a route to their formation must exist. Such a route is affected by specific, local (microscopic) and stochastic conditions. Investigating what is the generative mechanism underlying the reported observations is now a priority in our research.
In conclusion, the need of a detailed understanding of vesicle formation and solute encapsulation prompts for more extensive investigation of vesicle populations with techniques that do not average out the differences between vesicles.
Open Questions
Our studies continue since there are still several open questions around to the phenomena reported here, most of them related to its mechanism.
The elucidation of the mechanistic details not only would contribute to fully understanding the role of lipid membranes in the origin of life context, but could also allow the development of novel liposome preparation methods giving a more abundant number of super-filled vesicles, and this would be significant also for future applications based on synthetic cells that require high reaction efficiency.
A list of still open question includes:
-
Do small solutes behave like macromolecules (ferritin, ribosome)?
-
Does the entrapment of nucleic acids polymers or oligomers also obey to a power law? If yes, does nucleic acids length play a role?
-
Since fatty acid vesicles are considered the most plausible model of primitive membranes, will they be able to produce the same phenomenology as the shown cases of phospholipid vesicles? More in general, what is the role of the chemical structure and of the properties of the vesicle lipids?
-
It has been observed that the highest enrichment factors are found in very small vesicles (diameter <60 nm). Why does vesicle size play a role? Are anomalous encapsulation events, like those reported for ferritin and ribosomes, observable also in giant vesicles (diameter >1–2 μm)? (see also Nomura et al. (2003) for a study on TX–TL reaction inside giant vesicle that possibly suggests solute overconcentration).
Additional evidence from experiments concerning the above-mentioned questions will help to understand the mechanistic details of solute self-concentration inside vesicles. For example, preliminary data have shown that anomalous concentration effects, similar to those described in “Ferritin encapsulation” and “Ribosomes and ribo-peptidic complexes encapsulation” sections, can be observed when tRNA and other nucleic acids are entrapped in phospholipid, as well as pure fatty acid vesicles (D’Aguanno et al., manuscript in preparation).
What is the Generative Mechanism Underlying Power Law Solute Distribution?
We have speculated that the anomalous entrapment derives from the interplay between vesicle formation and solute/membrane interaction (Souza et al. 2011). In particular, we proposed a mechanism whereby empty vesicles are formed when an open bilayer disc or surface (Lasic 1988) rapidly closes to give a spherical vesicle [estimated rate ~1 ms−1 (Hernández-Zapata et al. 2009)], whereas solute-filled vesicles derive from bilayer disc/surface that close more slowly because of non-specific interactions (transient adsorption?) of proteins on their surface or edge (Fig. 7). Some hints for this mechanism come also from the vesicle size dependence of internal concentration (Fig. 3b), which suggests that the number of encapsulated molecules scales with the area of open lipid bilayer discs/surfaces and/or with the length of their edge.
A hypothesis about the mechanism of empty and solute-filled vesicle formation. Open bilayers (discs—open surface in three dimensions) are unstable intermediate structures derived from lipid self assembly. These bilayer surfaces, after reaching a sufficient size, can close on themselves and form vesicles (Lasic 1988). Solute capture must occur before or during bilayer closure, bringing about to solute-filled vesicles. We assume that the fast closure of the discs/surfaces brings about empty vesicles, and this is the dominant process within the vesicle population. In contrast, possibly caused by stochastic and non-specific solute-bilayer interaction (which transiently “stabilizes” the disc/surface), a slow closure rate could allow further molecules to be entrapped. Taken from Matteo Allegretti’ Master Thesis (“Liposomes as cellular models: proteins compartmentation”, University of Roma Tre, May 2010)
According to this hypothesis, due to a slower closure rate, more solutes will be incorporated in those membranes that already bound some molecules (a cooperative step). The driving force for this mechanism can be the release of hydration shells, whereas, from a kinetic and physical viewpoint, one should consider that encapsulation is possible only if a pre-vesicle intermediates (lipid discs/surfaces) seals not too fast when compared with their encounter rate with solutes. The latter rate can be estimated by the Smoluchowski theory, and compared with the average closure rate of open lipid discs/surfaces (ca. 1 ms−1) (for a more detailed discussion, see Souza et al. (2011). Can this hypothetical mechanism be proven experimentally? Recently published coarse-grained simulations seem not to support it (van Hoof et al. 2012, 2014), but could stochastic simulations be more informative? Interesting simulations on PURE systems inside vesicles have been already reported (Lazzerini-Ospri et al. 2012; Calviello et al. 2013), but they were not referring to the physical aspects of solute encapsulation. On the other hand, Mavelli has shown that according to very simple hypotheses on the effect of solute adhesion on open bilayers (slowing their closure rate) it is indeed possible to obtain a power-law like distribution (Mavelli et al., manuscript in preparation).
Concluding Remarks
Going back to our initial considerations, one of the inherent difficulties to explain the origin of the first protocells starting with a metabolism outside is mostly due to the fact that the bilayer of phospholipid and fatty acid vesicles is not permeable to macromolecules. With the mechanisms described in this review, instead, the incorporation of biopolymers takes place during—and not after—the formation of the compartments.
Our data and propositions touch on the general scenario of the origin of life. The encapsulation of macromolecules above a threshold concentration, as documented in our work and argued above, can be the preliminary act of an original prebiotic metabolism. Of course our work is not relative to the more basic question, namely the biogenesis of the functional macromolecules, DNA and enzymes in particular. The origin of ordered sequences of DNA and or polypeptides in identical copies is still one of the unsolved questions within the field of the origin of life (Luisi 2007, 2012). Equally unsolved are more specific questions, whether the initial encapsulation for the pristine metabolism took place only once, or many times; and whether the original encapsulation was attended by a re-equilibration of the encapsulated material in the “filled” vesicles. These fascinating questions are still the subject of intense investigations in the field.
Another important aspect to note is that in certain circumstances we have observed intra-liposomal solute concentration reaching values similar to the macromolecular crowding regime of biological cells. It is known that in crowding conditions, the aqueous volume available for other molecules in solution is reduced, and this in turn modify (enhances) the molecular reactivity (Zhou et al. 2008) and facilitates the protein folding (Tokuriki et al. 2004).
In conclusion, we believe that the new finding of the spontaneous concentration and crowding may help to solve the old, unsolved question of the necessary high local concentration of reactants in protocellular systems and then, in principle, the origin of metabolism and cellular structures at the same time.
References
Bangham AD, Standish MM, Watkins JC (1965) Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol 13:238–252
Batzri S, Korn ED (1973) Single bilayer liposomes prepared without sonication. Biochim Biophys Acta 298:1015–1019. doi:10.1016/0005-2736(73)90408-2
Bell SH, Weir MP, Dickson DP et al (1984) Mössbauer spectroscopic studies of human haemosiderin and ferritin. Biochim Biophys Acta 787:227–236
Berclaz N, Blochliger E, Muller M, Luisi PL (2001a) Matrix effect of vesicle formation as investigated by cryotransmission electron microscopy. J Phys Chem B 105:1065–1071. doi:10.1021/jp002151u
Berclaz N, Muller M, Walde P, Luisi PL (2001b) Growth and transformation of vesicles studied by ferritin labeling and cryotransmission electron microscopy. J Phys Chem B 105:1056–1064. doi:10.1021/jp001298i
Blain JC, Szostak JW (2014) Progress toward synthetic cells. Annu Rev Biochem. doi:10.1146/annurev-biochem-080411-124036
Boal et al., Steering Group for the Workshop on Size Limits of Very Small Microorganisms, National Research Council (1999) Size limits of very small microorganisms: proceedings of a workshop. The National Academies Press, Washington, D.C
Calviello L, Stano P, Mavelli F et al (2013) Quasi-cellular systems: stochastic simulation analysis at nanoscale range. BMC Bioinformatics 14:S7. doi:10.1186/1471-2105-14-S7-S7
Caschera F, Sunami T, Matsuura T et al (2011) Programmed vesicle fusion triggers gene expression. Langmuir ACS J Surf Colloids 27:13082–13090. doi:10.1021/la202648h
Dominak LM, Keating CD (2007) Polymer encapsulation within giant lipid vesicles. Langmuir 23:7148–7154. doi:10.1021/la063687v
Dong H, Nilsson L, Kurland CG (1996) Co-variation of tRNA abundance and codon usage in Escherichia coli at different growth rates. J Mol Biol 260:649–663
Fischer A, Franco A, Oberholzer T (2002) Giant vesicles as microreactors for enzymatic mRNA synthesis. ChemBioChem 3:409–417. doi:10.1002/1439-7633
Hernández-Zapata E, Martínez-Balbuena L, Santamaría-Holek I (2009) Thermodynamics and dynamics of the formation of spherical lipid vesicles. J Biol Phys 35:297–308
Hosoda K, Sunami T, Kazuta Y et al (2008) Quantitative study of the structure of multilamellar giant liposomes as a container of protein synthesis reaction. Langmuir ACS J Surf Colloids 24:13540–13548. doi:10.1021/la802432f
Ichihashi N, Matsuura T, Kita H, Sunami T, Suzuki H, Yomo T (2012) Constructive approaches for the origin of life. In: Seckbach J (ed) Genesis-in the beginning: precursors of life, chemical models and early biological evolution. Cellular origin, life in extreme habitats and astrobiology (Springer) vol 22, pp 289–303. doi:10.1007/978-94-007-2941-4_17
Kato A, Yanagisawa M, Sato YT et al (2012) Cell-sized confinement in microspheres accelerates the reaction of gene expression. Sci Rep 2:283. doi:10.1038/srep00283
Kita H, Matsuura T, Sunami T et al (2008) Replication of genetic information with self-encoded replicase in liposomes. Chembiochem Eur J Chem Biol 9:2403–2410. doi:10.1002/cbic.200800360
Kurihara K, Tamura M, Shohda K, Toyota T, Suzuki K, Sugawara T (2011) Self-reproduction of supramolecular giant vesicles combined with the amplification of encapsulated DNA. Nat Chem 3:775–781. doi:10.1038/nchem.1127
Lasic DD (1988) The mechanism of vesicle formation. Biochem J 256:1–11
Lazzerini-Ospri L, Stano P, Luisi P, Marangoni R (2012) Characterization of the emergent properties of a synthetic quasi-cellular system. BMC Bioinformatics 13(Suppl 4):S9. doi:10.1186/1471-2105-13-S4-S9
Lohse B, Bolinger P-Y, Stamou D (2008) Encapsulation efficiency measured on single small unilamellar vesicles. J Am Chem Soc 130:14372–14373. doi:10.1021/ja805030w
Luisi PL (2006) The emergence of life: from origins of life to synthetic biology. Cambridge University Press, Cambridge
Luisi PL (2007) Question 3: the problem of macromolecular sequences: the forgotten stumbling block. Orig Life Evol Biosph 37:363–365
Luisi PL (2012) An open question on the origin of life: the first forms of metabolism. Chem Biodivers 9:2635–2647. doi:10.1002/cbdv.201200281
Luisi PL, Walde P, Oberholzer T (1999) Lipid vesicles as possible intermediates in the origin of life. Curr Opin Colloid Interface Sci 4:33–39. doi:10.1016/S1359-0294(99)00012-6
Luisi PL, Ferri F, Stano P (2006) Approaches to semi-synthetic minimal cells: a review. Naturwissenschaften 93:1–13. doi:10.1007/s00114-005-0056-z
Luisi PL, Allegretti M, Souza TP et al (2010) Spontaneous protein crowding in liposomes: a new vista for the origin of cellular metabolism. ChemBioChem 11:1989–1992. doi:10.1002/cbic.201000381
Maeda YT, Nakadai T, Shin J et al (2012) Assembly of MreB filaments on liposome membranes: a synthetic biology approach. ACS Synth Biol 1:53–59. doi:10.1021/sb200003v
Mansy SS, Schrum JP, Krishnamurthy M et al (2008) Template-directed synthesis of a genetic polymer in a model protocell. Nature 454:U10–U122. doi:10.1038/nature07018
Martini L, Mansy SS (2011) Cell-like systems with riboswitch controlled gene expression. Chem Commun 47:10734–10736. doi:10.1039/c1cc13930d
Mayer LD, Hope MJ, Cullis PR, Janoff AS (1985) Solute distributions and trapping efficiencies observed in freeze-thawed multilamellar vesicles. Biochim Biophys Acta 817:193–196
Monnard P-A, Deamer DW (2002) Membrane self-assembly processes: steps toward the first cellular life. Anat Rec 268:196–207. doi:10.1002/ar.10154
Moritani Y, Nomura SM, Morita I, Akiyoshi K (2010) Direct integration of cell-free-synthesized connexin-43 into liposomes and hemichannel formation. FEBS J 277:3343–3352. doi:10.1111/j.1742-4658.2010.07736.x
Murtas G, Kuruma Y, Bianchini P et al (2007) Protein synthesis in liposomes with a minimal set of enzymes. Biochem Biophys Res Commun 363:12–17. doi:10.1016/j.bbrc.2007.07.201
Nishimura K, Matsuura T, Nishimura K et al (2012) Cell-free protein synthesis inside giant unilamellar vesicles analyzed by flow cytometry. Langmuir 28:8426–8432. doi:10.1021/la3001703
Noireaux V, Libchaber A (2004) A vesicle bioreactor as a step toward an artificial cell assembly. Proc Natl Acad Sci USA 101:17669–17674. doi:10.1073/pnas.0408236101
Nomura S, Tsumoto K, Hamada T et al (2003) Gene expression within cell-sized lipid vesicles. ChemBioChem 4:1172–1175. doi:10.1002/cbic.200300630
Nourian Z, Danelon C (2013) Linking genotype and phenotype in protein synthesizing liposomes with external supply of resources. ACS Synth Biol 2:186–193. doi:10.1021/sb300125z
Oberholzer T, Luisi PL (2002) The use of liposomes for constructing cell models. J Biol Phys 28:733–744. doi:10.1023/A:1021267512805
Oberholzer T, Wick R, Luisi PL, Biebricher CK (1995) Enzymatic RNA replication in self-reproducing vesicles: an approach to a minimal cell. Biochem Biophys Res Commun 207:250–257. doi:10.1006/bbrc.1995.1180
Oberholzer T, Nierhaus KH, Luisi PL (1999) Protein expression in liposomes. Biochem Biophys Res Commun 261:238–241. doi:10.1006/bbrc.1999.0404
Pohorille A, Deamer D (2002) Artificial cells: prospects for biotechnology. Trends Biotechnol 20:123–128. doi:10.1016/S0167-7799(02)01909-1
Rasmussen S, Chen LH, Nilsson M, Abe S (2003) Bridging nonliving and living matter. Artif Life 9:269–316. doi:10.1162/106454603322392479
Rasmussen S, Bedau MA, Chen L, Deamer D, Krakauer DC, Packard NH, Stadler PF (eds) (2009) Protocells. bridging nonliving and living matter. MIT Press, Cambridge MA
Saito H, Kato Y, Le Berre M et al (2009) Time-resolved tracking of a minimum gene expression system reconstituted in giant liposomes. ChemBioChem 10:1640–1643. doi:10.1002/cbic.200900205
Sakakura T, Nishimura K, Suzuki H, Yomo T (2012) Statistical analysis of discrete encapsulation of nanomaterials in colloidal capsules. Anal Methods 4:1648–1655. doi:10.1039/C2AY25105A
Shimizu Y, Inoue A, Tomari Y et al (2001) Cell-free translation reconstituted with purified components. Nat Biotechnol 19:751–755. doi:10.1038/90802
Shimizu Y, Kanamori T, Ueda T (2005) Protein synthesis by pure translation systems. Methods 36:299–304. doi:10.1016/j.ymeth.2005.04.006
Souza TP, Stano P, Luisi PL (2009) The minimal size of liposome-based model cells brings about a remarkably enhanced entrapment and protein synthesis. ChemBioChem 10:1056–1063. doi:10.1002/cbic.200800810
Souza TP, Steiniger F, Stano P et al (2011) Spontaneous crowding of ribosomes and proteins inside vesicles: a possible mechanism for the origin of cell metabolism. ChemBioChem 12:2325–2330. doi:10.1002/cbic.201100306
Souza TP, Stano P, Steiniger F, D’Aguanno E, Altamura E, Fahr A, Luisi PL (2012) Encapsulation of ferritin, ribosomes, and ribo-peptidic complexes inside liposomes: insights into the origin of metabolism. Orig Life Evol Biosph 42:421–428. doi:10.1007/s11084-012-9303-4
Stano P, Kuruma Y, Souza TP, Luisi PL (2010) Biosynthesis of proteins inside liposomes. Methods Mol Biol 606:127–145. doi:10.1007/978-1-60761-447-0_11
Stano P, Carrara P, Kuruma Y et al (2011) Compartmentalized reactions as a case of soft-matter biotechnology: synthesis of proteins and nucleic acids inside lipid vesicles. J Mater Chem 21:18887–18902. doi:10.1039/c1jm12298c
Stano P, Rampioni G, Carrara P et al (2012) Semi-synthetic minimal cells as a tool for biochemical ICT. Biosystems 109:24–34. doi:10.1016/j.biosystems.2012.01.002
Stano P, D’Aguanno E, Bolz J et al (2013) A remarkable self-organization process as the origin of primitive functional cells. Angew Chem Int Ed Engl 52:13397–13400. doi:10.1002/anie.201306613
Stano P, Souza TP, Carrara P, Altamura E, D’Aguanno E, Caputo M, Luisi PL, Mavelli F (2014) Recent biophysical issues about the preparation of solute-filled lipid vesicles. Mech Adv Mater Struct. doi:10.1080/15376494.2013.857743
Sun BY, Chiu DT (2005) Determination of the encapsulation efficiency of individual vesicles using single-vesicle photolysis and confocal single-molecule detection. Anal Chem 77:2770–2776. doi:10.1021/ac048439n
Sunami T, Sato K, Matsuura T et al (2006) Femtoliter compartment in liposomes for in vitro selection of proteins. Anal Biochem 357:128–136. doi:10.1016/j.ab.2006.06.040
Szostak JW, Bartel DP, Luisi PL (2001) Synthesizing life. Nature 409:387–390. doi:10.1038/35053176
Tanford Charles (1973) The hydrophobic effect: formation of micelles and biological membranes. Wiley, New York, NY
Tokuriki N, Kinjo M, Negi S et al (2004) Protein folding by the effects of macromolecular crowding. Protein Sci 13:125–133. doi:10.1110/ps.03288104
Torino D, Martini L, Mansy SS (2013) Piecing together cell-like systems. Curr Org Chem 17:1751–1757. doi:10.2174/13852728113179990082
Van Hoof B, Markvoort AJ, van Santen RA, Hilbers PAJ (2012) On protein crowding and bilayer bulging in spontaneous vesicle formation. J Phys Chem B 116:12677–12683. doi:10.1021/jp3062306
Van Hoof B, Markvoort AJ, van Santen RA, Hilbers PAJ (2014) Molecular simulation of protein encapsulation in vesicle formation. J Phys Chem B 118:3346–3354. doi:10.1021/jp410612k
Van Nies P, Nourian Z, Kok M et al (2013) Unbiased tracking of the progression of mRNA and protein synthesis in bulk and in liposome-confined reactions. ChemBioChem 14:1963–1966. doi:10.1002/cbic.201300449
Walde P (2006) Surfactant assemblies and their various possible roles for the origin(s) of life. Orig Life Evol Biosph 36:109–150. doi:10.1007/s11084-005-9004-3
Walde P (2010) Building artificial cells and protocell models: experimental approaches with lipid vesicles. BioEssays 32:296–303. doi:10.1002/bies.200900141
Yamaji K, Kanai T, Nomura SM, Akiyoshi K, Negishi M, Chen Y, Atomi H, Yoshikawa K, Imanaka T (2009) Protein synthesis in giant liposomes using the in vitro translation system of Thermococcus kodakaraensis. IEEE Trans Nanobiosci 8:325–331
Yu W, Sato K, Wakabayashi M et al (2001) Synthesis of functional protein in liposome. J Biosci Bioeng 92:590–593
Zhou H-X, Rivas G, Minton AP (2008) Macromolecular crowding and confinement: biochemical, biophysical, and potential physiological consequences. Annu Rev Biophys 37:375–397. doi:10.1146/annurev.biophys.37.032807.125817
Acknowledgments
This review summarizes the results obtained in the last years by the joint efforts of P. L. Luisi and A. Fahr groups, starting within the EU-FP6 SYNTHCELLS project (Approaches to the Bioengineering Synthetic Minimal Cells, Nr. 043359). We thank Fabio Mavelli (University of Bari, Italy) for discussion on stochastic simulations. T. Pereira de Souza was supported by the Alexander von Humboldt Foundation as a post-doctoral fellow in Jena. A more extensive version of this review can be found in Luisi (2012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Souza, T.P., Fahr, A., Luisi, P.L. et al. Spontaneous Encapsulation and Concentration of Biological Macromolecules in Liposomes: An Intriguing Phenomenon and Its Relevance in Origins of Life. J Mol Evol 79, 179–192 (2014). https://doi.org/10.1007/s00239-014-9655-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-014-9655-7