Abstract
A model for abiotic photophosphorylation of adenosine diphosphate by orthophosphate with the formation of adenosine triphosphate was studied. The model was based on the photochemical activity of the abiogenic conjugates of pigments with the polymeric material formed after thermolysis of amino acid mixtures. The pigments formed showed different fluorescence parameters depending on the composition of the mixture of amino acid precursors. Thermolysis of the mixture of glutamic acid, glycine, and lysine (8:3:1) resulted in a predominant formation of a pigment fraction which had the fluorescence maximum at 525 nm and the excitation band maxima at 260, 375, and 450 nm and was identified as flavin. When glycine in the initial mixture was replaced with alanine, a product formed whose fluorescence parameters were typical to pteridines (excitation maximum at 350 nm, emission maximum at 440 nm). When irradiated with the quasi-monochromatic light (over the range 325–525 nm), microspheres in which flavin pigments were prevailing showed a maximum photophosphorylating activity at 375 and 450 nm, and pteridine-containing chromoproteinoid microspheres were most active at 350 nm. The positions and the relative height of maxima in the action spectra correlate with those in the excitation spectra of the pigments, which point to the involvement of abiogenic flavins and pteridines in photophosphorylation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adenosine-5′-triphosphate (ATP) is an energy-rich molecule and its main function in all modern organisms is the storage and transfer of chemical energy. This function is realized due to the formation and hydrolysis of phosphoanhydride bonds between the phosphoryl residues in ATP molecule. These bonds are formed upon the synthesis of ATP from adenosine diphosphate (ADP) and orthophosphate and this phosphorylation reaction needs the inflow of energy.
In the contemporary biosphere, the major energy source for ATP synthesis is solar radiation which is utilized by the photosynthesis apparatus. Through photosynthetic phosphorylation, which takes place in the thylakoid membranes of plant chloroplasts and in the chromatophores of photosynthetic bacteria, the photon energy is converted to the chemical energy of ATP. In non-photosynthesizing organisms, the energy source for ATP synthesis is oxidation of organic products formed during photosynthesis.
Along with ATP, there are other high-energy phosphates, which store energy in organisms. The question is debated whether the ATP is the oldest form of energy conservation or, in the primitive organisms, simpler molecules of inorganic condensed phosphates played this role. Some phototrophic bacteria in addition to membrane-bound ATP synthase contain a proton-pumping inorganic pyrophosphate synthase, which couples the energy liberated from light driven electron transport to synthesis of inorganic pyrophosphate (Baltscheffsky and Baltscheffsky 1995). However, even in these organisms pyrophosphate cannot substitute ATP as an essential chemical component of the modern-type cell, because in its absence neither metabolism nor the functioning of the genetic apparatus is possible.
Due to the key role of ATP in metabolism, the problem of abiotic formation of ATP attracted the attention of researchers of chemical evolution (Schwartz and Ponnamperuma 1968; Bahn and Fox 1981; Cheng et al. 2002; Julian and Beauchamp 2003; Holm and Andersson 2005). The question arises whether the chemical evolution could lead to the formation of a system that converts light energy into the energy of ATP?
In previous publications, we reported the development of a chemical model of the abiogenic photophosphorylating system which induces the phosphorylation of ADP by the orthophosphoric acid residue thus yielding ATP (Kolesnikov 1991; Kritsky et al. 2007; Kolesnikov et al. 2008). The photocatalyst for the system was an abiogenic supramolecular product, chromoproteinoid, arising in process of amino acid thermolysis. Besides unordered amino acid polymer, the chromoproteinoid contained flavin and pteridine pigments partially conjugated to proteinoid matrix (Heinz et al. 1979; Heinz and Ried 1981, 1984; Kolesnikov and Kritsky 2001). Similar to biological flavins, the abiogenic flavins demonstrated photochemical activity in electron transfer reactions (Kolesnikov and Kritsky 2001).
The pigmented chromoproteinoid material demonstrated photophosphorylating activity only when aggregated into supramolecular structures such as proteinoid microspheres (Fox and Dose 1972). This model of chemical processes occurring in the abiotic environment is an excellent source of photochemically active pigments immobilized on the matrix. The matrix is a complex of proteinoid microsphere with silicate. Under the influence of light, the suspension of these structures catalyzed the phosphorylation of ADP by orthophosphate to form ATP. The rate of 5′-adenosine monophosphate (AMP) phosphorylation to ADP was almost an order of magnitude slower than the conversion of ADP to ATP. A higher activity of the model in the presence of electron acceptor such as atmospheric dioxygen or hydrogen peroxide indicated that the photoprocess involved the reduction of the photoreceptor pigment and this pigment needed reoxidation to make the process cyclic. Under the condition of oxidative regeneration of the photoreceptor, the model could transform to ATP up to 20–30 % of the ADP molecules present in the reaction mixture (Kolesnikov et al. 2008). The photophosphorylation process depended on the blue light and ultraviolet radiation, i.e., on the spectral areas absorbed by the isoalloxazine heterocycle of the flavin molecule, which led us to assume that the abiogenic flavin pigments were involved in photophosphorylation.
The maxima of light absorption by a neutral aqueous solution of flavin, such as riboflavin or FMN, at room temperature are at 450, 375, and 260 nm. The absorption of a photon brings flavin molecule to an excited singlet state which can be converted into the triplet state. Flavins in the excited triplet state (3Fl) generate singlet oxygen (1O2) at a yield of about 50 % (Egorov et al. 1999). Besides, they possess a high photochemical activity and enter into reactions of electron transfer to yield free radical product—flavin semiquinone (FlH•). Excited flavin is much more electrophilic than the ground state molecule (E ’o of the pair 3Fl/FlH• is 1.8 V instead of −0.3 V for Fl/FlH•), and its interaction with a high potential donor yields products accumulating free energy (Heelis 1982). In modern organisms flavins display photochemical activity as chromophores of a number of photoenzymes and sensory photoreceptor proteins (Sancar 2003; Losi and Gärtner 2011, 2012).
During thermolysis of an amino acid mixture, not only flavins but also structurally related pteridines are formed (Heinz et al. 1979; Heinz and Ried 1981, 1984). Pteridines, as well as flavins, are photochemically active molecules (Kritsky et al. 1997; Lorente et al. 2011), and one might expect that they are also involved in photophosphorylation. Unlike flavins, pteridines do not absorb the visible light—their light absorption bands fall within the ultraviolet (UV-A and UV-B) region of the spectrum. The pteridine derivatives, pterins (2-amino-4-pteridinones) as well as flavins are involved in reception of light by organisms (Sancar 2003; Losi and Gärtner 2011).
The goal of the present research was to answer the question if abiogenic flavins and pteridines are really light receptors upon ATP synthesis in a proteinoid model. For this purpose we studied the dependence of ATP production rate on the wavelength of exciting light. The comparison of these action spectra with the excitation spectra of pigment fluorescence, as well as with the absorption spectra of standard preparations showed that the photophosphorylation was really related to the activity of abiogenic flavins and pteridines. A change in the composition of the precursor amino acid mixture influenced the structure of pigments present in the product thus resulting in shifts in the spectral parameters and in the efficiency of the photophosphorylation process.
Methods
Chemicals
Amino acid preparations, 5′-AMP (sodium salt), ADP (monopotassium salt), and ATP (disodium salt) were from Sigma-Aldrich and had 98 % purity. FMN, H2O2, and all other chemicals and mineral salts were also from Sigma-Aldrich and had purity no less than 98 %. Pterin (2-amino-4-oxopteridine) was from Schirks Laboratories, Switzerland. The solution of orthosilicic acid was prepared by fusion of calcined and ground SiO2 (granular silica gel from Silica Gel Ltd., UK) with dry Na2CO3 in a platinum melting pot at 900 °C, by subsequent solution of the cooled melt in water, and by acidification of the resulting solution with 2 N H2SO4 to pH ≈ 1.5.
Thermal Condensation of Amino Acids
Pigment-containing proteinoids were obtained by thermolysis of a finely dispersed homogenous mixture of dry amino acid preparations. As a starting material for the synthesis we used the combination of amino acids reduced to three components (glutamic acid, glycine, and lysine in a molar ratio of 8:3:1). This composition of the amino acid precursors mixture was empirically selected to provide the highest yield of flavin pigment products (Kolesnikov and Kritsky 2001). The yield of flavin (isoalloxasine) pigments was about 1.5–2.0 % of the initial amino acid mixture. Replacement of glycine in the above-mentioned substrate mixture to an equimolar quantity of alanine resulted in the fact that the predominant pigment product of thermolysis became not isoalloxazines, but pteridines.
The mixture was heated in an evacuated glass vessel at 185 °C for 6 h. Besides the amino acid polymer, the thermolysis product contained flavin derivatives (in a free state and in the form of conjugates of 8–10 kDa with amino acid polymers) (Kolesnikov and Kritsky 2001).
Preparation of Proteinoid Microsphere Suspensions
Proteinoid microspheres were obtained after the protocol described in our previous articles (Kritsky et al. 2007; Kolesnikov et al. 2008). Proteinoid preparation (32 mg of dark colored mass formed after thermolysis) was dissolved in 9 ml of water at 100 °C and 1 ml of a freshly prepared solution of 0.2 M sodium silicate was added. The solution of proteinoid and sodium silicate was cooled down to room temperature during 20 min. Sodium silicate solution was prepared by adding 2 N NaOH to ortholisic acid solution in 2 N H2SO4 up to pH 7. One milliliter of the obtained suspension contained 800 μg of proteinoid, and the silicate concentration was equivalent to 2 × 10−2 M of monomeric silicon dioxide. In the presence of silicate, the microspheres retained their structural stability at pH ≥ 7.8, which made possible studying photophosphorylation under pH values most favorable for this reaction. The proteinoid preparation obtained by thermolysis of the mixture of glutamic acid, alanine, and lysine was converted to microspheres according to an analogous technique, i.e., by treatment with hot water in the presence of silicate with subsequent cooling of the solution. All operations with the products of amino acids thermolysis used in photophosphorylation experiments were carried out in the sterile box.
Fluorescence Spectra of the Products of Thermolysis of Amino Acid Mixtures
To measure absorption and fluorescence spectra, aqueous solutions of proteinoids were used. The absorption spectra of pigments were measured on a SF 2000 spectrophotometer (Spektr, Russia). Fluorescence emission spectra and fluorescence excitation spectra were registered on a DU-650 spectrofluorimeter (Shimadzu, Japan) or on a Fluorat-02-Panorama spectrofluorimeter (Lumex, Russia). Based on the scanning results, three-dimensional (the 3D) diagrams were built, in which on the horizontal axes (X and Y) were the wavelengths of exciting and emitted light, respectively, and on the vertical axis (Z)—the intensity of fluorescence emission in relative units.
Assay of Photophosphorylating Activity of Pigment-Containing Microspheres
Experimental mixture of 2.0 ml contained 500 μl of microsphere suspension (0.8 mg chromoproteinoid per 1.0 ml of the mixture; the SiO2 concentration in this mixture was 5 × 10−3 M), 10−3 M ADP (ATP impurity did not exceed 0.3 %), and 0.2 M sodium phosphate buffer (pH 7.8). A lower concentration of phosphate buffer did not provide sufficient buffer capacity to maintain in the incubation mixture pH value ≥ 7.8 (at lower pH phosphorylation reaction does not take place), and we found the introduction of additional buffer compounds to the experimental mixture to be undesirable. Earlier we showed that in the presence of millimolar concentrations of H2O2, the photophosphorylation occurred more efficiently than in the presence of saturating concentration of atmospheric oxygen (the ATP molar yield was ca. 30 % against 20 %, respectively) (Kolesnikov et al. 2008). Because of this, H2O2 was added to the incubation mixture, as an oxidant. The concentration of H2O2 in experimental mixture was 5 × 10−3 M. To prevent microbial contamination, all working solutions used in the reaction mixture (except the microsphere suspension) were passed through the Millex GS filter with pore diameter 0.22 μm (Millipore, Ireland). Samples placed to quartz cuvettes (optical pathlength 1 cm) were evacuated prior to incubation and thermostated at 19–21 °C with water flow in the course of incubation.
Irradiation Conditions
For non-monochromatic irradiation of experimental mixtures we used a high-pressure mercury arc lamp DRL-400 (Lisma VNIIS, Russia) (the flux level in cuvette was 70 W m−2). The experimental mixtures were placed into the molybdenum glass cuvettes which transmitted radiation with λλ ≥ 0.320 nm.
To measure the spectral dependence of the reaction rate of phosphorylation reaction the samples were irradiated from a DKsSH-150 xenon arc lamp (Kontest, Russia) with a monochromator, which provided a quasi-monochromatic light flow at a slit width of 40 nm. The radiating power intensity at the level of sample to be irradiated varied within 1.2–1.6 W m−2 depending on wavelength. Measurements of the photophosphorylating activity of experimental samples containing a microsphere suspension and reaction substrates were performed under irradiation with the light in the wavelength range from 325 to 525 nm. Each version of the action spectra experiments was conducted at least three times. Preliminary experiments have shown that exposure to light of higher wavelengths did not induce phosphorylation.
After completion of irradiation, microspheres were removed from the aqueous phase by filtering through a Millex-HV filter with a pore diameter of 0.45 μm.
The Determination of ATP Content
The ATP content in the samples was determined by a luminescent method using the luciferin–luciferase reaction (Strehler and Totter 1954). The luminescence intensity was measured with a luminometer Biotox-7 (Biotox, Russia). The scatter of parallel measurements on the luminometer did not exceed ±5 %. The number of parallel luminometric measurements of ATP content in the samples varied from two to four (the variation in analytical data did not exceed ±5 % of the mean). The control samples incubated in the dark were treated similarly. In treating the data, the results of control dark experiments were subtracted from the experimental data before presenting in figures and table.
In a previous study of the model, in parallel with the luminometric measurement of the ATP concentration, we determined the concentration of ATP using HPLC separation of adenosine phosphates present in the reaction mixture (Kolesnikov et al. 2008). In the present research, the chromatographic verification of the luminometric measurements was also performed to validate the luminometric data, but not in each experiment. The substrates and reaction products were separated with HPLC Stayer chromatograph (Aquilon, Russsia); Synergi column, 4 μm, Hydro-RP 80A, 4.6 × 250 mm (Phenomenex, USA); elution with 0.1 M KH2PO4, pH 6.0, containing 0.5 % acetonitrile. The compounds eluting from the column were monitored from changes in optical density of the eluate at 260 nm and were identified by the time of their retention on the column. To estimate quantitatively the content of nucleoside phosphates, the column was calibrated with their standard preparations. The loss of ADP concentration in process of photoincubation was equimolar to the increase of ATP.
To check a possible formation of inorganic pyrophosphate (PPi) in photophosphorylation reaction we analyzed the reaction products by a colorimetric method (Heinonen et al. 1981) and two different chromatographic methods—HPLC separation (Yoza et al. 1991) and the paper chromatography (Grunze und Thilo 1954).
The Plotting of Action Spectra
Action spectra were represented as a graph, which reflects the dependence of the amount of ATP synthesized on the wavelength of the inducing light. The data for plotting were taken from the curves representing the time course of accumulation of ATP in the samples irradiated with light of a fixed wavelength (Ahmad et al. 2002; Van Vickle-Chavez and Van Gelder 2006). The measurement was performed in the wavelength range from 325 to 525 nm, in steps of 25 nm. Preliminary experiments have shown that light with longer wavelength did not induce phosphorylation. Duration of light exposure was chosen so that the time course of ATP accumulation was linear. For flavin-containing microspheres that time was 20 min, and for pterins-containing—40 min. Amount of ATP formed by irradiation with light of different wavelengths was normalized by the number of quanta of incident light, expressed in moles of photons (Einsteins). The diagram contains the arithmetical means for ATP amount formed upon irradiation with the light of each wavelength studied and the SEM values.
Results
Fluorescent Characteristics of Abiogenic Pigments
Pigments formed together with amino acid polymers during thermolysis of the amino acid mixture were studied by means of two-dimensional scanning of fluorescence excitation spectra and fluorescence emission spectra. Analysis of the 3D spectra permitted us to characterize positions of bands corresponding to the transition of pigment molecules present in proteinoid preparations from the ground state to excited singlet states (fluorescence excitation), as well as their transition from the excited singlet state (S1) to the ground state (fluorescence emission).
Figure 1 shows the results of scanning the fluorescence excitation and fluorescence emission spectra of chromoproteinoid solutions obtained by thermolysis of the mixture glutamic acid, glycine, and lysine (8:3:1). In the emission spectrum of the preparation, a band with a maximum of 525 nm prevailed, to which the excitation bands with maxima at 260, 375, and 450 nm corresponded. The maxima of the excitation spectra are practically coincident with the position of bands corresponding to the energies of the transition of flavins, such as riboflavin or riboflavin-5′-phosphate (FMN), from the ground to singlet excited state. The position of the fluorescence maximum corresponds to the energy of the radiative deactivation of the excited singlet state S1 of the flavin molecules (Heelis 1982). Analysis of the emission spectrum of this preparation also revealed a weak radiation with a maximum about 440 nm, to which an excitation band with a maximum of 350 nm, typical for the S0 to S1 transition of pteridines, including many biological pterins, corresponded.
Three-dimensional surfaces depicting fluorescence emission intensity (arbitrary units) as a function of excitation and emission wavelengths for aqueous solutions of pigment-containing products of thermolysis of amino acid mixtures. a Chromoproteinoid formed by thermolysis of the mixture Glu, Gly, and Lys (8:3:1). b Chromoproteinoid formed by thermolysis of the mixture Glu, Ala, and Lys (8:3:1)
The presence of two spectrally different pigments: flavins and pteridines in the proteinoid preparation is readily evidenced by its fluorescence emission spectra where peaks at 525 and 440 nm are present (Fig. 2). The maxima at λ excit 350, 375 и 450 nm in the excitation spectra correspond to these emission maxima. The position of the spectral maxima and their intensity ratio point to that flavin pigments dominated in this preparation and, besides, a minor pteridine component was present. Except for pigments, whose fluorescence correspond to flavins and pteridines, no other fluorescing compounds were found in amino acid thermolysis products.
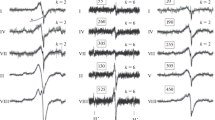
Fluorescence spectra of the aqueous solution of a chromoproteinoid preparation formed by thermolysis of the mixture Glu, Gly, and Lys (8:3:1). a Spectra of fluorescence emission excited by radiation at a wavelength of λ exc: 1–340 nm, 2–375 nm, and 3–450 nm. b Excitation spectra corresponding to fluorescence emission at: 1–440 nm and 2–525 nm
The Influence of the Precursors Composition on the Structure of the Pigment
Replacing glycine in the starting amino acid mixture (glutamic acid, lysine, and glycine; 8:3:1) with an equimolar amount of alanine resulted in a change in the composition of the thermolysis pigment product. As a consequence, in the fluorescence emission spectrum (Fig. 3) of the proteinoid preparation obtained in thermolysis of a mixture of glutamic acid, lysine, and alanine in the molar ratio 8:3:1, a band with a maximum at 440 nm prevailed, which is characteristic of pteridines fluorescence.
Fluorescence excitation and emission spectra of the aqueous solution of a chromoproteinoid formed by thermolysis of the mixture Glu, Ala, and Lys (8:3:1). 1. Fluorescence emission excited by radiation at a wavelength of 340 nm; 2. Fluorescence emission excited by radiation with a wavelength of 375 nm; 3. Fluorescence emission excited at a wavelength of 450 nm; 4. The fluorescence excitation spectrum corresponding to emission at a wavelength of 440 nm
The fluorescence emission with a maximum at 525 nm typical for flavins was in this case much less pronounced. As with the proteinoid product formed upon thermolysis of the mixture glutamic acid–lysine–glycine, the scanning of fluorescence emission and excitation spectra of this preparation did not show any other luminescence than that of flavins and pteridines. Thus, the chemical structure of pigments formed, i.e., the prevalence in the product of flavins or pteridines, depended on the composition of the initial amino acid mixture.
The Reaction of Photophosphorylation and Its Spectral Sensitivity
When irradiated, the suspension of flavoproteinoid microspheres demonstrated photophosphorylating activity. After 30-min irradiation about 30 % of ADP was converted to ATP. The formation of ATP was demonstrated by the luminescent luciferin–luciferase method for ATP estimation and by the HPLC assay of reaction products (Fig. 4). Determination of ATP by these two methods gave similar results (Table 1). The formation of ATP did not take place in the absence of irradiation, without microspheres or ADP. Three different methods used in our research did not reveal the formation of pyrophosphate and other condensed inorganic phosphates in process of irradiation.
HPLC separation of substrates and products of photophosphorylation reaction sensitized by flavoproteinoid miscrospheres: a experimental mixture after 30-min irradiation. b Reaction mixture incubated without irradiation. Reaction mixture of standard composition (see “Methods”) was irradiated with high-pressure mercury arc lamp DRL-400 “Lisma VNIIS” (Russia) (the flux level in cuvette was 70 W m−2 during 30 min. Chromatograph “Stayer” “Aquilon”, Russia). Column “Synergi” Hydro-RP 80A, 4 μm, 4.6 × 250 mm; eluent 0.1 M KH2PO4 pH 6.0, containing 1 % acetonitrile. The separation was monitored at 260 nm. The column was calibrated with standard preparations of ATP, ADP, and AMP to determine retention times
To obtain information on the chemical nature of pigments sensitizing the phosphorylation of ADP by orthophosphate to yield ATP, we studied the manifestation of the photophosphorylating activity upon irradiation of microsphere suspensions with the quasi-monochromatic light in a wavelength range of 325–525 nm.
Figure 5 presents curves reflecting the time course of ATP accumulation in the photophosphorylation reaction under the action of radiation of different wavelengths on suspensions of microspheres obtained by thermolysis of the mixture of glutamic acid, glycine, and lysine (Fig. 5a) and the mixture of glutamic acid, alanine, and lysine (Fig. 5b).
a Time course of ATP accumulation in a photophosphorylation reaction with participation of pigment-containing proteinoid microspheres formed by products of thermolysis of the mixture Glu, Gly, and Lys (8:3:1). b Time course of ATP accumulation in a photophosphorylation reaction with participation of pigment-containing proteinoid microspheres formed by products of thermolysis of the mixture Glu, Ala, and Lys (8:3:1). The content of ATP produced is presented with the subtraction of the results of the dark control
On the basis of these curves the action spectra were constructed (Fig. 6, solid lines). In Fig. 6a in parallel with the action spectrum, the spectrum of light absorption by the aqueous solution of FMN is given, its absorption maxima being at 260, 375, and 450 nm (molar extinction coefficients 27.1, 10.4, and 12.2 mM−1 cm−1, respectively). The position of the maxima in the action spectrum, as well as the ratio of photophosphorylating activity in these peaks coincided with the absorption spectrum of the flavin standard. This led to the conclusion that phosphorylation was photosensitized by flavin pigment. As noted above, it was flavins that prevailed quantitatively in the pigment fraction of chromoproteinoids obtained upon thermolysis of the mixture of lysine, glutamic acid, and glycine.
Action spectra for the reaction of photophosphorylation of ADP to ATP. a Microspheres containing the chromoproteinoid product of thermolysis of the mixture Glu, Gly, and Lys (8:3:1). The amount of ATP formed during 20 min of irradiation (minus the results of the dark experiment) was normalized to the number of quanta of incident light expressed in Einsteins. The dotted line shows the absorption spectrum for a standard solution—a flavin mononucleotide solution (the optical density is given in arbitrary units). b Microspheres containing the chromoproteinoid product of thermolysis of the mixture Glu, Ala, and Lys (8:3:1). The content of ATP formed during 40 min of irradiation (minus the results of the dark experiment) was normalized to the number of quanta of incident light expressed in Einsteins. The dotted line shows the absorption spectrum for a pterin solution (the optical density is given in arbitrary units)
In the action spectrum given in Fig. 6a, along with light absorption maxima at 375 and 450 nm, an additional shoulder is present between λλ 320 and 350 nm, i.e., in the region corresponding to the long-wave absorption band of typical pteridine spectra (λ max 340–350 nm). The presence of this band points to that, besides flavins, pteridines were also functioning as photosensitizers within proteinoid microspheres. Thus, not only abiogenic flavin but also pteridine molecules could sensitize the reaction of ADP photophosphorylation.
This suggestion was confirmed in the measurements of the action spectrum of photophosphorylation occurring in the presence of microspheres formed from proteinoids enriched in pteridine pigments (a product of thermolysis of the mixture of glutamate acid, alanine, and lysine at the ratio 8:3:1). The long-wave maximum of the action spectrum of this reaction was at 350 nm, i.e., it was similar to typical absorption (or fluorescence excitation) spectra of pteridines (Fig. 6b).
The results suggest that abiogenic flavins and pteridines within proteinoid microspheres are able to sensitize ATP formation in the model of photophosphorylation. On irradiation of samples with relatively low intensities of the quasi-monochromatic light, the molar yield of ATP was lower than in our previous studies where a more powerful irradiation source with a wider spectral range was used (Kolesnikov et al. 2008; Telegina et al. 2010). Under the conditions of the experiment described here, i.e., under irradiation of reaction mixture with quasi-monochromatic light, in the samples with abiogenic flavin photosensitizer, the ATP yield reached 15 %, and in the system with pteridine photosensitizer it did not exceed 7 %.
Discussion
The results point to the possibility of spontaneous formation in the enzyme-free medium of a photochemical system that converts the photon energy to chemical energy of ATP. In the experimental conditions this system formed from a mixture of amino acids—glutamic acid, lysine, and glycine (or alanine). For each of these amino acids there is evidence pointing to the possibility of its abiogenic formation from simpler molecules (Harada and Fox 1964; Grossenbacher and Knight 1965; Oro 1965; Pavlovskaya and Telegina 1974; Hennet et al. 1992; Holm and Andersson 2005). In the development of photochemical model we focused on getting of abiogenic product enriched with flavin and pteridine chromophores. In this context, the question of how the conditions of the early Earth could provide a high concentration of lysine and glutamic acid in the mixture of precursors has shifted somewhat to the background. However, this question remains relevant and the possibility of formation of these pigments from mixtures of different compositions requires further study.
The presence of flavin or pteridine pigment was of fundamental importance for the functioning of the system. Flavin molecules were more active in conversion of light energy as compared to pteridines. They absorb photons over a broader spectrum than pteridines and the upper limit of the light absorption by their molecules lies in blue-green region at about 530 nm, while most biological pteridines do not absorb photons with a wavelengths above 400 nm. Besides, flavins have higher values of molar extinction coefficient.
The abiogenic pigments do not show photophosphorylating activity in the absence of a structured matrix to which these chromophores are bound (Telegina et al. 2010). Amino acid polymers additionally structured with silicate performed the role of this matrix. Pigment-containing proteinoids, when not aggregated to microsphere, are essentially inactive as sensitizers of photophosphorylation. In this respect, the results of the present study are consistent with the evidence that flavin molecules in solution exhibit only a very low photosensitizing activity in the reaction of phosphorylation of ADP to ATP (Losinova et al. 1983, 1986).
The question arises, what physicochemical environment could be optimal for the functioning of flavins as sensitizers of photochemical energy-converting process? The absorption spectrum of flavins nearly complements the emission spectrum of the F- and G-class stars. As for the Sun, it is a G2 V star according to stellar classification (Kaler 1997). Its surface emits maximum energy in the range of 475–505 nm (Ahmed 2002). The long-wavelength flavin absorption maximum at about 450 nm is close to the maximum of solar radiation outside the Earth’s atmosphere, i.e., the flavin-based energy converter is adjusted to the spectral profile of solar radiation reaching the early Earth. The emergence of an ozone layer has decreased the access of blue-violet light and UV-A and UV-B radiation, which are absorbed by flavins, and the radiation spectrum shifted toward the red region. The ratio of energy input reaching sea level at the borders of the visible spectrum in the UV and IR ranges (E 400/E 750) fell from approximately 1.5 to 0.9. This change in light conditions had to deprive flavins of an important selective advantage as potential light energy as compared to photosynthetic pigments (Kritsky et al. 2013).
The other important issue is the redox condition of the reaction medium. When discussing the properties of abiogenic converter of light energy we focus on the oxidized form of flavins as potential photoreceptor. The reduced flavins, both dihydro- and the free radical (semiquinone) forms, absorb light significantly more weakly than the oxidized flavin (Ehrenberg et al. 1967; Hemmerich and Haas 1975). To be efficient in photocatalytic processes, the anionic didehydroFAD molecule in the catalytic center of photoenzyme DNA–photolyase uses an inflow of energy from the light harvesting chromophore with higher absorptive capacity (Sancar 2003). As to the flavin semiquinone, the serious obstacle to the participation in the energy converter is its instability. Binding to a specific protein (or starch films) can dramatically increase the semiquinone stability (Knappe et al. 1972; Edwards 2006; Penzkofer 2012); however, there is no evidence that the matrices available in the epoch of primitive evolution could stabilize the semiquinone. In the absence of conditions providing stable and sufficiently high concentration of the semiquinone, its functioning as a converter of light energy becomes unlikely.
Since the photophosphorylation sensitized by oxidized flavin involves the formation of reduced flavin species, an important prerequisite for the implementation of the flavin photocycle is the availability of a reoxidizing agent, which does not necessarily need to be atmospheric dioxygen. A suitable candidate for this role is hydrogen peroxide, which ensures the functioning of the flavin-based photophosphorylation in our model (Kolesnikov et al. 2008). Curiously, the low oxygen content in the atmosphere is not an obstacle for the geochemical formation of hydrogen peroxide. For instance, an effective source of hydrogen peroxide can be water photolysis catalyzed by iron-sulfur minerals (Borda et al. 2001). In astrobiological context, the detection of hydrogen peroxide in the atmosphere of Mars deserves special attention. It has been suggested that in addition to photolytic dissociation of water, large amounts of hydrogen peroxide could be generated by a mechanism based on the production of large-scale electrostatic fields during dust storms and dust devils (Encrenaz et al. 2012).
As to the electron donors for the reduction of an electrophilic molecule of excited flavin in the abiotic environment; these compounds include carboxylic acids, alcohols, and sulfides (Heelis 1982). In this respect, the flavin model is comparable to early (anoxygenic) versions of photosynthesis that cannot utilize water as an electron source, and obtain electrons from the oxidation of sulfides, organic acids, and hydrogen.
Isoalloxazine derivatives, flavins, could form in abiotic chemical processes and along with the other coenzymes, they are regarded as a kind of metabolic fossils (White 1976). In modern organisms, flavins such as FMN or flavin adenine dinucleotide (FAD) combine their cofactor function in “dark” biocatalysis with the participation in photobiological processes. They are not involved in the transformation of light energy to the energy of chemical bonds but act as chromophores of photoenzymes DNA-photolyases and a number of sensory photoreceptors (Sancar 2003; Losi 2007; Losi and Gärtner, 2011, 2012). The structural differences between protein families to which these flavoproteins belong point out that the evolution repeatedly stopped its choice on flavins as participants of a photobiological mechanism. Some biological pterins (2-amino-4-pterdinones) also act as chromophores in non-photosynthetic physiological receptors of light (Sancar 2003; Losi and Gärtner 2011).
It is difficult the discuss the model studied as a direct evolutionary progenitor of flavin- or pterin-containing photoreceptor proteins, since the structure of the model is not genetically determined and its formation depends solely on the physicochemical conditions of the environment. Similarly, it is also difficult to relate the phosphorylating mechanism of the model with the enzymes, catalyzing in organisms photophosphorylation of ADP to form ATP. In photosynthetic pro- and eukaryotes, this function is performed by a membrane-bound F-type H+-ATPases (or, in some organisms, Na+-ATPases). To drive its catalytic activity, the enzyme uses the energy of the transmembrane electrochemical ion gradient formed by the light-induced electron transport. A similar mechanism underlies the photophosphorylation to form inorganic pyrophosphate. The enzyme that catalyzes the reaction, H+-pyrophosphatase, is structurally close to the V-ATPase (vacuolar ATPase in eukaryotes) (Baltscheffsky and Baltschefffsky 1995; Baltschefsky et al. 1999). The latter, in evolutionary terms, are, probably, more primitive than F-type ATPases (Mulkidjanian et al. 2007).
Today we are not able to trace the continuity between the development of a chemical model which is based on the activity of an abiogenic matrix-bound chromophore and the evolution of the membrane-bound enzymes, the structure of which is genetically determined and the synthesis presumes the operation of informational macromolecules. As to the structure of the abiogenic model, its characteristics exclusively depend on the environment such as an availability of these or others precursor molecules. So, abiogenic prototypes of metabolic devices could not be the direct ancestors of modern bioenergetic mechanisms. However, the fact that abiotic process could form a rather complex mechanism that transforms light energy into the energy of ATP may expand our understanding of the possibilities of chemical evolution.
Abbreviations
- ADP:
-
Adenosine diphosphate
- ATP:
-
Adenosine triphosphate
- Fl:
-
Oxidized form of flavin
- FlH• :
-
Free radical reduced flavin
- 1Fl and 3Fl:
-
Singlet and triplet excited states of flavin
- FMN:
-
Riboflavin-5′-phosphate (flavin mononucleotide)
- FAD:
-
Flavin adenine dinucleotide
- Pi :
-
Inorganic orthophosphate
- PPi :
-
Inorganic pyrophosphate
- 1O2 :
-
Singlet oxygen
References
Ahmad M, Grancher N, Heil M, Black RC, Giovani B, Gailland P, Lardemer D (2002) Action spectrum for cryptochrome-dependent hypocotyl growth inhibition in Arabidopsis. Plant Physiol 129(2):774–785
Ahmed T (2002) The wavelength of the sun’s peak radiation output. In: Elert G (ed) The physics factbook—an encyclopedia of scientific essays. Available online: http://hypertextbook.com/facts/2002/TahirAhmed.shtml. Accessed on 24 February 2013.
Bahn PR, Fox SW (1981) Models for protocellular photophosphorylation. BioSystems 14(1):3–14
Baltscheffsky M, Baltscheffsky H (1995) Alternative photophosphorylation, inorganic pyrophosphate synthase and inorganic pyrophosphate. Photosynth Res 46(1):87–91
Baltschefsky M, Schultz A, Baltschefsky H (1999) H+-proton-pumping inorganic pyrophosphatase: a tightly membrane-bound family. FEBS Lett 452(3):121–127
Borda MJ, Elsetinow AR, Schoonen MA, Strongin DR (2001) Pyrite-induced hydrogen peroxide formation as a driving force in the evolution of photosynthetic organisms on an early Earth. Astrobiology 1(3):283–288
Cheng CF, Chang W, Rong CT, Chunyuan M, Zhiwei CJ, Zhao Y (2002) Phosphorylation of adenosine with trimetaphosphate under simulated prebiotic conditions. Orig Life Evol Biosph 32(3):219–224
Edwards AM (2006) General properties of flavins. In: Silva E, Edwards AM (eds) Flavins: photochemistry and photobiology. Comprehensive series in photochemistry and photobiology, vol 6. Royal Society of Chemistry, London, pp 1–11
Egorov SY, Krasnovsky AA Jr, Bashtanov MY, Lyudnikova TA, Kritsky MS (1999) Photosensitization of singlet oxygen formation by pterins and flavins. Time-resolved studies of oxygen phosphorescence under laser excitation. Biochemistry (Moscow) 64(10):1117–1121
Ehrenberg A, Müller F, Hemmerich P (1967) Basicity, visible spectra, and electron spin resonance of flavosemiquinone anions. Eur J Biochem 2(3):286–293
Encrenaz T, Greathouse TK, Lefévre F, Atreya SK (2012) Hydrogen peroxide on Mars: Observations, interpretation and future plans. Planet Space Sci 68(1):3–17
Fox SW, Dose K (1972) Molecular evolution and the origins of life. W.H. Freeman, San Francisco
Grossenbacher KA, Knight CA (1965) Amino acids, peptides, and spherules obtained from “primitive Earth” gases in a sparking system. In: Fox SW (ed) The origins of prebiological systems and their molecular matrices. Academic Press, N.-Y., London., pp 173–186
Grunze H und Thilo E (1954) Die Papierchromatographie der kondensierten Phosphate. Berlin Akademie Verlag, 25 S
Harada K, Fox SW (1964) Thermal synthesis of natural amino acids from a postulated primitive terrestrial atmosphere. Nature 201(4917):335–336
Heelis PF (1982) The photophysical and photochemical properties of flavins (izoalloxazines). Chem Soc Rev 11(1):15–39
Heinonen JK, Honkasalo SH, Kukko EI (1981) A method for the concentration and for the colorimetric determination of nanomoles of inorganic pyrophosphate. Anal Biochem 117(2):293–300
Heinz B, Ried W (1981) The formation of chromophores through amino acid thermolysis and their possible role as prebiotic photoreceptors. BioSystems 14(1):33–40
Heinz B, Ried W (1984) The molecular structure of “chromoproteinoids”—amino acid thermolysates containing abiogenetically produced flavins and deazaflavins as prosthetic groups. Orig Life 14(1–4):279–289
Heinz B, Ried W, Dose K (1979) Thermische Erzeugung von Pteridinen und Flavinen aus Aminosäueregemischen. Angew Chem 91(6):510–511
Hemmerich P, Haas W (1975) Recent developments in the study of “Fully reduced flavin”. In: Yagi K (ed) Reactivity of flavins. University of Tokyo Press, Tokyo, Japan, pp 1–13
Hennet RJ-C, Holm NG, Engel MH (1992) Abiotic synthesis of amino acids under hydrothermal conditions and the origin of life: a perpetual phenomenon? Naturwissenschaften 79(8):361–365
Holm NG, Andersson E (2005) Hydrothermal simulation experiments as a tool for studies of the origin of life on the Earth and other terrestrial planets: a review. Astrobiology 5(4):444–460
Julian RR, Beauchamp JL (2003) Abiotic synthesis of ATP from AMP in the gas phase: implications for the origin of biologically important molecules from small molecular clusters. Int J Mass Spectrometry 227(1):147–159
Kaler JB (1997) Stars and their spectra: an introduction to the spectral sequence. Cambridge University Press, Cambridge, p 300
Knappe WR, Hemmerich P (1972) Covalent intermediates in flavin-sensitized photodehydrogenation and photodecarboxylation. Z Naturforsch B 27(9):1032–1035
Kolesnikov MP (1991) Proteinoid microspheres and the process of prebiological photophosphorylation. Orig Life Evol Biosph 21(6):31–37
Kolesnikov MP, Kritsky MS (2001) Study of chemical structure and of photochemical activity of abiogenic flavin pigment. J Evol Biochem Physiol 37(5):507–514
Kolesnikov MP, Telegina TA, Lyudnikova TA, Kritsky MS (2008) Abiogenic photophosphorylation of ADP to ATP sensitized by flavoproteinoid microspheres. Orig Life Evol Biosph 38(3):243–255
Kritsky MS, Telegina TA (2004) Role of nucleotide-like coenzymes in primitive evolution. In: Seckbach F (ed) Origins: genesis, evolution and diversity of life. Kluwer, Dordrecht, pp 215–231
Kritsky MS, Lyudnikova TA, Mironov EA, Moskaleva IV (1997) The UV radiation-driven reduction of pterins in aqueous solution. J Photochem Photobiol B:Biol 39(1):43–48
Kritsky MS, Kolesnikov MP, Telegina TA (2007) Modeling of abiogenic synthesis of ATP. Doklady Biochem Biophys 417(1):313–315
Kritsky MS, Telegina TA, Vechtomova YL, Kolesnikov MP, Lyudnikova TA, Golub OA (2010) Excited flavin and pterin coenzyme molecules in evolution. Biochemistry (Moscow) 75(10):1200–1216
Kritsky MS, Telegina TA, Vechtomova YL, Buglak AA (2013) Why flavins are not competitors of chlorophyll in the evolution of biological converters of solar energy. Int J Mol Sci 14(1):575–593
Lorente C, Petroselli G, Dántola ML, Oliveros E, Thomas AH (2011) Electron transfer initiated reactions photoinduced by pterins. Pteridines 22(4):111–119
Losi A (2007) Flavin-based blue-light photosensors: a photobiophysics update. Photochem Photobiol 83(6):1283–1300
Losi A, Gärtner W (2011) Old chromophores, new photoactivation paradigms, trendy applications: flavins in blue light-sensing photoreceptors. Photochem Photobiol 87(3):491–510
Losi A, Gärtner W (2012) The evolution of flavin-binding photoreceptors: an ancient chromophore serving trendy blue-light sensors. Annu Rev Plant Biol 63:49–72
Losinova TA, Diomina OV, Nedelina OS, Brzhevskaya ON, Kayushin LP (1983) Photosensitized synthesis of adenosine triphosphate. Biophysica (Russian) 28(5):555–557
Losinova TA, Nedelina OS, Kayushin LP (1986) Adenosine diphosphate effect on light-induced oxygen absorption by flavins. Biophysica (Russian) 31(1):10–15
Mulkidjanian AY, Makarova KS, Galperin MY, Koonin EV (2007) Inventing the dynamo machine: the evolution of the F-type and V-type ATPases. Nat Rev Microbiol 5(11):892–899
Oro J (1965) Stages and mechanisms of prebiological organic synthesis. In: Fox SW (ed) The origins of prebiological systems and their molecular matrices. Academic Press, New York, pp 137–171
Pavlovskaya TE, Telegina TA (1974) Photochemical conversions of lower aldehydes in aqueous solutions and in fog. Orig Life 5(3/4):303–309
Penzkofer A (2012) Reduction-oxidation photocycle dynamics of flavins in starch films. Int J Mol Sci 13:9157–9183
Sancar A (2003) Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem Rev 103(6):2203–2237
Schwartz A, Ponnamperuma C (1968) Phosphorylation of adenosine with linear polyphosphate salts in aqueous solution. Nature 218(5140):443
Strehler BL, Totter JR (1954) Determination of ATP and related compounds: firefly luminescence and other methods. Methods Biochem Anal N.Y. 1:341–356
Telegina TA, Kolesnikov MP, Vechtomova YL (2010) Photoactivated matrices in prebiotic evolution processes. High Energy Chem 44(3):228–233
Van Vickle-Chavez SJ, Van Gelder RN (2006) Action spectrum of Drosophila cryptochrome. J Biol Chem 282(14):10561–10566
White HB (1976) Coenzymes as fossils of an earlier metabolic state. J Mol Evol 7(2):101–104
Yoza N, Akazaki I, Ueda N, Kodama H, Tateda A (1991) High-performance liquid chromatographic determination of pyrophosphate in the presence of a 20,000-fold excess of orthophosphate. Anal Biochem 199(2):279–285
Acknowledgments
The financial support from the Presidium of Russian Academy of Sciences (Basic Research Program No. 28) and from the Russian Foundation for Basic Research (Project 11-04-01007a) is appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00239-015-9671-2.
Rights and permissions
About this article
Cite this article
Telegina, T.A., Kolesnikov, M.P., Vechtomova, Y.L. et al. Abiotic Photophosphorylation Model Based on Abiogenic Flavin and Pteridine Pigments. J Mol Evol 76, 332–342 (2013). https://doi.org/10.1007/s00239-013-9562-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-013-9562-3