Abstract
Identifying the molecular basis for complex adaptations such as the toxic proteins used by venomous snakes to subdue and digest prey is an important step in understanding the evolutionary and functional basis for such traits. Recent proteomics-based analyses have made possible the identification of all constituent proteins in whole venom samples. Here we exploit this advance to study patterns of population-level variation in venom proteins from 254 adult eastern massasauga rattlesnakes (Sistrurus c. catenatus) collected from 10 populations. Analysis of presence–absence variation in specific proteins from 1D PAGE gels shows that: (1) The frequency spectra for individual protein bands is U-shaped with a large number of specific proteins either being consistently “common” or “rare” across populations possibly reflecting functional differences. (2) Multivariate axes which summarize whole venom variation consist of bands from all major types of proteins implying the integration of functionally distinct components within the overall venom phenotype. (3) There is significant differentiation in venom proteins across populations and the specific classes of proteins contributing to this differentiation have been identified. (4) Levels of population differentiation in venom proteins are not correlated with levels of neutral genetic differentiation, or genetically effective population sizes which argues that patterns of venom variation are not simply a consequence of population structure but leaves open the role of selection in generating population differences in venom. Our results identify particular classes of venom proteins and their associated genes as being fruitful targets for future studies of the molecular and functional basis for this complex adaptive phenotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Identifying the molecular basis for complex adaptations in wild organisms represents an important step in understanding how such traits evolve and function (Mitchell-Olds et al. 2007). For traits composed of sets of interacting proteins, identifying which proteins vary within and between populations can suggest candidate genes that underlie adaptive phenotypic variation in the form of loci encoding these proteins. This opens the door to studies of how selection molds variation at the DNA level (Nachman 2006) and how genetic variation is linked to phenotypic variation in adaptive traits. For example, levels of protein variation within populations can highlight the relative importance of different types of evolutionary forces such as genetic drift and selection as influences on polymorphism (Nei 1987) while patterns of population differentiation can suggest if local adaptation due to divergent selection occurs (Mitchell-Olds et al. 2007). Finally, patterns of covariation and relative abundance of specific proteins can provide hypotheses about how individual proteins influence the function of the complex adaptation which they make up (Benfey and Mitchell-Olds 2008).
Toxic venoms produced by snakes in the family Viperidae are one of the most widely studied types of animal venoms. Venomous species such as rattlesnakes produce a diverse mixture of enzymes, peptides, and other molecules which, upon envenomation, incapacitate prey leading to death and initiation of digestion (Mackessy 2009). Although snake venoms can contain a large number of distinct toxic proteins, proteomics analyses (e.g., Sanz et al. 2006) have shown that most belong to a limited set of protein families. For example, in rattlesnakes, the majority of protein-based toxins belong to four classes of proteins (metalloproteinases, phospholipase A2s, serine proteases, and disintegrins) that have hemotoxic, myotoxic, and neurotoxic affects on potential prey (Mackessy 2009).
Variation in venom composition is hypothesized to be adaptive in that it allows snakes with different venoms to kill and digest preferred prey (Mackessy 1988; Daltry et al. 1996). Support for this hypothesis comes from work showing associations between ontogenetic shifts in diet and venom composition (Mackessy 1988), and direct tests that show venom produced by a particular species is most toxic to its preferred prey (Mackessy et al. 2006; Barlow et al. 2009). In addition, the repeated finding of population differentiation in venom composition within species (Daltry et al. 1996; Forstner et al. 1997; Salazar et al. 2007) has been argued to represent local adaptation due to divergent selection on venom genes. Evidence for this interpretation comes from studies show that population differences in venom composition correlate with differences in toxic effects on organisms (e.g., Forstner et al. 1997; Aguilar et al. 2007) or demonstrate that venom differentiation is correlated with population differences in diet (Daltry et al. 1996). However, other studies have found no such correlation and suggest that neutral evolutionary processes such as genetic drift have a greater impact on levels of venom variation than does selection (Williams et al. 1988). Thus evolutionary and ecological causes and functional consequences of intraspecific variation in venom composition remain unclear and in need of additional work and new approaches (Wuster 1999).
Many studies of venom variation to date have used individual venom profiles generated from 1D PAGE gel electrophoresis in which the specific venom proteins showing divergence have not been identified nor is the molecular mechanism responsible for generating observed variation understood (Forstner et al. 1997; see Chippaux et al. 1991 for review). The major reason for the lack of detailed knowledge was methodological difficulties in comprehensively assaying complex mixtures of proteins. More recently more comprehensive assays of venom variation have been conducted using a combination of 2D gel electrophoresis (Serrano et al. 2005) and newly developed proteomics techniques the combination of which allow the rapid and accurate quantification of all proteins present in a given venom sample (Calvete et al. 2007). This has resulted in an explosion of “venomics” studies which have used detailed characterization of venom composition in a wide variety of snakes to investigate a broad range of questions in evolutionary biology and biomedical science (Calvete 2009). We see an opportunity to combine the speed and cost efficiency of 1D gel analyses of venom with the detailed knowledge about protein composition from venomics techniques to gain new insights into the evolutionary processes and mechanisms that determine snake venom protein variation at the population level. In particular, we feel that the detailed information on protein sizes from venomics studies can be used to provisionally identify the protein family represented by a particular size band in the 1D venom profile of individual snakes. This information can then be used to study patterns of protein-specific variation within and between snake populations to an unprecedented level of detail.
Here, we use this approach to study venom protein variation in eastern massasauga rattlesnakes (Sistrurus c. catenatus). These snakes occupy a diverse set of habitats in central and eastern North America and eat a variety of endothermic (mammals) and ectothermic (frogs and lizards) prey (Campbell and Lamar 2004). Previous work on venom composition and function in Sistrurus as a whole includes a detailed venomics-based analysis of venom composition in 4 of 6 named subspecies of S. catenatus and S. miliarius including S. c. catenatus (Sanz et al. 2006) and evidence that both structural (sequence-based) differences in protein coding sequences and gene regulation contribute to venom variation in this group (Gibbs and Rossiter 2008; Gibbs et al. 2009). Intraspecific studies of venom composition are more limited. Sanz et al. (2006) conducted detailed proteomics analyses on venom samples from two individual S. c. catenatus providing limited but detailed information on venom protein variation. Gibbs et al. (2009) used a “population venomics” approach to study venom variation from 34 wild-caught S. c. catenatus from three populations but did not attempt to assess patterns of differentiation between populations due to low sample sizes.
In this study, we generate a larger data set on population variation in specific venom proteins in this snake by combining data from 1D PAGE gel analyses from more than 250 snakes sampled from 10 populations with information on protein identity of specific bands based on molecular weight data from the LC MS/MS-based proteomics analyses of Sanz et al. (2006). We use this data to (1) estimate frequency spectra of individual protein bands and use this information to assess the possible function of specific proteins, (2) use non-parametric statistical techniques from community ecology to summarize overall variation in venom composition, test for population differentiation and identify the specific classes of proteins that contribute to that differentiation, and (3) test hypotheses for the patterns of population differentiation by estimating correlations between levels of venom differentiation and neutral genetic variation.
Materials and Methods
Sample Collection
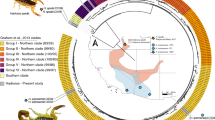
Venom samples were collected from 254 wild-caught Sistrurus c. catenatus from 10 sites that span the distribution of this snake in eastern North America (Fig. 1). Samples were collected when snakes were active (April to August) in single years from 2004 to 2008. At most sites, samples were collected from a single geographically restricted (<5 km) area. However, to increase sample size, the samples from the Western Pennsylvania “population” (PA) represent pooled samples from three geographically distinct locations all within ~40 km of each other. After capture in the field, each animal was constrained using a set of telescoping clear plastic tubes and then induced to bite the top of a 50 ml glass beaker that had been covered with Parafilm. Secreted venom (between 10 and 200 μl) was immediately pipetted into a 2 ml cryovial and stored either in a −80°C freezer or in liquid nitrogen until further processing. Only samples from adult snakes (>70 g in body weight) were analyzed to avoid the possibility that ontogenetic shifts in venom composition might influence venom composition (Mackessy 1988). This value was chosen as the cutoff because unpublished data from our work suggests massasaugas are at this size during their third year of life and all gravid females we have captured weigh this much or more.
a Map showing S. c. catenatus population locations from which venom samples were collected. Sample sizes and abbreviations for each population were as follows: ILL Carlyle Lake State Park, Illinois (n = 32); PRF Prairie Road Fen, Ohio (21), KL Killdeer Plains, Ohio (75); GRL-1 Grand River Lowlands 1, Ohio (17); GRL-2 Grand River Lowlands 2, Ohio (12); GRL-3 Grand River Lowlands 3 (19); WM Willard Marsh Wildlife Area, Ohio (14); PA Butler County, Pennsylvania (24); CS Cicero Swamp, New York (18); BPNP Bruce Peninsula National Park, Ontario (22). bInset showing the relative location of the sampling localities within the United States and Canada
In most cases only a single sample from each individual was analyzed and we assume that the venom profile for an adult is largely stable across time. Other studies have limited evidence for temporal variation in venom composition (see Chippaux et al. 1991 for review). However, Gregory-Dwyer et al. (1986) found little change in the 1D gel venom profiles of captive individuals from three species of rattlesnakes sampled over a 20-month period. Further, we were able to collect multiple venom samples from the same individual in different years for a small number of adults (n = 3). When analyzed, profiles for samples from the same snake collected in different years were indistinguishable (Gibbs and Chiucchi, unpublished data). Second, comparisons of the venom profiles of captive adults of a closely related species (S. miliarius barbouri) fed on constant diets show little change in the presence or absence of specific proteins over a 2-year period (Gibbs et al., unpublished data).
Gel Electrophoresis and Imaging
Our methods for analyzing venom using gel electrophoresis generally followed those of Huang and Mackessy (2004). Briefly, we first used a Bradford assay (Bradford 1976) as modified by BioRad Laboratories (San Diego, CA, USA) to determine the protein concentration of each sample. Next, venom profiles for individual snakes were generated by loading 10 μg of protein into a single well of a 15-well 10% NuPAGE Novex Bis-Tris gel (Invitrogen) (Fig. 2). To maximize the resolution of different size venom proteins each sample was run twice: once in a gel run in MES buffer (optimal resolution of proteins between ~2.5 and 200 kDa) and a second time in a gel run in MOPS buffer (optimal resolution of proteins between 14 and >200 kDa) (Invitrogen). After scoring band sizes (see below) data from both gels was then combined to create a single venom profile for each individual snake. To determine venom protein sizes, lanes 1, 7, and 15 on each gel contained 5 μl of Mark 12 Unstained Protein Standard (Invitrogen). Gels were run, stained with Coomassie blue, and then destained overnight. After destaining, photographs of the gels were taken using the Kodak Gel Logic 200 Imaging System. After recording an image of each gel we estimated a molecular weight for each distinct band using the Lane and Band Analysis module in the 1D Image Analysis Software Package included with the Gel Logic System in combination with the in-lane size markers.
Protein Band Comparisons and Identities
Our analyses are based on our ability to accurately identify distinct bands as the same or different in the venom profiles of different individuals. To do this, we constructed a series of size-related bins that spanned the size range of all detected bands. Initially, bins were set up using the size estimates of bands present in the profiles of four individuals. These individuals were run multiple times (3 times each on both of two gels for a total of six replicates per individual) and each distinct band in the profile of an individual was sized as above and the standard deviation (SD) based on the repeated measurements of the same band across all gels calculated. A size bin was then defined as ±1.96 SD the mean size of each band found in the replicated individual and these measurements were then used to establish a series of bins for scoring band sizes across all individuals. When the estimated sizes of bands from the profiles of other snakes fell within one of these bins the bands were classified as the same protein. Occasionally, new bands were identified that fell outside of any existing bin. In this case a new bin was constructed using the estimated size of the novel band and the SD from band closest in molecular weight that was present in the replicated individual. This procedure was used to align the venom profiles of all individuals and then generate a presence-absence matrix for all scoreable bands observed in at least one snake in the sample. Finally, the detailed LC MS/MS-based proteomics analysis of Sistrurus venoms by Sanz et al. (2006) provides precise sizes in kD for the proteins found in this taxon. Mackessy (2009) also used information on the sizes of general classes of proteins to identify bands in 1D gel profiles of rattlesnake venoms in general. We used both sources in combination with our estimates of the molecular weights of specific bands to classify bands found in individual venom profiles into particular venom protein families (see Appendix). We acknowledge that the size ranges of some classes of venom proteins were not entirely exclusive (e.g., PLA2s and disintegrins) and so this might result in the misclassification of a small number of bands.
Statistical Analyses: Patterns of Variation
We detected a large number of distinct proteins (n = 48) in the venom profiles of individual snakes. To estimate the frequency spectra for individual bands within each population we first calculated the relative frequency of each of the 48 distinct bands in the venom profiles of individuals in each of the 10 populations. We then used a LOESS curve-fitting procedure to obtain a qualitative description of the shape of the band venom frequency spectra. We were also interested in determining whether particular bands showed consistent patterns of relative abundance in different populations. To statistically evaluate if bands were consistently present at high, medium, or low frequency in different populations we classified specific bands in each population as falling into low (frequency of between 0.0 and 0.35), medium (0.36–0.64), or high (0.65–1.0) categories and then carried out a sign test on the number of times that a band fell into a specific category across all 10 populations.
To conduct a multivariate analysis of venom variation we used non-metric multidimensional scaling (NMS: Mather 1976) to estimate axes that describe overall variation in venom band presence/absence across all individuals. We choose this approach over more traditionally used parametric ordination techniques such as Principle Component Analyses (PCA) (e.g., Daltry et al. 1996; Forstner et al. 1997) because we feel that the non-normal presence/absence band data are more appropriately analyzed using multivariate non-parametric methods. We used the analysis package PC-ORD (McCune and Grace 2002) to generate NMS scores using Sørenson (Bray–Curtis) distances to estimate similarities among venom profiles. We used a trimmed data set which only contained bands present at an overall frequency of ≥5% to avoid potential biases due to the presence of a large number of zero entries in the data matrix (McCune and Grace 2002). This resulted in the elimination of 7 bands from the analysis hence the final data set for the NMS analysis consisted of data for 41 bands. To generate NMS scores we used the “slow and through” autopilot mode of NMS in PC-ORD and generated factor scores based on the best of 250 runs of real data to generate the axis and 250 runs of random data for a Monte Carlo test of significance. A stress analysis in relation to the dimensionality in the data set was used to determine the appropriate number of axes that best described variation in the data set. Variance explained by each axis was expressed by the coefficient of determination between Sørensen distances in the original venom band space. The biological interpretation of each axis was inferred by examining the magnitude of the correlations between specific axis scores and specific venom band abundance across all individuals in the analyses.
Statistical Analyses: Population Differentiation
We used several approaches to assess differentiation in venom composition across populations. First, we compared NMS factor scores for individuals in different populations using ANOVA followed by pairwise comparisons for each set of populations. Second, we used Multi-Response Permutation Procedures (MRPP) and Indicator Species Analysis (McCune and Grace 2002) as another way of assessing differentiation. MRPP (Mielke 1984) provides a nonparametric multivariate test of differences between groups. The A statistic from MRPP describes effect size, or the ‘‘chance-corrected within-group agreement’’ for a particular set of variables. When A = 0, the groups are no more or less different than expected by chance; when A = 1, all sample units are identical within each group. Significance of the overall and pairwise A statistics for each population were assessed using Monte Carlo based tests of significance.
While MRPP analyses provide an overall assessment of whether venom composition differs between populations they do not identify which bands are responsible for observed differences. Indicator Species Analysis (ISA) (Dufrêne and Legendre 1997) provides this information by describing how well each variable (venom band in this case) differentiates groups. This is done by calculating an IV statistic for each band which is based on both the specificity of the band to particular populations and how abundant a band is in snakes from particular populations where the band is found. IV values are highest when bands are found at high abundance in single populations and lowest when bands are found in all populations but only in a small number of individuals in each. We conducted ISA on the venom data using PC-ORD and assessed the significance of each estimated IV value for a given band using Monte Carlo based tests of significance.
Correlations Between Venom and Genetic Variation
Finally, to assess whether venom variation was correlated with genetic variation, we tested for a correlation between a measure of within population venom variation (mean Sørenson distance for all venom bands across all individuals) and long-term genetically effective population size (θ [4Neμ]) as estimated using data from 19 microsatellite DNA loci and the program MIGRATE (Beerli 2009) (for details see Chiucchi and Gibbs 2010). If genetic drift had a significant effect on levels of venom variation then we would expect to find a positive correlation between observed levels of venom and genetic variation. We also used Mantel matrix randomization tests as implemented in the program ZT test (Bonnet and Van de Peer 2002) to determine if significant correlations were present between a matrix consisting of a measure of overall population differences in venom composition (pairwise A values as determined by the MRPP analyses), and matrices containing population measures of genetic differentiation (pairwise population F st values based on the microsatellite data set described above) and linear distances (in km) between all pairs of populations. We expect a positive relationship between pairwise F st values and A values if the degree to which populations are genetically similar has an influence on the degree to which populations differ in venom composition.
Results
Venom Bands
We identified 48 distinct scoreable venom protein bands across 254 individual snakes (Appendix). Using our size-based criteria, we classified 8 bands (16.7%) as l-amino acid oxidase nucleases (abbreviated as LAAO-nuclease), 22 (45.8%) as type I and III metalloproteinases (MP), 4 (8.3%) as serine proteases (SP), 8 (16.7%) as type 2 phospholipases (PLA2s), 3 (6.0%) as disintegrins (DIS), 2 (4.1%) as myotoxins (MYO), and 1 band each (2.0%) as cystine rich secretory protein (CRISP) and lectin proteins, respectively. The mean frequency of different types of proteins across all individuals varies considerably (Table 1): most bands are present at intermediate frequencies (mean: 0.35–0.47) while serine proteases are consistently found at high frequencies (0.94) whereas myotoxins and the C-type lectin band were consistently uncommon (mean frequencies of 0.16 and 0.004, respectively).
However, mean frequency values mask the wide range of variation in the frequency of specific bands within four of the six types of proteins (Fig. 3a, Table 1). Where multiple bands are present, mean frequencies for metalloproteinase, phospholipases, LAAO, and disintegrin proteins span values from 0.12 to 0.88. The exceptions are serine protease bands, which are found in most individuals and myotoxins, which are always uncommon (Fig. 3e, g). When overall frequency data across all bands is pooled (Fig. 3a), the overall distribution is bimodal with a significantly greater number of bands found at high (0.9–1.0) and low (0.01–0.1) frequencies than predicted from random expectation (comparison of expected vs. observed number of bands at high, mid-range (0.1–0.8) and low frequency classes; χ2 = 33.1; df = 2; P < 0.0001). Consistent with this pattern, the best-fit LOESS line is also bimodal with peaks at either extreme of the distribution.
a Frequency distribution of protein bands across a pooled sample of all individuals. Distributions are shown for all bands (a) and for bands for specific protein families only (b–g). b Frequency distribution for all venom bands within each population. Lines on all plots represent the LOESS best-fit curve
The tendency for specific bands to be at high (frequency ≥ 0.65), medium (0.35–0.64), or low (0.0–0.34) frequency seems to be conserved across different populations for many proteins. All populations show the bimodal frequency spectra across all proteins seen in Fig. 3a and, in each case, the best-fit LOESS line is approximately U-shaped (Fig. 3b). When the frequencies of specific bands are compared across all 10 populations, 73% of all proteins show a significant (P < 0.05) tendency to be consistently at high or low abundance and this proportion is similar across most types of proteins (Table 2). Thus, most venom proteins expressed in S. c. catenatus tend to fall into two classes: those that are consistently expressed in the venom of most individuals regardless of geographic location and another class of proteins which tend to be less frequently expressed in snakes in any given population. This pattern is present across all types of proteins for which multiple distinct proteins are observed with the exception of serine protease proteins which are present in most snakes in all populations.
Patterns of Venom Band Variation
We used NMS to describe band presence/absence variation across all individuals. NMS Autopilot in PC-ORD chose a three-dimensional representation of the data as providing a substantial and statistically significant reduction in stress as compared with randomized data (results not shown). The overall stress value for the best-fit three-dimensional representation of the data was 21.9. Overall, the three axes chosen explain a total of 70% of the presence/absence variation among individuals with each axis explaining a roughly equivalent amount of overall variation (coefficients of determination for Axis 1: 26%; Axis 2: 23%; Axis 3: 21%; Table 3).
To gain a biological interpretation of each axis we calculated Spearman’s rank correlation coefficients (r) between axis scores for each individual and the presence or absence of a particular band. We used the magnitude of the r value as a measure of the strength of the covariation between axis and individual band variation and arbitrarily chose a cut-off of r ≥ 0.3 for assessing which bands had biologically meaningful correlations with axis scores. Based on this criterion, each of the three axes shows correlations with distinct sets of nine individual bands each from a variety of protein families (Table 3). Five of these bands (5-lnuc, 6-lnuc, 11-mp, 19-mp, 22-mp, and 34-crisp—see Appendix) show correlations with two different axes and therefore represent proteins which have a disproportionate impact on overall patterns of venom band variation. Specific axes show distinct patterns of covariation with particular bands: Axis 1 has positive correlations with 3 metalloproteinase and 2 LAAO-nuclease bands and negative correlations with a myotoxin and 2 PLA2 bands. Axis 2 summarizes variation involving a tradeoff in the frequency of the CRISP protein band relative to the frequencies of 8 other metalloproteinase and LAAO-nuclease bands. Finally, Axis 3 scores show positive correlations with nine bands from a diverse set of proteins (LAAO-nuclease, metalloproteinase, serine proteases, PLA2s, and disintegrins). Our results indicate an important component of overall venom variation in S. c. catenatus is covariation in the occurrence of multiple individual bands from all major classes of venom proteins.
Population Differentiation
We assessed population differentiation in venom composition in a number of ways. First, we compared NMS axis scores across populations both graphically and statistically. Figure 4 shows mean population scores (±95% CI) for different combinations of axes. Most differentiation occurs along the joint distribution of axes 1 and 2 (Fig. 4a) but separation between populations is also present along other combinations of axes (Fig. 4b, c). One-way ANOVAs showed significant differences in individual scores between populations for all three axes (all P < 0.001) and post-hoc comparisons show that many pairs of populations differ in individual scores especially with respect to Axis 1 (see Appendix).
Plots of mean NMS scores (+95% CI) for 10 populations of S. c. catenatus. a Axis 1 and 2 scores. b Axis 1 and 3 scores. c Axis 2 and 3 scores. Abbreviations of population names are as in Fig. 1
MRPP analyses show a similar pattern of wide-spread differentiation in venom composition between populations. The A statistic, which is a measure of the overall level of differentiation between populations similar to F st in population genetics was equal to 0.27 and was significantly greater than chance based on Monte Carlo sampling (P < 0.00001). Values for A from pairwise population comparisons ranged from 0.02 to 0.40 (Table 4); values for all but three comparisons (involving nearby populations GRL1-3) were significantly different from chance (all P < 0.011; critical P value adjusted for multiple tests using Benjamini-Hochberg experiment-wide correction [Narum 2006]). Inspection of mean A values for each population shows that the overall level of differentiation for each population is roughly similar with the exception that two population in the center of the geographic distribution of sampled populations show somewhat higher (GRL-1) and lower (KL) levels of differentiation from the other populations (Table 4).
Finally, ISA showed that 16 of 41 bands analyzed had IV values that were significantly greater than expected by chance (P < 0.012; B-Y adjusted critical value) (Table 5). These bands represent 5 of the 7 different types of venom proteins and make up a substantial portion of the bands from each type (LAAO-nucleases: 4/7 total bands [57%]; metalloproteinases: 7/18 [39%]; CRISP: 1/1; [100%]; PLA2: 3/6 [50%]; myotoxins: 1/2 [50%]). If we use the difference between observed and expected IV values as a measure of the magnitude of differentiation, then the five bands showing the largest differentiation all come from different classes of venom proteins. The most distinct is 39-PLA, followed by 9-mp, 1-lnuc, 51-myo, and 31-CRISP, respectively. This supports the idea that differentiation occurs across multiple, potentially different, functional types of venom proteins.
Covariation of Venom and Genetic Variation
We found no correlation between population levels of venom or genetic variation. There was no significant correlation between within-population levels of venom diversity and genetically effective population size estimated as θ (range of θ values: 0.14–2.2; r = 0.068; P = 0.85). We also detected no significant correlation between venom differentiation and either genetic differentiation or distance between populations. All Mantel tests had relatively low r values and none were significantly greater than random expectation (venom [pairwise A values] versus genetic differentiation [F st; range of values = 0.08–0.38; r = 0.26; P = 0.16]; venom differentiation vs. distance in km [range: 13–1218 km; r = 0.18; P = 0.27]). A partial Mantel test which examined the correlation between venom differentiation and genetic differentiation while holding distance constant was also non-significant (partial r = 0.24; P = 0.20). Thus, venom differentiation appears to be independent of the overall genetic divergence between S. c. catenatus populations and is not related to a measure of the potential for gene flow between populations (distance). However, this conclusion is provisional given the relatively small sample size used in the analysis (see Legendre and Fortin 2010).
Discussion
Mechanisms Responsible for Venom Band Variation
As with other gel-based studies of venom variation at the population level, we quantified variation across individuals using the presence–absence of specific bands. Our interpretations of the results rest on a number of assumptions and caveats. First, we assume that the same size band represents the same protein or sets of proteins in different snakes. This could be tested via proteomics-based analyses of proteins harvested from the same size band in different snakes as described by Sanz et al. (2006) although we did not carry out such analyses. Second, our measure of venom variation is limited in several ways that underestimates the overall variation in venom composition within and between individuals. Specifically, we only analyzed variation in terms of presence or absence of a specific band and did not attempt to account for variation in the relative amount of protein present when a given band occurred in different individuals even though Gibbs et al. (2009) has shown that the relative amount of a specific protein can vary among individuals. However, Gibbs et al. (2009) also showed that presence/absence variation was an important source of variation in proteins from all major classes of proteins. Further, 2D gel electrophoresis on specific bands isolated from 1D gels has shown that such “bands” may consist of multiple proteins of different polarities (Sanz et al. 2006). Thus, protein “bands” that we use in our analysis may actually consist of multiple isoforms which we assume consist largely of the same protein type.
We emphasize that our goal in this paper was to characterize phenotypic variation in venom both within and between populations. To understand the evolutionary significance of venom band variation it is necessary to clarify the molecular mechanisms responsible for generating presence/absence variation in specific bands and at this stage we do not claim to have made such an assessment. Comparisons between small numbers of parents and offspring have repeatedly identified similarities in venom composition across generations that imply genetic control of some portion of the venom phenotype (see review in Chippaux et al. 1991). One genetic mechanism that could lead to specific proteins showing patterns of segregating variation is allelic variation at specific loci that results in structurally distinct proteins that cause changes in protein sizes (e.g., Wooldridge et al. 2001). However, as described by Gibbs et al. (2009), presence and/or absence of a specific band could also be due to whether or not an individual expresses the proteins encoded by a particular locus or set of loci (gene expression). Under the first mechanism, population differentiation in venom composition would be due to the fixation of alternate alleles encoding distinct proteins or the gain and/or loss of particular loci encoding different proteins (Gibbs and Rossiter 2008). As such, the evolutionary question of interest becomes explaining why selection favors alternate alleles in different populations possibly due to local adaptation (Mitchell-Olds et al. 2007). In contrast, if differences in expression are responsible for population differences then the outstanding question is different, namely why are individual snakes showing high levels of phenotypic plasticity in this adaptive trait (Gibbs et al. 2009)? As discussed in Gibbs et al. (2009), we suspect that both mechanisms are responsible for venom variation in S. c. catenatus and so an important step forward in understanding the evolutionary basis for this variation is assessing the relative importance of regulation versus structural changes as causes of variation in specific proteins.
Population Frequencies of Venom Bands
A striking feature of frequency spectra of venom bands is that within and across all populations, individual bands show a U-shaped distribution with a disproportionately high number of bands either being common or rare with relatively few bands present at intermediate frequencies. This pattern is similar to that observed for allozyme variation across loci in populations of many organisms and has been interpreted as the distribution predicted under a neutral evolutionary model in which allozyme frequencies evolve via the joint effect of drift and mutation (Fuerst et al. 1977). The superficial match between the venom distribution and the predicted distribution suggests the possibility that a substantial amount of the venom variation has an allelic basis suggesting neutral evolutionary processes and not selection may play a large role in molding variation within populations. However, under the drift-mutation model, the identity of the specific proteins at high or low frequency in different populations should be random. Instead, we find that roughly 73% of all proteins are consistently either common or rare in different populations. This rejects the hypothesis that random processes are responsible for determining the relative frequencies of different proteins in different populations and suggests that the repeatable characterization of proteins as either common or rare may have some deterministic basis possibly related to the function of the proteins.
As a testable hypothesis, we propose that the frequency of specific proteins reflects differences in their functional role in the overall venom phenotype in terms of killing and digesting different prey (Gibbs et al. 2009). In particular, high frequency venom proteins found in most snakes in most populations (e.g., most serine proteases) may be analogous to highly expressed ‘‘housekeeping’’ proteins which perform a variety of essential cellular functions (e.g., Warrington et al. 2000) and may perform generic killing and digestive functions that are not prey specific. In contrast, proteins present at low frequencies (e.g., myotoxins and many PLA2s) may be more plastic in either evolutionary or ecological timescales and serve to “customize” an individual snake’s venom to feeding on particular prey requiring a specific venom protein (Mackessy et al. 2006). This hypothesis could be tested experimentally by monitoring changes in the frequencies of different venom proteins in snakes fed over long periods of time on distinct diets combined with functional tests of the effects of specific high and low frequency venom proteins on different prey.
Population Differentiation
There is strong evidence for population differentiation in venom proteins yet the evolutionary cause of this pattern remains unclear. The lack of an association between patterns of venom and genetic variation argues that population structure alone acting through genetic drift and/or gene flow has limited, if any influence, on venom composition at the population level (e.g., Williams et al. 1988). Local adaptation to population-specific differences in prey (e.g., Daltry et al. 1996) remains a leading hypothesis to explain differences in venom composition between S. c. catenatus populations yet we were unable to test if this mechanism explains the observed differentiation. One prediction is that if local adaptation in venom is present then snakes in different populations should show differences in diet. In fact, there is limited evidence to suggest that this is not the case. Specifically, Weatherhead et al. (2009) reported an analysis of diet using fecal samples for two populations (BPNP and KL) that show substantial differences in venom composition with the result that the prey consumed by snakes in each population was markedly similar. This is inconsistent with the idea that local adaptation to prey differences is responsible for venom differences. A thorough exploration of the possible role of selection as a cause of population differences in venom will require establishing the molecular mechanism responsible for presence/absence variation in venom bands (see below), more detailed analyses of diet across a broader range of populations using a more sophisticated method of prey identification such as stable isotope analysis (Urton and Hobson 2005), and direct analysis of levels of differentiation in loci coding for venom proteins that contribute to differences in venom between populations (Duda and Lee 2009). We are currently exploring all these approaches.
Regardless of the mechanism responsible, it is clear that a diversity of proteins are responsible for population-level differences in venom composition. As with the multivariate analysis of venom variation, differentiation involves a variety of distinct proteins suggesting that the population differences are functionally complex in terms of potential impact on prey. These results also identify potential candidate genes and proteins that may be responsible for local adaptation in these snakes and which can be targeted for future in-depth studies at the population level (e.g., Duda and Lee 2009).
Integration of the Venom Phenotype
Previous work (e.g., Daltry et al. 1996; Forstner et al. 1997) has used multivariate techniques to summarize phenotypic variation in venom composition across populations but this work is the first to identify at a crude level, the specific proteins that contribute to each axis of variation. As such, it provides new insights into the degree to which the overall venom phenotype is integrated across functionally distinct types of proteins. As summarized by Mackessy (2009—see Table 1), proteins from different families play distinct roles in the overall function of venom in terms of the capture and processing of prey. Our multivariate analysis of venom variation suggests that the overall venom phenotype can be broken down into a small number of axes which show a moderate degree of integration across functionally distinct sets of proteins. For example, as shown in Table 3, Axis 1 scores from the NMS analyses show high correlations with bands from four types of proteins each of which has a different function in capturing and digesting prey (see Mackessy 2009 for review). The other two NMS-defined axes show high correlations with a similarly diverse set of specific proteins.
The significance of these results is that it argues that at least part of the venom phenotype is organized into a relatively small number of modules each consisting of a suite of distinct proteins each of which differs in their effect on prey. Our analysis identifies these proteins and provides an empirical framework for testing whether these modules represent functionally distinct sets of proteins that have prey specific effects and whether synergistic interactions between specific proteins play a role in the function of venom (e.g., Chaim-Matyas et al. 1995).
References
Aguilar I, Guerrero B, Salazar AM et al (2007) Individual venom variability in the South American rattlesnake, Crotalus durissus cumanensis. Toxicon 50:214–224
Barlow A, Pook CE, Harrison RA et al (2009) Co-evolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc R Soc B 276:2443–2449
Beerli P (2009) MIGRATE-N: estimation of population sizes and gene flow using the coalescent. http://popgen.scs.fsu.edu/Migrate-n.html
Benfey PN, Mitchell-Olds T (2008) From genotype to phenotype: systems biology meets natural variation. Science 320:495–497
Bonnet E, Van de Peer Y (2002) zt: a software tool for simple and partial Mantel tests. J Stat Softw 7:1–12
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem 72:248–254
Calvete JJ (2009) Venomics: digging into the evolution of venomous systems and learning to twist nature to fight pathology. J Proteomics 72:121–126
Calvete JJ, Juarez P, Sanz L (2007) Snake venomics: strategy and applications. J Mass Spectrom 42:1405–1414
Campbell JA, Lamar W (2004) The venomous reptiles of the western hemisphere. Cornell University Press, Ithaca, NY
Chaim-Matyas A, Borkow G, Ovadia M (1995) Synergism between cytotoxin P4 from the snake venom of Naja nigricollis nigricollis and various phospholipases. Comp Biochem Physiol B: Biochem Mol Biol 110:83–89
Chippaux J-P, Williams V, White J (1991) Snake venom variability: methods of study, results and interpretation. Toxicon 29:1279–1303
Chiucchi J, Gibbs HL (2010) Similarity of contemporary and historical levels of gene flow among highly-fragmented populations of a rattlesnake. Mol Ecol 19:5345–5358
Daltry JC, Wuster W, Thrope RS (1996) Diet and snake venom evolution. Nature 379:537–540
Duda TF Jr, Lee T (2009) Ecological release and venom evolution of a predatory marine snail at Easter Island. PLoS ONE 4:e5558
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366
Forstner MRJ, Hilsenbeck RA, Scudday JF (1997) Geographic variation in whole venom profiles from the mottled rock rattlesnake (Crotalus l. lepidus) (Kennicott) in Texas. J Herpetol 31:277–285
Fuerst PA, Chakraborty R, Nei M (1977) Statistical studies on protein polymorphism in natural populations. I. Distribution of single locus heterozygosity. Genetics 86:455–483
Gibbs HL, Rossiter W (2008) Rapid evolution by positive selection and gene gain and loss: PLA2 venom genes in closely related Sistrurus rattlesnakes with divergent diets. J Mol Evol 66:151–166
Gibbs HL, Sanz L, Calvete JJ (2009) Snake population venomics: proteomics-based analyses of individual variation reveals gene regulation effects on venom protein expression in Sistrurus rattlesnakes. J Mol Evol 68:113–125
Gregory-Dwyer VM, Egen NB, Bosisio AB et al (1986) An isolectric focusing study of seasonal variation in rattlesnake venom proteins. Toxicon 24:995–1000
Huang P, Mackessy SP (2004) Biochemical characterization of phospholipase A2 (trimorphin) from the venom of the Sonoran lyre snake Trimorphodon biscutatus lambda (family Colubridae). Toxicon 44:25–34
Legendre P, Fortin M-J (2010) Comparison of the Mantel test and alternative approaches for detecting complex multivariate relationships in the spatial analysis of genetic data. Mol Ecol Resour 10:831–844
Mackessy SP (1988) Venom ontogeny in the Pacific rattlesnakes, Crotalus viridis helleri and C. viridis oreganus. Copeia 1988:92–101
Mackessy SP (2009) Venom composition in rattlesnakes: trends and biological significance. In: Hayes WK, Beaman KR, Cardwell MD, Bush SP (eds) The biology of rattlesnakes. Loma Linda University Press, Loma Linda, CA, pp 495–510
Mackessy SP, Sixberry NM, Heyborne WH et al (2006) Venom of the Brown Treesnake, Boiga irregularis: ontogenetic shifts and taxa-specific toxicity. Toxicon 47:537–548
Mather PM (1976) Computational methods of multivariate analysis in physical geography. Wiley, Chichester
McCune B, Grace JB (2002) Analysis of ecological communities. MjM Software, Gleneden Beach, Oregon, USA, 304 pp. (www.pcord.com)
Mielke PW (1984) Meteorological applications of permutation techniques based on distance functions. In: Krishnaiah PR, Sen PK (eds) Handbook of statistics, vol 4. North-Holland, Amsterdam, pp 813–830
Mitchell-Olds T, Willis J, Goldstein D (2007) Which evolutionary processes influence natural genetic variation for phenotypic traits? Nat Genet 8:845–856
Nachman MW (2006) Detecting selection at the molecular level. In: Fox CW, Wolf JB (eds) Evolutionary genetics case concepts studies. Oxford University Press, Oxford
Narum S (2006) Beyond Bonferroni: less conservative analyses for conservation genetics. Conserv Genet 7:783–787
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Salazar AM, Rodriguez-Acosta A, Giron ME et al (2007) A comparative analysis of the clotting and fibrinolytic activities of the mapanare (Bothrops atrox) snake venom from different geographical areas in Venezuela. Thromb Res 120:95–104
Sanz L, Gibbs HL, Mackessy SP et al (2006) Venom proteomes of closely-related Sistrurus rattlesnakes with divergent diets. J Proteome Res 5:2098–2112
Serrano SMT, Shannon JD, Wang D et al (2005) A multifaceted analysis of viperid snake venoms by two-dimensional gel electrophoresis: an approach to understanding venom proteomics. Proteomics 5:501–510
Urton EJM, Hobson KA (2005) Intrapopulation variation in gray wolf isotope (15N and 13C) profiles: implications for the ecology of individuals. Oecologia 145:317–326
Warrington JA, Nair A, Mahadevappa M (2000) Comparison of human adult and fetal expression and identification of 535 housekeeping/maintenance genes. Physiol Genomics 2:143–147
Weatherhead PJ, Knox JM, Harvey DS et al (2009) Diet of Sistrurus catenatus in Ontario and Ohio: effects of body size and habitat. J Herpetol 43:693–697
Williams V, White J, Schwaner TD et al (1988) Variation in venom proteins from isolated populations of tiger snakes (Notechis ater niger, N. scutatus) in south Australia. Toxicon 26:1067–1075
Wooldridge BJ, Pineda G, Banuelas-Ornelas JJ et al (2001) Mojave rattlesnakes (Crotalus scutulatus scutulatus) lacking the acidic subunit DNA sequence lack Mojave toxin in their venom. Comp Biochem Physiol Part B 130:169–179
Wuster W (1999) Testing causal hypotheses for venom variation in snakes. Toxicon 37:289
Acknowledgments
Most importantly, we thank Jeff Davis, Mike Dreslik, Dan Harvey, Matt Kowalski, Greg Lipps, Kevin Shoemaker, and Doug Wynn for their generous help with collecting samples. Without their assistance this study would not have been possible. Steve Mackessy, Jose Diaz, and Andrea Doseff provided important assistance with lab procedures, Stuart Ludsin, and Laura Kubatko gave advice on statistical analyses, and the Gibbs lab, Paul Fuerst and an anonymous reviewer provided comments. This work was supported by funds from Ohio State University and the Ohio Division of Wildlife.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lisle Gibbs, H., Chiucchi, J.E. Deconstructing a Complex Molecular Phenotype: Population-Level Variation in Individual Venom Proteins in Eastern Massasauga Rattlesnakes (Sistrurus c. catenatus). J Mol Evol 72, 383–397 (2011). https://doi.org/10.1007/s00239-011-9437-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-011-9437-4