Abstract
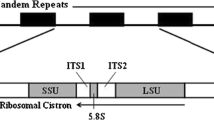
This study investigates the ribosomal RNA transcript secondary structure in corals as confirmed by compensatory base changes in Isopora/Acropora species. These species are unique versus all other corals in the absence of a eukaryote-wide conserved structural component, the helix III in internal transcriber spacer (ITS) 2, and their variability in the 5.8S-LSU helix basal to ITS2, a helix with pairings identical among all other scleractinian corals. Furthermore, Isopora/Acropora individuals display at least two, and as many as three, ITS sequence isotypes in their genome which appear to be capable of function. From consideration of the conserved elements in ITS2 and flanking regions, it appears that there are three major groups within the IsoporaAcropora lineage: the Isopora + Acropora “longi” group, the large group including Caribbean Acropora + the Acropora “carib” types plus the bulk of the Indo-Pacific Acropora species, and the remaining enigmatic “pseudo” group found in the Pacific. Interbreeding is possible among Caribbean A. palmata and A. cervicornis and among some species of Indo-Pacific Acropora. Recombinant ITS sequences are obvious among these latter, such that morphology (as represented by species name) does not correlate with common ITS sequence. The combination of characters revealed by RNA secondary structure analyses suggests a recent past/current history of interbreeding among the Indo-Pacific Acropora species and a shared ancestry of some of these with the Caribbean Acropora. The unusual absence of helix III of ITS2 of Isopora/Acropora species may have some causative role in the equally unusual instability in the 5.8S-LSU helix basal to ITS2 of this species complex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reef-building scleractinian (stony) corals are key components of tropical coral reefs; they deposit the three-dimensional structure that provides habitats for many coral reef inhabitants and are the main primary producers on coral reefs due to photosynthesis by their endosymbiotic algae. Scleractinian corals first appeared in the fossil record in the mid-Triassic (~240 million years [my] ago) after a 14-my gap in the coral fossil record (Veron 1995; Stanley and Fautin 2001). This suggests that modern corals have not evolved from Paleozoic corals and has led to the hypothesis that the scleractinians have evolved from soft-bodied ancestors (the “naked-coral” hypothesis [Medina et al. 2006]).
Molecular data have revealed at least two different hypotheses regarding the origin of the Scleractinia. Early molecular phylogenies were not resolved at the base of the scleractinian tree, and later molecular phylogenies showed an origin of the Scleractinia that predates their earliest fossils (~300 my ago), both supporting the naked-coral hypothesis and suggesting that scleractinian corals evolved more than once and are thus not monophyletic (Romano and Palumbi 1996, 1997; Romano and Cairns 2000; Chen et al. 2002; Cuif et al. 2003). However, a recent phylogeny based on full mitochondrial genome sequences (Medina et al. 2006) places the origin of the Scleractinia much closer to their first appearance in the fossil record (~240–288 My ago), refuting the multiple origin hypothesis and supporting monophyly of the Scleractinia.
Within the Scleractinia, molecular data have revealed several relationships that differ from the traditional taxonomic groups based on morphological characteristics. For example, DNA sequence analysis has shown an unexpected division of the Scleractinia into ‘robust’ and ‘complex’ corals (Romano and Palumbi 1997; Romano and Cairns 2000), as well as nonmonophyly of certain Atlantic scleractinian families (e.g., Faviidae, Pectiniidae) (Fukami et al. 2004). Furthermore, genetic studies (in combination with in vitro crossing experiments and data on spawning times) have shown that interspecific hybridization is important in the evolution of some corals, particularly in the genus Acropora (reviewed by Willis et al. 2006), and that many species in the genus Acropora are poly- or paraphyletic (van Oppen et al. 2001).
Despite the hugely valuable insights into the evolutionary history of reef corals provided by molecular approaches, the development of molecular markers suitable for such studies has been slow. Initially, most studies were based on allozymes and nuclear ribosomal DNA (nrDNA) (reviewed by van Oppen et al. 2002a). Mitochondrial DNA shows an unusually slow rate of nucleotide substitution in corals and other anthozoans (van Oppen et al. 1999; Shearer et al. 2002; Hellberg 2006) and, therefore, lacks the power to distinguish between some taxa (Concepcion et al. 2006). For example, only 25 of 16,134 sites, representing the full mtDNA genome, were found to be variable among the three members of the Montastraea annularis species complex (Fukami and Knowlton 2005). A small number of single-copy nuclear DNA markers for the study of species boundaries and evolutionary relationships have recently been developed for several coral species (Hatta et al. 1999; van Oppen et al. 2001; Vollmer and Palumbi 2002; Fukami et al. 2004). The use of nuclear rDNA, and the internal transcribed spacer (ITS) regions, in particular, for coral systematics has been discouraged because of the extremely high level of intragenomic and intraspecific diversity observed in Acropora, some of which predates the evolution of several species (Vollmer and Palumbi 2004). Chen et al. (2004) and Wei et al. (2006) have shown that these ITS characteristics are restricted to the genus Acropora and have advocated the use of this marker as appropriate for phylogenetic analyses in other corals.
Here we show that, despite the large amount of noise in the primary sequence of Acropora ITS regions, the ITS2 RNA secondary structure reveals valuable information about evolutionary patterns in the Acroporidae. Chen et al. (2004) already recognized that the Acropora ITS2 secondary structures are strikingly different from those of other hard corals; however, their study lacked an analysis of compensatory base substitutions in the secondary structures presented. In the analyses here, we utilize all available ITS2 sequences of scleractinian corals, as well as other Anthozoa, and align those using RNA transcript secondary structure as confirmed by compensatory base changes (CBCs and hemi-CBCs), emphasizing the relatively conserved regions.
Materials and Methods
All the ITS2 sequences used are available from GenBank (search under “nucleotide,” using “ITS2 and anthozoa,” and then Blast with the 5.8S sequences to obtain the remainder). The list is available from the corresponding author upon request. Note that these were obtained originally by cloning PCR products, rather than direct sequencing of the PCR products. The Indo-Pacific Acropora carrying the “carib” motif can be found by Blast of that motif. Secondary structures were derived by comparing RNA transcript folds calculated by MulFold (http://frontend.bioinfo.rpi.edu/applications/mfold/cgi-bin/rna-form1.cgi) to find the fold common to a genus and related genera. A list of “pseudo” GenBank entries is available upon request, as well as an alignment based on pairing in the secondary structure, such that any two nucleotides that pair with each other are in the same two columns. The proposed secondary structure was confirmed by the presence of CBCs. ITS1 sequences were also examined and compared; no secondary structure was discerned in the Acropora RNA transcripts.
Results and Discussion
Standard Coral ITS2 RNA Transcript Secondary Structure
Analysis of the secondary structure of scleractinian coral ITS2 transcripts was first presented by Chen et al. (2004). Our secondary structures are related but not identical because we find that all corals (and all Anthozoa) except Isopora and Acropora have the basic four-helix structure, helices I, II, III, and, usually, IV. For all of them, the basal pairings of helix I and of helix II are highly conserved, and helix II has the characteristic pyrimidine-pyrimidine bulge near the base. In helix III, on the 5′ side near the tip, one finds the sequence of greatest overall conservation (GCGRAGGCTAAA), as expected from other eukaryote ITS2 analyses. Helix IV, as also expected, shows the greatest variation, being essentially identical only within a species; its secondary structure is not always obvious, for lack of comparative sequences that provide the CBC information to confirm the correctness of the fold. The above ignores one unique hard coral sequence in GenBank, the sole sequence available for Alveopora. This particular sequence has the standard helices I and II, and a recognizable start of III, but there are obvious errors scattered in various places and it appears that some sequence is missing toward the 3′ end.
By contrast, within Isopora and Acropora, all these descriptions apply, except that helix III is absent. This molecular accident is extremely rare among eukaryotes, known only for two other groups, the siphonaceous marine green algae and a small group of parasites of the Valkampfiidae (Coleman 2007). Lacking biochemical knowledge, as yet, of the precise motif requirements for successful ITS2 processing in the nucleolus, it is difficult to evaluate this phenomenon in evolutionary terms.
The retained ITS2 secondary structures (helices I and II) suggest Astreopora as the closest genus to the Isopora/Acropora clade. This contradicts molecular phylogenies which suggest that the Montipora/Anacropora clade is the sister group to Acropora/Isopora (Fukami et al. 2000; Le Goff-Vitry et al. 2004). However, all three of these Astrocoeniina genera have a significantly truncated helix III of ITS2, albeit while still retaining the universally conserved sequence on the 5′ side.
Special Case of the Isopora and Acropora Species
The genera Isopora and Acropora of Astrocoeniina corals are exceptional with respect to all other Anthozoa. Helices I and II are standard and obvious in these two genera and largely conserved between them. The lack of helix III, with its 5′ highly conserved nucleotide motif, is striking (Fig. 1c and d). From helix II to the LSU, there may be as many as 40 nucleotides among these species, the initial 12 or so obviously dedicated to the single-stranded region bridging the junction between II and III as in the other coral genera. The 3′ region may or may not be capable of forming a helix IV (no CBC support), but there is clearly no discernible helix III type as found in other scleractinian corals (Fig. 1). The ITS1 sequences of these two genera also differ from the rest of the corals; all of the Isopora and Acropora sequences have a very foreshortened ITS1, with no apparent helices, in distinct contrast to ITS1 of all other scleractinian corals.
ITS2 RNA secondary structures of scleractinian corals. Foldings of (a) Platygyra sinensis (AF481901), (b) Montipora taiwanensis (AY722778), (c) Acropora valida (U82727), and (d) Acropora palmata (AF239100). Arrows designate the pyrimidine-pyrimidine nonpairing that is characteristic of helix II in eukaryotes. The most highly conserved nucleotide sequence, on the 5′ side of helix III, is bounded by the heavy line. The bracket indicates the region of 18 nucleotide pairs maintained identical among suspected interbreeders of, for example, Platygyra. The sequence in d represents the longer of the two isoforms found in Caribbean Acropora species. The sequence there surrounded by a dashed line is the “carib” motif, varying by only a few nucleotide alterations in the end loop positions or just following them (see text)
Isopora
Helices I and II have identical sequence among the Isopora species except at their tips. Interestingly, the sequences of Isopora cuneata available from the Great Barrier Reef are essentially identical in sequence for the region of helix I, helix II, and the II–III bridge, but the remainder is remarkably different in sequence from the one Isopora cuneata sequence from Indonesia (GenBank AY722738), which in turn is nearly identical to all the other sequences of Indonesian Isopora species (I. cuneata, I. togianensis, I. palifera, and I. brueggemanii).
Isopora and Acropora sequences are nearly identical for all 11 basal pairings of helix II (three hemi-CBCs in total), but in helix I, there is a CBC at pairing number 7. This is G-C in all Isopora and in one group of Acropora, the only unique shared characteristic; all other Acropora have a C-G there. One further point is that there is no evidence that Isopora individuals might have two loci of rDNA repeats per haploid genome (see below).
The 5.8S-LSU Pairing in Isopora/Acropora
One additional region of secondary structure emphasizes the uniqueness of the Isopora and Acropora species. As in all other eukaryotes, there is a helix formed by the 3′ end of the 5.8S gene with the 5′ end of the LSU that provides a base for the ITS2 folding (Fig. 2). While all other scleractinian corals, and even other hexacorals, have 11 pairings identical in this helix (high-lighted blue in Fig. 2), the Isopora and Acropora versions display five different variants of this helix.
Helix formed by 5.8S-LSU pairing basal to ITS2. For Acropora valida (U82727; IndoPacific coral type), Acropora palmata (AF239100; Caribbean Acropora type), and Platygyra sinensis (AF481885; typical scleractinian coral type), the 3′ end of 5.8S RNA is shown paired with the 5′ end of the LSU. For the 26 genera of the typical scleractinian coral type, the few nucleotide changes found either are in the bulge or are single nucleotide changes such that the pairing remains intact, i.e., a U-G becomes U-A (a hemi-CBC). There is a single case of a full CBC; the genus Galaxea has C-G at the basalmost pairing, which is U-A in all other genera. For comparisons of Acropora/Isopora with other scleractinian corals, the 11 highly conserved pairings are highlighted in blue. An asterisk marks the pairing (U-A) that is uniform in other Scleractinia but C-G (a CBC) in all Isopora/Acropora except the “pseudo” isotype. To the left of the scleractinian coral helix is the 5.8S sequence of Isopora (e.g., DQ251736). No LSU sequence of Isopora is available for this comparison but two nucleotide changes in 5.8S suggest that Isopora LSU might differ. On either side of the Caribbean Acropora helix, in red, are the only nucleotide changes found in either the 5.8S or the LSU of the “longi” isotype. This isotype of ITS2 differs from the other acroporids by a CBC. To the left of the Indo-Pacific Acropora helix, in red, are the altered nucleotides found in the “pseudo” isotype (e.g., AF540616). No “pseudo” LSU is available, but at least two changes can be anticipated
The 11 highly conserved 5.8S-LSU helix pairings are identical in the vast majority of Acropora for which the LSU component is available but differ from the “scleractinian coral” helix by one CBC (asterisk in Fig. 2). In addition, the Caribbean helix has lost one pairing, forming a larger bulge. The third variant characterizes one category (“longi”) of ITS2 sequences, found among A. longicyathus, A. tenuis, A. cytherea, A. hyacinthus, and A. spicifera sequences. Its 5.8S-LSU helix changes are illustrated in Fig. 2 adjacent to the Caribbean Acropora diagram, fundamentally just a single CBC. The “longi” Acropora sequences are the ones that share with Isopora the same pairing at the seventh position in helix I.
The additional two variants lack the LSU information as yet, but clearly suggest a necessity for its alteration. The Isopora 5.8S sequence (adjacent to the “scleractinian coral” diagram in Fig. 2) appears to have lost one pairing and has an additional change suggesting a unique CBC among the most conserved positions. The 5.8S region of the final variant, called “pseudo,” appears in Fig. 2 adjacent to the I-P Acropora helix. Two of the otherwise conserved pairings are changed in the 5.8S region in such a way as to suggest that CBCs are required in the LSU. Exactly this altered version of 5.8S RNA, “pseudo,” is found in some individuals of seven species (A. aspera, A. cerealis, A. millepora, A. muricata, A. pulchra, A. spathulata, and A. spicifera).
These synapomorphies in the 5.8S region of Isopora and Acropora are all the more striking because the 5.8S-LSU helix basal to ITS2 is absolutely conserved at the family or higher level in eukaryotes generally. This helix may contain diagnostic information to help the cell decide whether or not a primary transcript can be processed (Cote et al. 2002). The significance of variation here in Isopora and Acropora remains uncertain, but might suggest that it has taken on an additionally detailed role in sequence recognition for rRNA transcript processing, since the selectivity provided normally by helix III is absent.
Isotype Nomenclature for Acropora
There is no uniformity of sequence within a species of Acropora, unlike the situation in other eukaryotes or even Isopora, where ITS2 sequences are more similar within species than between, and the 5.8S sequences within a genus are essentially identical. Instead, all the many available sequences of the Acropora species can be categorized easily into just a few isotypes, according to their 5.8S + ITS2 helix 1 sequences, and further subdivided into groupings, subisotypes, by their sequences 3′ to helix II. Figure 3 illustrates the relative conservation levels of these different regions of 5.8S and ITS2.
Color diagram indicating regions of different nucleotide conservation levels in ITS2. Levels range from <95% identity within species (uncolored), through >95% identity in species to genus, to >95% identity in genus, to > 95% identity above genus and even family level (darkest blue). Position of the 5.8S region pairing with LSU (see Fig. 4) indicated by large arrowheads. As indicated in the lower diagram, Acropora/Isopora ITS2 sequences have no helix III
Caribbean Acropora
There are >200 ITS2 sequences in GenBank for the three Caribbean Acropora species, A. cervicornis, A. palmate, and A. prolifera. Except at their tips, the sequences of helices I and II are near-identical for all (one hemi-CBC), and the 5.8S region is likewise identical. Thus there is but one isotype in the Caribbean species. The region 3′ to helix II, however, displays two sequence types differing in both length and sequence; hence there are two subisotypes, each quite uniform in sequence. Both subisotypes have so far been found within all individual animals but one of all three species. This suggests that the three species were and/or are currently interbreeding, which is supported by analysis of single-copy nuclear and mitochondrial DNA markers (van Oppen et al. 2001; Vollmer and Palumbi 2002).
The shorter sequence of these three species is restricted to the Caribbean, but the longer sequence has a motif found elsewhere. There is a totally conserved sequence AAAGGTGATCACACAT in the post-helix-II ITS2, not present in the short isotype, but present in certain Indo-Pacific Acropora. In Fig. 1d (dashed line), this appears as the 5′ side of helix IV plus the terminal loop plus three nucleotides on the 3′ side of the helix.
Indo-Pacific Acropora
All Indo-Pacific Acropora ITS2 sequences are remarkably homogeneous in their helices I and II, but differ in the 3′ region of ITS2 and in aspects of 5.8S. Among the 18 species with sequences available for this study (Fig. 4), there are three different isotypes defined by the 5.8S-ITS2 helix I sequences (“I-P Acro,” “longi,” and “pseudo”), and these share six 3′ ITS2 variant sequences (A, B, C, “carib,” longi Y, and longi Z). The isotypes are invariant, but for the 3′ ITS2 categories (subisotypes) there can be some nucleotide variation within a subtype, but each subtype is a discrete cluster of very similar sequences, clearly distinct from any of the others. To simplify the discussion, Fig. 4 illustrates this situation for just the Indo-Pacific Acropora sequences. Solid lines indicate the fundamental sequence categories, e.g., isotype “I-P Acro,” subtype A; or isotype “I-P Acro,” subtype ‘carib.’ Note that, for example, “I-P Acro” subtype A shares the same 3′ terminal sequence category as “pseudo” subtype A.
Diagramatic representation of 5.8S-ITS2 isotypes known among Indo-Pacific Acropora species. The three 5′ isotypes (parentheses) are “I-P Acro,” “longi,” and “pseudo.” The six subtypes of 3′ termini (in color), the region of potential ITS2 helix IV, are A, B, C, “carib,” “longi Y,” and “longi Z.” Solid lines show the known types, connecting 5′ types with 3′ types. Abbreviated species designations (aspera, cerealis, cytherea, digitifera, florida, formosa, gemmifera, humilis, hyacinthus, longicyathus, millepora, muricata, papillare, pulchra, spathulata, spicifera, tenuis, valida) are superimposed on the diagram to illustrate which types are known so far in each species. Where the species name appears over a dashed line, the two connected types are found in the same species. A box around an abbreviated species name indicates that it is known that the two connected types can be found in a single animal
The “longi” isotype, named for A. longicyathus, is the only Acropora isotype that shares a unique secondary structure characteristic with Isopora, the CBC at position 7 of ITS2 helix I; however, Isopora 5.8S sequences differ from those of Acropora. There are only two subisotypes of “longi,” “longi Y” and “longi Z,” each quite uniform in sequence.
The “pseudo” isotype corresponds with subclade IVC in Fig. 1 of Márquez et al. (2003). Like “longi,” it displays only two variant subtypes in the ITS2 3′ sequences, A and B. It was suggested earlier that some of the variability of ITS sequences among Acropora arises from the presence of pseudogenes (Márquez et al. 2003). The evidence was the presence of substitutions predicted to disrupt pairings in the secondary structure of the 5.8S RNA and the larger number of substitutions at methylation sensitive sites in the “pseudo” 5.8S, relative to other sequences. The 5.8S/LSU helix pairing for these was not assessed by Márquez et al. (2003) because the LSU component was not available. Examination of ITS2 sequences and secondary structures of the “pseudo” isotype sequences reveals no sign of deterioration in the sequence or secondary structure that might be expected in pseudogenes. ITS2 is fully capable of forming the standard helices I and II like the other Acropora, suggesting that these are likely to be functional copies rather than pseudogenes relieved of selective pressure.
All other Indo-Pacific Acropora ITS2 sequences, which lack the “longi” or “pseudo” 5.8S characters, constitute the “I-P Acro” isotype. This is the largest group, and it has four subisotypes, A, B, C, and “carib.” The most interesting of these is “carib,” for here is found the 3′ sequence highlighted in the long Caribbean subisotype (Fig. 1d; dashed line). This nucleotide motif appears in the same position in the ITS2 of many individuals of Indo-Pacific species A. aspera, A. digitifera, A. florida, A. humilis, and A. pulchra. The “carib” subtype roughly corresponds to phylogenetic clade IV, based on a single-copy nuclear and a mitochondrial locus as described by van Oppen et al. (2001). These occur from Taiwan to the Great Barrier Reef of Australia. The Indo-Pacific individuals containing the Caribbean sequence motif show variant nucleotides in the motif only in the positions that are in the tip loop or 3′ of it, and no obvious helix IV can be formed; the “carib” subtype of “I-P Acro” isotype also has a slightly changed and longer helix I sequence.
Viewed solely from sequences of ITS2 and its adjacent regions, two generalizations arise. All scleractinian corals except Isopora and Acropora have the “standard” ITS2 secondary structure of other eukaryotes, with all the hallmark characteristics conserved. Isopora and Acropora ITS2 sequences retain standard secondary structure only through the helix II–III bridge, and lack the further 3′ characteristics, except for retention of a potential helix IV in some.
The Polyploidy Factor
There is one further major difference that is unique to Acropora animals. Apparently nearly all Acropora individuals, but not those of Isopora, have multiple copies of at least two quite different ITS2 isotypes, so far as known from the current data. The nuclear rDNA locus is a region notorious for its number of repeats, generally subject to strict homogenization except in known or suspected hybrids. In plants, animals, and protists, ITS2 variation within an individual (and usually within a species) is mostly limited to one to five transitions scattered among single-stranded regions, including apices of helices, except in hybrid lineages, where larger numbers of substitutions may be present (Proschold et al. 2005). Our analysis is limited in some cases by the availability of only one sequence per animal. Are we missing intermediate types differing from and/or bridging those present? The answer appears to be no. The most egregious example is the A. pulchra 79 animal (see Fig. 1 of Márquez et al. 2003), for which cloning and sequencing have revealed two “pseudo” sequences (79.2 and 79.5) and three of the “I-P Acro” isotype. Of these three “I-P Acro,” two are alike (79.3 and 79.4), falling in the A subtype, and the third (79.1) has a different 3′ terminal sequence, that of the B subtype. The two “pseudo” isotype sequences are both subtype A. Thus there are three total 5.8-ITS2 types in this animal, and none of intermediate character.
The only example of a probable exception to the general rule of two sequence types per animal involves a few individuals identified as A. florida and A. humilis. In these, all sequences so far are of the “I-P Acro” isotype, “carib” subtype. This might be due to the small number of clones sequenced, or it might be that these two species are unusual in this character.
The possibility of two loci of repeats in the genome was suggested by Vollmer and Palumbi (2004) in the context of Caribbean species, but not discussed further. This possibility (see Kenyon 1997 on the widespread putative polyploidy in Acropora species) is intriguing, for it would help to explain the two or more isotypes present. Since in corals all animals are at least diploid, if not at some higher ploidy level, the genome of an animal can have (in a hybrid) at least two ribosomal repeat cistrons. There could theoretically exist hybrid/polyploid animals with two, four, or even more different 5.8S-ITS2 types. We have uncovered none so great, and only a few animals with three subtypes (e.g., A. pulchra 79). Judging from the absence of high numbers of subtypes per animal, much of what we see may therefore be the consequence of past hybridizations that have already begun the sorting-out process, or may reflect the sporadic occurrence of hybridization events (Willis et al. 2006). Further chromosome counts and/or in situ cytological analyses of numbers of ribosomal repeat positions will help to resolve this.
Problems persist in distinguishing ancient lineage sorting from current/recent hybridization, the issues emphasized by Vollmer and Palumbi (2004), which they consider the weak point of using ITS sequence information. However, those authors ignored the secondary structure of ITS and, thus, the information concerning which sequence variation is relatively trivial versus more consequential. We agree with the conclusion of Vollmer and Palumbi (2004) that the Acropora are exceptional among all corals in their ITS2 variability, a point further emphasized by Chen et al. (2004) and by Wei et al. (2006). However, this variability is almost solely in the region between the end of helix II and the beginning of the LSU (Fig. 3).
The Species Problem
If every Acropora animal has at least two types of 5.8S-ITS2, then obviously every Acropora species does also. ITS2 types from two different species may be identical, but each may contain a different array of additional types. As can be seen from Fig. 4, just three species so far have only one subtype (A. florida and A. humilis have the “carib” type and A. longicyathus has the “longi” type); by contrast, individuals of A. pulchra display in toto five different sequence subtypes (“I-P Acro” subtypes “carib,” A, B, and C, and “pseudo” subtype A). The overall picture, from the ITS point of view, is of one interbreeding superspecies or syngameon in the Pacific Acropora—perhaps all cannot interbreed freely now, but a reticulate history might be inferred, and at least some direct experimental evidence for occasional current species interbreeding is available (reviewed by Willis et al. 2006). The relict nucleotide sequence shared by types found in Caribbean Acropora and one group of Pacific Acropora is evidence for a common Atlantic-Pacific ancestry, at some time in the past. No other biogeographic correlation is obvious, but as yet we have no sequences from the Indian Ocean, much less Africa. Sequence attention has mostly concentrated on the Australia-Indonesian region of the Indo-Pacific.
It is in the Pacific Ocean mixture that one encounters the clearest examples of probable evidence for hybridization. Some pairs of individual animals have sequences identical in the ITS2, while differing in their ITS1, and vice versa, possibly an indication of crossover resulting from interbreeding (e.g., A. hyacinthus AF538439 versus AF538444) (see also van Oppen et al. 2002b). In another case, ITS1 and ITS2 are almost identical in some sequences of both A. tenuis and A. hyacinthus (AF538475 and AF538485) but differ greatly from other examples of these species. One cannot help but suspect that morphology in these species is controlled by only a few genes with major effects, a possibility that has yet to be tested.
Summary
In sum, the Isopora/Acropora scleractinian corals represent one large clade, having branched from the precursor of the Isopora/Acropora clade some time in the past, since the ITS2 of essentially all eukaryotes, including other corals, has helices I, II, and III in ITS2. By contrast, Isopora and Acropora ITS2 sequences retain helices I and II but lack helix III, and each individual appears to have at least two recognizably different ITS2 types. The simplest explanation (though many more are possible) is that either a long period of evolution or a sudden molecular accident resulted in the Isopora/Acropora clade, with its unique types of 5.8S-LSU helix and also loss of helix III, plus acquisition of at least two cistrons of ribosomal repeats per haploid genome. In a later event, the species bearing the Caribbean signature sequence were split by the rise of the Panamanian barrier. Perhaps over a similar time span and perhaps only in the Pacific, one of the two rRNA cistrons diverged into the “pseudo” 5.8S isotype and another diverged into the “longi” variant. It seems there must be some further biogeographic pattern not yet discernible, since sequencing efforts are geographically limited. There are apparently only ca. eight Acropora sequence ITS2 subtypes in the Pacific, and their distribution does not seem to be random, though it does not correlate with species nomenclature. Thus the information gleaned from the ITS region is rich, though still enigmatic; at least it tells us what is needed next.
References
Chen CA, Wallace CC, Wolstenholme J (2002) Analysis of the mitochondrial 12S rRNA gene supports a two-clade hypothesis of the evolutionary history of scleractinian corals. Mol Phylogenet Evol 23:137–149
Chen CA, Chang CC VWN, Chen CH, Lein YT, Lin HE, Dai CF, Wallace CC (2004) Secondary structure and phylogenetic utility of the ribosomal internal transcribed spacer 2 (ITS2) in scleractinian corals. Zool Stud 43:759–771
Coleman AW (2007) Pan-eukaryote ITS2 homologies revealed by RNA secondary structure. Nucleic Acids Res 35:3322–3329
Concepcion GT, Medina M, Toonen RJ (2006) Noncoding mitochondrial loci for corals. Mol Ecol Notes 6:1208–1211
Cote CA, Greer CL, Peculis BA (2002) Dynamic conformational model for the role of ITS2 in pre-RNA processing in yeast. RNA 8:786–797
Cuif JP, Lecointre G, Perrin C, Tillier A, Tillier S (2003) Patterns of septal biomineralization in Scleractinia compared with their 28S rRNA phylogeny: a dual approach for a new taxonomic framework. Zool Scripta 32:459–473
Fukami H, Knowlton N (2005) Analysis of complete mitochondrial DNA sequences of three members of the Montastraea annularis coral species complex (Cnidaria, Anthozoa, Scleractinia). Coral Reefs 24:410–417
Fukami H, Omori M, Hatta M (2000) Phylogenetic relationships in the coral family Acroporidae, reassessed by inference from mitochondrial genes. Zool Sci 17:689–696
Fukami H, Budd AF, Paulay G, Sola-Cava A, Chen CA, Iwao K, Knowlton N (2004) Conventional taxonomy obscures deep divergence between Pacific and Atlantic corals. Nature 427:832–835
Hatta M, Fukami H, Wang WQ, Omori M, Shimoike K, Hayashibara T, Ina Y, Sugiyama T (1999) Reproductive and genetic evidence for a reticulate evolutionary history of mass-spawning corals. Mol Biol Evol 16:1607–1613
Hellberg ME (2006) No variation and low synonymous substitution rates in coral mtDNA despite high nuclear variation. BMC Evol Biol 6:24
Kenyon JC (1997) Models of reticulate evolution in the coral genus Acropora based on chromosome numbers: parallels with plants. Evolution 51:756–767
Le Goff-Vitry MC, Rogers AD, Baglow D (2004) A deep-sea slant on the molecular phylogeny of Scleractinia. Mol Phylogenet Evol 30:167–177
Márquez LM, Miller DJ, MacKenzie JB, van Oppen MJH (2003) Pseudogenes contribute to the extreme diversity of nuclear ribosomal DNA in the hard coral Acropora. Mol Biol Evol 20:1077–1086
Medina M, Collins AG, Takaoka TL, Kuehl JV, Boore JL (2006) Naked corals: skeleton loss in Scleractinia. Proc Natl Acad Sci USA 103:9096–9100
Proschold T, Harris EH, Coleman AW (2005) Portrait of a species: Chlamydomas reinhardtii. Genetics 170:1601–1610
Romano SL, Cairns SD (2000) Molecular phylogenetic hypotheses for the evolution of scleractinian corals. Bull Mar Sci 67:1043–1068
Romano SL, Palumbi SR (1996) Evolution of scleractinian corals inferred from molecular systematics. Science 271:640–642
Romano SL, Palumbi SR (1997) Molecular evolution of a portion of the mitochondrial 16S ribosomal gene region in scleractinian corals. J Mol Evol 45:397–411
Shearer TL, van Oppen MJH, Romano SL, Woerheide G (2002) Slow mitochondrial DNA sequence evolution in the Anthozoa (Cnidaria). Mol Ecol 11:2475–2487
Stanley GDJ, Fautin DG (2001) The origins of modern corals. Science 291:1913–1914
van Oppen MJH, Willis BL, Miller DJ (1999) Atypically low rate of cytochrome b evolution in the scleractinian coral genus Acropora. Proc Roy Soc Lond B Biol Sci 266:179–183
van Oppen MJH, Willis BL, van Vugt HWJA, Miller DJ (2000) Examination of species boundaries in the Acropora cervicornis group (Scleractinia, Cnidaria) using nuclear DNA sequence analyses. Mol Ecol 9:1363–1373
van Oppen MJH, McDonald BJ, Willis B, Miller DJ (2001) The evolutionary history of the coral genus Acropora (Scleractinia, Cnidaria) based on a mitochondrial and a nuclear marker: reticulation, incomplete lineage sorting, or morphological convergence? Mol Biol Evol 18:1315–1329
van Oppen MJH, Woerheide G, Takabayashi M (2002a) Nuclear markers in evolutionary and population genetic studies of scleractinian corals and sponges. Proc 9th Int Coral Reef Symp Bali 1:131–138
van Oppen MJH, Willis BL, van Rheede T, Miller DJ (2002b) Differences in spawning time and genetic structuring among reproductively compatible corals: an example from the Acropora aspera species group. Mol Ecol 11:1363–1376
Veron JEN (1995) Corals in space and time. UNSW Press, Sydney
Vollmer SV, Palumbi SR (2002) Hybridization and the evolution of reef coral diversity. Science 296:2023–2025
Vollmer SV, Palumbi SR (2004) Testing the utility of internally transcribed spacer sequences in coral phylogenetics. Mol Ecol 13:2763–2772
Wallace CC, Chen CA, Fukami H, Muir PR (2007) Recognition of separate genera within Acropora based on new morphological, reproductive and genetic evidence from Acropora togianensis, and elevation of the subgenus Isopora Studer, 1878 to genus (Scleractinia: Astrocoeniidae; Acroporidae). Coral Reefs 26:231–239
Wei NV, Wallace CC, Dai C-F, Moothien Pillay KR, Chen CA (2006) Analyses of the ribosomal internal transcribed spacers (ITS) and the 5.8S gene indicate that extremely high rDNA heterogeneity is a unique feature in the scleractinian coral genus Acropora (Scleractinia; Acroporidae). Zool Stud 45:404–418
Willis BL, van Oppen MJH, Miller DJ, Vollmer SV, Ayre DJ (2006) The role of hybridization in the evolution of reef corals. Annu Rev Ecol Syst 37:489–517
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coleman, A.W., van Oppen, M.J.H. Secondary Structure of the rRNA ITS2 Region Reveals Key Evolutionary Patterns in Acroporid Corals. J Mol Evol 67, 389–396 (2008). https://doi.org/10.1007/s00239-008-9160-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-008-9160-y