Abstract
Two types of gene encoding small subunits (SSU) of ADP-glucose pyrophosphorylase, a starch-biosynthetic enzyme, have been found in cereals and other grasses. One of these genes encodes two SSU proteins. These are targeted to different subcellular compartments and expressed in different organs of the plant: the endosperm cytosol and the leaf plastids. The SSU gene encoding two proteins evolved from an ancestral gene encoding a single protein by the acquisition of an alternative first exon. Prior to the work reported here, this type of SSU gene had been found in all grasses examined except maize. In maize, two separate genes, Bt2 and L2, were known to have the same roles as the alternatively spliced gene found in other grasses. The evolutionary origin of these maize genes and their relationship to the SSU genes in other grasses were unclear. Here we show that Bt2 and L2 are paralogous genes that arose as a result of the tetraploidization of the maize genome. Both genes derive from an ancestral alternatively spliced SSU gene orthologous to that found in other grasses. Following duplication, the Bt2 and L2 genes diverged in function. Each took a different one of the two functions of the ancestral gene. Now Bt2 encodes the endosperm cytosolic SSU but does not contribute significantly to leaf AGPase activity. Similarly, L2 has lost the use of one of its two alternative first exons. It can no longer contribute to the endosperm cytosolic SSU but is probably responsible for the bulk of the leaf AGPase SSU.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The enzyme ADP-glucose pyrophosphorylase (AGPase) catalyses the first committed step in the pathway of starch synthesis in plants. It synthesizes the nucleotide sugar ADP-glucose from glucose 1-phosphate and ATP. ADP-glucose is the glucose donor in the reaction catalyzed by starch synthase. Plant AGPase is a heterotetrameric enzyme composed of two small subunits (SSU) and two large subunits (LSU) (Giroux and Hannah 1994 and references within), whereas most of the bacterial AGPases, which are involved in glycogen synthesis, are composed of four identical subunits (Ballicora et al. 2003). The plant subunits are closely related to one another and to the bacterial subunit and they are believed to have evolved from a common prokaryotic ancestral gene by duplication and divergence. Many plant species have multiple genes encoding both of the subunits. For example in rice, there are two genes encoding AGPase SSUs and four genes encoding LSUs (Akihiro et al. 2005). The SSU sequences from monocots are more closely related to one another than to the SSU sequences from dicots, suggesting that duplication of the small subunit gene occurred after the separation of monocots and dicots (Hannah et al. 2001; Johnson et al. 2003; Patron and Keeling 2005). The LSU sequences, however, form three distinct groups each with both monocot and dicot members (Patron and Keeling 2005). This suggests that the LSU genes duplicated prior to the dicot-monocot split and that after this, in some lineages, there were additional duplications (Patron and Keeling 2005).
In most plant cells, ADP-glucose, like starch, is synthesized exclusively in the plastids. However, in the endosperm of cereals and other grasses (Poaceae), ADP-glucose is also synthesized in the cytosol by an additional cytosolic form of AGPase (Fig. 1A). Like ADP-glucose in the plastids, ADP-glucose synthesized in the cytosol is also destined entirely for starch synthesis. It is imported into the plastids of the endosperm via a specific ADP-glucose transporter (Li et al. 1992; Shannon et al. 1998; Patron et al. 2004). In all grass endosperms examined, the cytosolic form accounts for the majority of the total AGPase activity (e.g., >95% is cytosolic in maize [Denyer et al. 1996]). The importance of cytosolic ADP-glucose for starch synthesis has also been demonstrated by low-starch mutants of maize and barley which lack either the cytosolic form of AGPase (Tsai and Nelson 1966; Dickinson and Priess 1969; Denyer et al. 1996; Johnson et al. 2003) or the ADP-glucose transporter (Shannon et al. 1998; Patron et al. 2004).
A The pathway of starch synthesis in a grass endosperm cell. In most plant cells, the enzyme ADP-glucose pyrophosphorylase (AGPase) exists exclusively in the plastids. However, uniquely in grass endosperm cells, there is also an additional form of AGPase in the cytosol. Both forms carry out the same reaction and the product, ADP-glucose (ADPG), of both is destined entirely for starch synthesis. Filled circles indicate specific transport proteins for the import of metabolites across the plastid membrane. B The structure of the Type 1 AGPase SSU gene in barley, Hv1, and its two alternative transcripts, Hv1a and Hv1b. Hv1b encodes a protein that is expressed mainly in barley leaves. Exon 1b contains a transit-peptide-coding sequence and so Hv1b is targeted to the plastids. Hv1a is expressed mainly in the endosperm of barley seeds. Exon 1a does not contain a transit-peptide-coding sequence and so Hv1a encodes a cytosolic protein
The existence of cytosolic and plastidial forms of AGPase has been demonstrated in a wide range of grasses, including economically important cereal crop plants and wild grass species, for example, maize (Zea mays [Denyer et al. 1996]), rice (Oryza sativa [Sikka et al. 2001]), barley (Hordeum vulgare [Thorbjørnsen et al. 1996a]), wheat (Triticum aestivum [Tetlow et al. 2003]), wild barleys (Hordeum murinum and Hordeum vulgare subsp. spontaneum [Beckles et al. 2001]). No grass species lacking a cytosolic form of AGPase has been identified so far and no cytosolic AGPase has yet been found in a nongrass species. It is therefore likely that the genes encoding the cytosolic subunits of AGPase evolved from genes encoding plastidial subunits in an immediate ancestor of the grasses. As far as we know this ancestor did not give rise to any plant groups other than the grasses.
The genes that are responsible for encoding the small and large subunits of the cytosolic form of AGPase have been identified in some grass species. In maize, the gene encoding the cytosolic LSU in the endosperm is called Shrunken2 (Sh2) (Bhave et al. 1990) and homologues of Sh2 have been identified in sorghum and rice (Chen et al. 1998). It is possible that genes with the same function as Sh2 exist in all grass species. However, very little information is available for the LSU genes in other grasses. Comparatively more is known about the identity and roles of the genes encoding SSUs of AGPase in grasses. In rice, where the genome sequence is known, there are two genes encoding SSU of AGPase (Ohdan et al. 2005). Orthologues of these two genes exist in other grasses, for example, barley (Thorbjornsen et al. 1996b; Johnson et al. 2003), and these two types of SSU gene are probably widespread within the grasses. One of the barley genes (Hv2) encodes the SSU in the embryo (Rösti et al. 2006). The other (Hv1) was shown to encode both the cytosolic SSU in the endosperm and the plastidial SSU in the leaves (Thorbjornsen et al. 1996b; Rösti et al. 2006). This is achieved by the use of alternative first exons to generate two slightly different transcripts (Fig. 1B). One of the alternative first exons encodes a transit peptide that directs the protein to the plastid and the other has no signal sequence and so the protein remains in the cytosol.
The genes encoding the SSU of AGPase in maize differ from those in other grass species in several respects. First, whereas there are only two types of SSU gene in most grass species, in maize there are three known SSU genes (Hannah et al. 2001). The maize genes are Bt2, which encodes the cytosolic SSU in the endosperm (Bae et al. 1990); L2 (also called Agpslzm), which encodes a plastidial SSU that is expressed in leaves (Prioul et al. 1994); and Agp2 (also called Agpsemzm), which encodes the plastidial SSU in the embryo (Giroux and Hannah 1994). Second, in maize two separate genes (L2 and Bt2) encode the major leaf and endosperm SSUs, whereas in other cereals these proteins are encoded by a single gene that produces two alternative transcripts. Third, in a study of known AGPase SSU sequences Hannah et al. (2001) suggested that the first exon of Bt2 in maize was distinctly different from those of the other known grass SSU genes. The aim of the work presented here was to investigate further the evolutionary origin of the three genes encoding the SSUs of AGPase in maize, as well as their relationship to one another and to the two types of SSU genes in other grass species.
Materials and Methods
Plant Material
Wheat cultivar Savannah was obtained from Syngenta Ltd. Barley cultivar Bomi was from the John Innes Centre Germplasm Collection. Wheat and barley plants were grown in individual pots in a controlled-environment room at a constant temperature of 15°C, with 16 h light:8 h dark and 70% humidity. Maize cultivars B73 and Mo17 were from Lieve Laurens, John Innes Centre. Maize cultivar Gaspie Flint was from Ming-Tang Chang, Iowa State University, Ames, USA. Maize mutant bt2-7503 was from Professor Curt Hannah, University of Florida, Gainsville, USA. Maize mutant bt2-414A was from Professor Martha James, Iowa State University, Ames, USA. Maize plants for most experiments were grown in individual pots in a greenhouse at a minimum temperature of 12°C. Maize (B73) plants used for reverse transcriptase PCR analysis were grown in individual pots in a controlled-environment room at a constant temperature of 15°C, with 16 h light:8 h dark and 70% humidity. Rice cultivar Nipponbare was from Barbara Worland, John Innes Centre. Rice plants were grown in individual pots in a controlled-environment room with 12 h light (28°C) and 12 h dark (24°C) and 90% humidity. Tissues were used immediately or harvested directly into liquid nitrogen and stored at −80°C prior to use.
Reverse Transcriptase PCR
RNA was purified from endosperm and leaf tissues of barley (cv. Bomi), wheat (cv. Savannah), rice (cv. Nipponbare) and maize (cv. Gaspie Flint) using TRIzol reagent (Invitrogen, Paisley, UK) as described by the manufacturer. cDNA was synthesized from 3 μg of total RNA using an oligo-dT20 primer at 58°C with Thermoscript reverse transcriptase (Invitrogen) in a total volume of 20 μl.
A 1-μl aliquot of single-stranded cDNA was used as a template for the PCR amplification of each product. Primer pairs were as in Table 1. Primers were designed to be transcript-specific (this was confirmed by sequencing the products) and to span introns (to check that amplification of contaminating DNA did not contribute to the products).
PCR was done using 2.5 U of Platinum Taq polymerase (Invitrogen). PCR conditions were optimized for each primer pair: 10% DMSO was required for amplification of Hv1b, Ta1b, Os1b, Bt2b, and L2b, and 35 cycles of amplification were used. PCR products were purified using the QIAquick PCR purification kit (Qiagen, Hilden, Germany) and sequenced directly with each oligonucleotide primer.
Cloning and Sequencing of Ta2 cDNA
RNA was purified from developing wheat endosperm harvested at approximately 10 days after emergence of the anthers using Concert Plant RNA reagent (Invitrogen) as described by the manufacturer. cDNA was synthesized from 10 μg of total RNA using a poly(T) primer and the Gene Racer Kit for Race (Invitrogen) according to the manufacturer’s instructions except that Thermoscript reverse transcriptase was used and cDNA was synthesized at 58°C. Forward (5′-TCCTAGCGGGACAGTCATATAGAGAGGC-3′ and 5′-GCAACCGTCACCGCCATGTATATAAGCC-3′) and reverse (5′-AAGGCGTCCTTGATCACCGTG-3′and 5′-AACTGCGTGAGAACATAGATCTTGGACACA-3′) primers were used for 3′- and 5′-RACE. Forward (5′-ACGAGTGTTCTTGGATCATCCTG-3′) and reverse (5′-TTGAGGCAGTTGCTGACCGGGATATCTAT-3′) primers were used for PCR amplification of the cDNA. All PCR products were cloned into pGEM-T Easy vector (Promega, Madison, WI, USA) for sequencing.
Amplification and Sequencing of Bt2 DNA Sequence and Encoded Transcripts
RNA was purified from leaf tissue using TRIzol reagent (Invitrogen) as described by the manufacturer. cDNA was synthesized from 3 μg of total RNA using an oligo-dT20 primer at 42°C with SuperscriptII reverse transcriptase (SuperScript II RNase H Reverse Transcriptase; Invitrogen) in a total volume of 20 μl. Forward (5′- CATAATTCTCGAGTTGCAAACCATG-3′) and reverse (5′-GCTGTGCAGCTAAGACTTCAAC-3′) primers were used for PCR amplification of the Bt2a cDNA and forward (5′-GATAGCCTCAGCTTCGCCCAG-3′) and reverse (5′- TAACCAGGTGCTGATCATTGTCG-3′) primers were used for PCR amplification of the Bt2b cDNA. DNA was prepared from leaf tissue and forward (5′-CAGGGTACTGCAGATGCTGTAAGGC-3′) and reverse (5′-CTGGTTGCACCTGGAATAACCT-3′) primers were used for PCR amplification of Bt2 genomic sequence. PCR was done using high-fidelity polymerase Pfu Turbo DNA polymerase (Strategene, Cambridge, UK). DMSO (10%) was required for amplification of Bt2b cDNA. PCR products were purified using the QIAquick PCR purification kit (Qiagen) and sequenced directly with each oligonucleotide primer.
Extraction and Assay of AGPase
All steps were performed at 4°C or on ice. Approximately 0.2 g leaf tissue from maize cultivar B73, Mo17, bt2-7503, or bt2-414A (harvested 30 days after germination) was homogenized in 3 ml of extraction buffer containing 50 mM HEPES (pH 7.4), 2 mM MgCl2, and 1 mM EDTA. After centrifugation for 15 min at 10,000g, the supernatant was assayed for AGPase activity. Protein was estimated using the Bio-Rad Protein Assay Reagent (Bio-Rad Laboratories Ltd., Hemel Hemsted, UK) according to the manufacturer’s instructions.
AGPase activity at 37°C was measured in the direction of ADP-glucose synthesis. Assays contained, in a volume of 200 μl, 100 mM HEPES (pH 7.6 for barley, pH 7.4 for maize), 15 mM MgCl2, 0.25% (w/v) BSA, 0.5 U inorganic pyrophosphatase (from baker’s yeast; Roche, Mannheim, Germany), 0.5 mM [U-14C]glucose 1-P (500 cpm nmol−1), 1.5 mM ATP, and 15 mM 3-PGA. After 10 min, the reactions were stopped by boiling for 2 min. Enzyme activity was determined by measuring the ADP-[14C]glucose adsorbed by DE81 paper (Whatman, Kent, UK) following treatment with alkaline phosphatase (from E. coli C75, Amersham, Buckinghamshire, UK) according to the method of Ghosh and Preiss (1966). Under these optimized assay conditions, the activity was linear with respect to time and amount of extract added. Each extract was assayed in triplicate.
Phylogenetic Analysis
The coding regions of AGPase SSU cDNAs were aligned using AlignX, from Vector NTI Suite 8.0 (Invitrogen). Final adjustments to the alignments were made by eye. The multiple sequence files were converted for use in the Phylip v3.6 package on a UNIX platform. Trees were generated using the DNAPARS program. To obtain bootstrap values, datasets of 100 bootstraps of the aligned sequences were produced using SEQBOOT. The resulting datasets were analyzed using the DNAPARS program with 10 jumbles. The CONSENSE program was used to make the consensus trees.
Results
Nomenclature
The known genes encoding SSU of AGPase in grass species are listed in Table 2. For simplification and consistency, we have assigned abbreviated gene names for all species other than maize. These names indicate whether the gene is Type 1 (e.g., Hv1) or Type 2 (e.g., Hv2). The two alternative transcripts encoded by the Type 1 genes are differentiated by an “a” or “b” (e.g., Hv1a and Hv1b), indicating that they include either exon 1a or exon 1b, respectively. Following convention, the gene names are italicized but the transcript names are not.
An AGPase SSU Gene Encoding Two Alternative Transcripts Also Exists in Maize
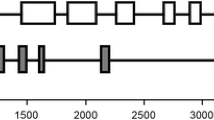
We reexamined the genomic sequences of the three AGPase SSU genes of maize looking for alternative first exons similar to those in the Type 1 genes of barley, wheat, and rice. In the Bt2 gene we found a previously undiscovered exon between exon 1 and exon 2 (Fig. 2). This gene therefore has 11 exons in total rather than 10 as described by Hannah et al. (2001). The newly discovered exon, which we call exon 1b, is similar to exon 1b in the Type 1 genes of barley, wheat, and rice. It has an in-frame start codon and a predicted transit peptide of 67 amino acids (ChloroP [Emanuelsson et al. 1999], score 0.580; Target P [Emanuelsson et al. 2000], score 0.971; Predotar [Small et al. 2004], probability 0.97), and there are consensus splice sites in the intron between exon 1b and exon 2. This suggests that maize Bt2 is a Type 1 gene, and like the Type 1 genes in the other grass species, it encodes both a plastidial and a cytosolic SSU.
Comparison of the structures and sequences of grass AGPase SSUs. The gene names are as given in Table 2. A The intron/exon structures of the genes in wheat, rice, and maize orthologous to Hv1 of barley. Exons are shown as boxes (numbers of first and last bases are indicated) and introns are shown as lines. The newly discovered exon 1b of Bt2 is shown in gray. The nonfunctional exon 1a-like sequence of gene L2 is hatched. Numbers indicate the positions of the exons in the nucleotide sequence in the EMBL accession. Note that the introns and exons are not drawn to scale. B Comparison of the exon 1b sequences of Hv1, Ta1, Os1, and Bt2 and the exon 1b-like sequence of L2. Sequences were aligned using the program for multiple sequence alignments AlignX, from Vector NTI Suite 8.0 (Invitrogen). Bases identical in all the sequences are shown in white on black; bases identical in at least 50% of the sequences are shown in black on gray. C Comparison of the nucleotide and predicted protein sequences of the exon 1a-like sequence of L2 with those of exon 1a of Bt2. Identical bases are shown in white on black. The asterisk in the L2 predicted protein sequence indicates a stop codon
Analysis of the sequence upstream of the first exon of the maize L2 gene revealed an exon-like sequence similar to exon 1a in the Type 1 genes (Fig. 2). Thus, L2 has a structure similar to that of the Type 1 genes with two alternative first exons. However, exon 1a of L2 is unlikely to be functional. Compared to exon 1a in Bt2, it has deletions and insertions that introduce frameshifts and a stop codon (Fig. 2C). Thus L2, which appears to encode one functional transcript only, probably evolved from a Type 1 gene that encoded two transcripts.
Bt2 and L2 Arose as a Result of the Tetraploidization of the Maize Genome
Having discovered that there are two Type 1-like genes in maize rather than one as in other grass species, we considered the possibility that these genes, Bt2 and L2, may have arisen as a result of the tetraploidization of the maize genome that occurred approximately 12 million years ago (Swigoňová et al. 2004a). Genome rearrangements and diploidization followed tetraploidization, but approximately 72% of the modern maize genome remains duplicated (Swigoňová et al. 2004a). To determine whether the Bt2 and L2 genes are located within duplicated chromosomal regions of the maize genome, we determined the extent to which the neighboring molecular markers are colinear.
The Bt2 gene is located on chromosome 4S (Teas and Teas 1953), whereas the L2 gene is on chromosome 1L at locus bnl17.15b (bt2) (http://www.maizegdb.org; Prioul et al. 1994). An RFLP probe for the L2 gene (p-bt2; relating to cDNA S72425) was shown previously to cross-hybridize strongly with the L2 gene on chromosome 1L and also to cross-hybridize with Bt2 on chromosome 4S (Prioul et al. 1994). This is presumably because of the high degree of identity (94%) between the Bt2 and the L2 sequences. The L2 probe was also shown previously to weakly cross-hybridize with an unknown locus, bnl17.15 (bt2) on chromosome 8L (http://www.maizegdb.org).
Examination of duplicated chromosomal regions within the maize genome that have been identified (Gaut et al. 2001; Hampson et al. 2003) showed that the region of chromosome 1 containing L2 was paralogous to a region of chromosome 4. This region showed no significant homology to any other chromosomal region in the maize genome. Comparison of the genetic maps available in the Gramene database (http://www.gramene.org) showed that this region of chromosome 4 contains Bt2 and it also showed that molecular markers neighboring Bt2 and L2 are colinear (Fig. 3A).
Colinearity among regions of the maize, rice, and sorghum genomes containing Bt2-like genes. A diagram of the positions of loci (shown in boldface) cross-hybridizing with an RFLP probe to the L2 gene (p-bt2; related to cDNA S72425), together with the positions of neighboring molecular markers. The bt2 locus on maize chromosome 4 is the Bt2 gene and the bnl17.15b (bt2) locus on maize chromosome 1 is the L2 gene. Orthologous sequences are linked by solid lines. The information on map positions was compiled from genetic maps available in the following databases: Maize DB (http://www.maizemap.org), Gramene (http://www.gramene.org), and The Plant Genome Mapping Laboratory (http://www.plantgenome.uga.edu/-projects.htm#Sorghum).
We considered whether duplication of the Type 1 genes, Bt2 and L2, in maize is likely to be confined to this species or could be more widespread within the grass family. Maize is a C4 plant and a member of the subgroup of grass species known as the PACC clade (Grass Phylogeny Working Group 2001). The duplication of Type 1 genes in maize could be a restricted to maize alone or it may be a feature of species with C4 photosynthesis or of members of the PACC clade. To examine these possibilities, we looked at the synteny among maize, sorghum, and rice (Figs. 3B and C). Sorghum is closely related to maize, although their ancestors diverged prior to the tetraploidization of maize (Swigoňová et al. 2004b). Like maize, sorghum is a member of the PACC clade and is a C4 plant. Rice is not a member of the PACC clade but belongs to the BEP clade (like wheat and barley [Grass Phylogeny Working Group 2001]), and it is a C3 plant. Single syntenous regions corresponding to the duplicated regions containing the Bt2 and L2 genes of maize were found in sorghum and rice. Since there is no complete genome sequence in sorghum, we cannot absolutely rule out the presence of another Type 1 gene in this species. However, the available information strongly supports the hypothesis that Bt2 and L2 are likely to be unique to maize rather than a feature of the PACC clade in general, that they are not essential for C4 photosynthesis, and that they arose as a result of tetraploidization in maize.
Phylogenetic Analysis of the Grass SSU cDNAs
Before attempting phylogenetic analysis of the AGPase SSU transcript sequences, we obtained three new grass SSU transcript sequences: Ta2, encoded by a wheat (Triticum aestivum) Type 2 gene; and two SSU transcripts from sorghum (Sorghum bicolor [L.] Moench), Sb1 and Sb2. The protein encoded by Ta2 had been shown to exist previously (Burton et al. 2002). It was partially purified from plastids of wheat endosperm and its sequence was shown to be very similar to that encoded by Hv2. Using the Hv2 sequence, a full-length cDNA encoding Ta2 was cloned (GenBank accession no. AY727927). The sorghum SSU sequences were assembled from published EST sequences (see Table 2).
A phylogenetic comparison of partial SSU transcripts, lacking their first exons, is shown in Fig. 4A. This shows that there are two distinct clades: one comprising the Type 1 transcripts and the other comprising the Type 2 transcripts. Note that the two alternative transcripts encoded by the Type 1 genes are identical in sequence when the first exon is excluded. This analysis suggests that the Type 1 and Type 2 genes diverged before separation of these grass lineages.
Phylogeny of AGPase SSUs. The trees were generated using a parsimony algorithm (program DNAPARS; Phylip v3.6 software package). Numbers are bootstrap values as a percentage of 100 bootstrap replicates. The sequences used are summarized in Table 2. A Phylogenetic tree of grass cDNA sequences excluding the first exon. The Arabidopsis SSU sequence (U70616) was used to root the tree. Note that the two alternative Type 1 transcripts for each species (e.g., Hv1a and Hv1b) are identical in sequence when the first exon is excluded and so “a” and “b” have been omitted from the name (e.g., Hv1). B Phylogenetic tree of the first exons of grass AGPase SSUs. Note that the two alternative first exons of Bt2 (Bt2a and Bt2b) and L2 (L2a and L2b) are as shown in Fig. 2. L2a is included in this analysis even though it is not functional
A second phylogenetic comparison of the first exons alone is shown in Fig. 4B. This analysis revealed three clades: one comprises the Type 1a sequences, the second comprises the Type 1b sequences, and the third comprises the Type 2 sequences. The Type 1b and Type 2 sequences, which include predicted transit peptides, are similar to one another but neither has significant similarity to the Type 1a sequences. The origin of exon 1a is unknown. The fact that the first exons of the Type 1a transcripts form a single clade suggests that these exon 1a sequences (which encode the cytosolic SSUs) have a common evolutionary origin. Thus, the cytosolic SSU evolved only once in the grass family.
The Spatial Patterns of Expression of AGPase SSUs
To discover whether the functions of the SSUs encoded by the Type 1 and Type 2 genes in rice, wheat, barley, and maize are likely to be similar, we compared the expression of the SSU transcripts in different organs of these species. We designed primers specific for each of the known transcripts from all four grass species and performed reverse transcriptase PCR on cDNA prepared from endosperm and leaves (Fig. 5). For transcript nomenclature see Table 2. For Type 1 genes, an “a” after the transcript name indicates that it contains exon 1a and a “b” indicates that it contains exon 1b.
The expression of AGPase SSU genes in the leaves and endosperm was determined using reverse transcriptase PCR analysis. Transcript names are as in Table 2. Details of the materials, primers, and PCR conditions are given under Materials and Methods. Each reaction was repeated at least three times using different RNA samples and the results of a typical experiment are shown. Actin was used as a positive control to show that cDNA synthesis was successful. E1 = “young” endosperm dissected from grains of approximately 15 (barley), 12 (wheat), 17 (rice), and 74 (maize) mg fresh weight. E2 = “old” endosperm dissected from grains of approximately 37 (barley), 28 (wheat), 31 (rice), and 143 (maize) mg fresh weight. L = leaves from young plants, prior to flowering (barley, wheat and maize), or from flowering plants (rice). C = control reactions (contained primers, but no cDNA) to show that products were dependent on the presence of template
These experiments showed similar patterns of expression among species of the Type 1a, Type 1b, and Type 2 transcripts. The Type 1a and Type 2 transcripts were present in endosperm and absent or detected with difficulty in the leaves. In contrast, the Type 1b transcripts (including those from the maize genes Bt2 and L2) were expressed in both the endosperm and the leaves. These findings are in agreement with previous quantitative measurements of the abundance of Type 1a, 1b, and 2 transcripts in barley (Rösti et al. 2006) and rice (Ohdan et al. 2005). The similarity of expression patterns supports the idea that the orthologous AGPase SSU transcripts in grass species are likely to have similar functions: namely, that Type 1a transcripts encode the cytosolic SSU in the endosperm, Type 1b encode the plastidial SSU in the leaves, and Type 2 encode the plastidial SSU in the seeds.
Bt2 Makes Little or No Contribution to the AGPase Activity in Maize Leaves
In barley leaves, the Type 1 gene Hv1 is required for >90% of the AGPase activity (Rösti et al. 2006). In maize, we have shown that due to the duplication of the Type 1 gene, there are two Type 1b transcripts: Bt2b, encoded by Bt2; and L2b, encoded by L2. RT-PCR (Fig. 5) showed that both of these transcripts were present in maize leaves and so both could potentially contribute to the leaf AGPase activity. To discover to what extent the Bt2b transcript encoded by Bt2 was required for leaf AGPase activity, we compared the AGPase activity in the leaves of two bt2 mutants with that in the leaves of two wild-type maize lines.
The bt2 mutant alleles chosen for this experiment, bt2-414A and bt2-7503, were ones containing lesions in the Bt2 gene that would prevent expression of the plastidial SSU protein in the leaves as well as expression of the cytosolic SSU protein in the endosperm. RT-PCR analysis of mutant bt2-414A confirmed that neither the Bt2a nor the Bt2b transcripts were produced in the leaves (data not shown). Previous analysis of mutant bt2-7503 revealed a point mutation in the 5′ splicing site of intron 3 changing GT to AT (Lal et al. 1999). This mutation abolishes use of the splice site and activates cryptic splice sites. To confirm the nature of the mutation in bt2-7503, the mutant Bt2b transcript was sequenced and compared to the Bt2b sequences from wild-type lines B73 and Mo17 (submitted to GenBank: DQ118037, DQ118038). We found that as a result of abnormal splicing in the mutant, the last 23 nucleotides of exon 3 were missing. This creates a frameshift and the appearance of a stop codon immediately downstream from the deletion in the predicted protein sequence. Thus neither bt2 mutant is capable of producing a Brittle-2 protein.
The AGPase activity in the leaves of two wild-type maize lines, B73 and Mo17, was similar: 134 ± 8 and 110 ± 11 nmol min−1 mg−1 protein, respectively (values are means ± SE of measurements on extracts of the mid part of the fourth leaf of five different plants harvested 4 weeks after germination). Loss of Bt2 gene function in maize did not result in a large decrease in AGPase activity in the leaves. The activity in the leaves of the two bt2 mutant lines, bt2-7503 and bt2414a, was 144 ± 10 and 109 ± 9 nmol min−1 mg−1 protein, respectively. Neither of the two bt2 mutants had substantially less AGPase activity than either of the two wild types studied. This suggests that the Bt2 gene contributes very little, if at all, to the AGPase activity in maize leaves. The bulk of the AGPase activity in leaves is therefore probably contributed by the other Type 1 gene that is expressed in the leaves, L2.
Discussion
Here we report an investigation of the evolutionary origin of the three genes encoding the SSUs of AGPase in maize and of their relationship to one another and to the two types of SSU genes in other grass species. We have concentrated on two of the maize genes, Bt2 and L2, which replace the single alternatively spliced SSU gene found in all other grass species studied. We have shown that Bt2 and L2 are paralogous genes produced as a result of the duplication of the maize genome. Both Bt2 and L2 have (or have remnants of) two alternative first exons, like those of the alternatively spliced SSU genes in other cereal species. We have called this type of SSU gene (with alternative first exons) Type 1 and we have called the third maize SSU gene, Agp2, and its orthologues Type 2. The inclusion of new sequence information in our phylogenetic study compared to that of Hannah et al. (2001) showed that exon 1a of the maize Bt2 gene, which encodes the N-terminus of the Brittle-2 protein, is not unique but is orthologous with Type 1 SSU sequences in all other cereal species examined. Thus, all of the Type 1 genes (Bt2, L2, and the alternatively spliced SSU genes of other grasses) clearly have the same evolutionary origin.
Despite their origins, Bt2 and L2 have both become essentially single-function genes, each encoding one (major) protein. Whereas in cereal species such as barley, a single Type 1 gene encodes both the cytosolic SSU in the endosperm and the plastidial SSU in the leaf (Rösti et al. 2006), in maize each Type 1 gene ceased to produce a different one of the two original alternative proteins. With Bt2, exon 1a is functional but the transcript including exon 1b may have little or no functional importance. Thus Bt2 encodes the cytosolic SSU in the endosperm but does not contribute significantly to the AGPase activity in leaves. With L2, exon 1b is functional but the accumulated mutations in exon 1a make it nonfunctional. L2, therefore, encodes only one transcript—for a plastidial SSU—and it is likely that it accounts for much of the SSU in the leaf.
The results presented here suggest that the genes encoding SSUs of AGPase in grasses evolved as shown in Fig. 6. In the ancestor of the grass family, duplication of the ancestral SSU gene gave rise to the progenitors of the Type 1 and Type 2 genes. Previous analysis showed that this duplication probably occurred after the separation of eudicots and monocots (Hannah et al. 2001). This duplication provided an opportunity for the subsequent specialization of the genes into organ-specific types (i.e., seed and leaf). Early in the evolution of the grasses, before the divergence of the ancestors of the modern grasses, the progenitor of the Type 1 genes acquired another first exon and gained the capacity to encode two transcripts. The independent origin of this new exon was noted by Thorbjornsen et al. (1996b) and Hannah et al. (2001). The original first exon, exon 1b, encoded a transit peptide and the protein it produced was plastidial. The new exon, exon 1a, did not encode a transit peptide and the protein produced from it was therefore cytosolic. At present, the Type 1a transcript, encoding a cytosolic protein, appears to have little or no function in the leaves, and similarly the Type 1b transcript, encoding a plastidial protein, has little or no function in the endosperm. Thus, transcriptional and translational regulatory mechanisms have arisen that restrict the plastidial protein to the leaves and the cytosolic protein to the endosperm. After the divergence of the ancestor of maize from those of rice, wheat, barley, and sorghum, the maize genome duplicated giving rise to the paralogous genes, Bt2 and L2. Hannah et al. (2001) noted the high level of sequence identity in the introns and the third codon position and also concluded that these genes were probably the result of a recent duplication. After duplication, the two Type 1 genes in maize diverged in function as described above.
In maize as in other grasses, the Type 2 gene, Agp2, encodes a plastidial SSU in the seeds. Although tetraploidization in maize would have resulted in the duplication of the Type 2 gene, no Type 2 gene other than Agp2 has been described in modern maize. Possibly, after tetraploidization, one of the duplicate Type 2 genes was lost or became dysfunctional. The maize genome sequencing project, launched in 2002 (Chandler and Brendel 2002), may provide information that will shed light on this.
Thus, the evolution of the cytosolic AGPase SSU in maize appears to have progressed beyond that in other grasses. The duplication of the maize genome enabled paralogous genes to partition the functions of an ancestral alternatively spliced gene. In maize, we find two paralogous genes encoding proteins with similar enzymatic functions but different subcellular and organ-specific patterns of expression. The rapid divergence of function in paralogous genes following genome duplication in this manner is not uncommon (Kellogg 2003; Blanc and Wolfe 2004; Taylor and Raes 2004 and references therein). Another example of the evolution of different subcellular targeting information in duplicated maize genes has been noted recently for enzymes in the porphyrin pathway (Williams et al. 2006).
In grass species with only one Type 1 gene, the cytosolic SSU protein has only a short sequence at the N-terminus (encoded by exon 1a) that differs from the sequence of the mature plastidial SSU protein encoded by the same gene. For this reason, the evolution of different kinetic and regulatory properties for the two SSUs in these species must have been severely constrained. These proteins operate in different subcellular compartments and very different organs: one in a photosynthetic organ and one in a nonphotosynthetic organ. As the conditions in the cytosol of the endosperm are likely to be quite different from those in the leaf chloroplasts, it would not be surprising if isoforms in these two subcellular compartments had different kinetic properties. It would be interesting to compare the properties of the cytosolic and chloroplastic SSUs in maize, which are encoded by separate genes, with those of other grass species that are encoded by a single Type 1 gene. The cytosolic and chloroplastic SSU proteins in maize may differ in kinetic properties more than those in other grass species.
Maize is not the only polyploid grass species and it is possible that duplication of the Type 1 SSU in other polyploid grasses has also enabled divergence of function. Wheat is one of the best-studied polyploid grass species and has a Ta.1 gene on chromosome 7 of each of its three genomes (Ainsworth et al. 1993, 1995). However, the sequence of only one Ta.1 gene is known at present (Table 2). Our preliminary studies using nullisomic-tetrasomic lines each lacking one of the three group 7 homeologues (data not shown) suggest that at least two of the three genomes of wheat possess transcriptionally active Ta.1 genes and that these Ta.1 genes each produce two alternative transcripts. The primers used in Fig. 5 amplify products of identical size from these homologous Ta1 genes—which explains why we saw products of only one size in these experiments (Fig. 5). Thus in wheat there is as yet no evidence for functional divergence between the Ta1 genes. Perhaps this is because polyploidy in wheat is a relatively recent event compared to that in maize. The maize genome duplicated 12 million years ago (Swigoňová et al. 2004a), whereas tetraploid wheat arose less than 0.5 million years ago and hexaploid wheat only 8000 years ago (Huang et al. 2002). Studies of AGPase in a wide range of polyploid grasses will be necessary to determine whether maize is truly unique in this respect or not.
As described in the Introduction, the available evidence suggests that cytosolic AGPase is unique to grass endosperms. Cytosolic AGPase has not been found in any organ of the grass plant other than the endosperm (e.g., barley leaves [Rosti et al. 2006]) or in any organ of a eudicot. However, only a limited number of species/organs have been studied so far and a wider examination of species/organs may be appropriate. For example, to our knowledge the endosperms of eudicots have not been examined. Phylogenetic analysis suggests that the Type 1 gene encoding the cytosolic SSU of AGPase in grasses diverged from a gene encoding a plastidial SSU after the divergence of monocots and eudicots (Hannah et al. 2001). Thus, if a cytosolic AGPase is discovered in a eudicot organ, perhaps the endosperm, this work predicts that it must have evolved completely independently from that in the grasses, i.e., by parallel evolution. Rather than in eudicots, it is more likely that other monocots might possess a cytosolic AGPase evolutionarily related to that in the grasses. In the future, we would like to examine the AGPase SSU genes in the endosperms of monocots closely related to grasses, such as sedges and rushes. This will allow the age of exon 1a in the Type 1 SSU gene to be estimated as well as showing whether cytosolic AGPase is truly restricted to the grasses or also present in other monocots.
References
Ainsworth C, Tarvis M, Clark J (1993) Isolation and analysis of a cDNA clone encoding the small subunit of ADP-glucose pyrophosphorylase from wheat. Plant Mol Biol 23:23–33
Ainsworth C, Hosein F, Tarvis M, Weir F, Burrell M, Devos KM, Gale MD (1995) Adenosine diphosphate glucose pyrophosphorylase genes in wheat: differential expression and gene mapping. Planta 197:1–10
Akihiro T, Mizuno K, Fujimura T (2005) Gene expression of ADP-glucose pyrophosphorylase and starch contents in rice cultured cells are cooperatively regulated by sucrose and ABA. Plant Cell Physiol 46:937–946
Bae JM, Giroux M, Hannah LC (1990) Cloning and characterization of the brittle-2 gene of maize. Maydica 35:317–322
Ballicora MA, Iglesias AA, Preiss J (2003) ADP-glucose pyrophosphorylase, a regulatory enzyme for bacterial glycogen synthesis. Microbiol Mol Biol Rev 67:213–225
Beckles DM, Smith AM, ap Tees T (2001) A cytosolic ADP-glucose pyrophosphorylase is a feature of graminaceous endosperms, but not of other starch-storing organs. Plant Physiol 125:818–827
Bhave MR, Lawrence S, Barton C, Hannah LC (1990) Identification and molecular characterization of shrunken-2 cDNA clones of maize. Plant Cell 2:581–588
Blanc G, Wolfe KH (2004) Functional divergence of duplicated genes formed by polyploidy during Arabidopsis evolution. Plant Cell 16:1679–1691
Burton RA, Johnson PE, Beckles DM, Fincher GB, Jenner HL, Naldrett MJ, Denyer K (2002) Characterization of the genes encoding the cytosolic and plastidial forms of ADP-glucose pyrophosphorylase in wheat endosperm. Plant Physiol 130:1464–1475
Chandler VL, Brendel V (2002) The maize genome sequencing project. Plant Physiol 130:1594–1597
Denyer K, Dunlap F, Thorbjørnsen T, Keeling P, Smith AM (1996) The major form of ADP-glucose pyrophosphorylase in maize endosperm is extra-plastidial. Plant Physiol 112:779–785
Dickinson DB, Preiss J (1969) Presence of ADP-glucose pyrophosphorylase in shrunken-2 and brittle-2 mutants of maize endosperm. Plant Physiol 44:1058–1062
Emanuelsson O, Nielsen H, von Heijne G (1999) ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci 8:978–984
Emanuelsson O, Nielsen H, Brunak S, von Heijne G (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300:1005–1016
Gaut BS, d’Ennequin ML, Peek A, Sawkins MC (2001) Maize as a model for the evolution of plant nuclear genomes. Proc Natl Acad Sci USA 97:7008–7015
Geigenberger P, Kolbe A, Tiessen A (2005) Redox regulation of carbon storage and partitioning in response to light and sugars. J Exp Bot 56:1469–1479
Giroux MJ, Hannah LC (1994) ADP-glucose pyrophosphorylase in shrunken-2 and brittle-2 mutants of maize. Mol Gen Genet 243:400–408
Ghosh HP, Preiss J (1966) Adenosine diphosphate glucose pyrophosphorylase—a regulatory enzyme in biosynthesis of starch in spinach leaf chloroplasts. J Biol Chem 241:4491–4504
Grass Phylogeny Working Group (2001) Phylogeny and subfamilial classification of the grasses (Poaceae). Ann Mo Bot Gard 88:373–457
Hampson S, McLysaght A, Gaut B, Baldi P (2003) LineUp: statistical detection of chromosomal homology with application to plant comparative genomics. Genome Res 13:999–1010
Hannah LC (1997) Starch synthesis in the maize endosperm. In Larkins BA, Vasil IK (eds) Advances in cellular and molecular biology of plants, Vol 4. Kluwer Academic, Dordrecht, The Netherlands, pp 375–405
Hannah LC, Shaw JR, Giroux MJ, Reyss A, Prioul JL, Bae JM, Lee JY (2001) Maize genes encoding the small subunit of ADP–glucose pyrophosphorylase. Plant Physiol 127:173–183
Huang S, Sirikhachornkit A, Su X, Faris J, Gill B, Haselkorn R, Gornicki P (2002) Genes encoding plastidial acetyl-CoA carboxylase and 3-phosphoglycerate kinase of the Triticum/Aegilops complex and the evolutionary history of polyploidy wheat. Proc Natl Acad Sci USA 99:8133–8138
Johnson PE, Patron NJ, Bottrill AR, Dinges JR, Fahy BF, Parker ML, Waite DN, Denyer K (2003) A low-starch barley mutant, Risø 16, lacking the cytosolic small subunit of ADP-glucose pyrophosphorylase, reveals the importance of the cytosolic isoform and the identity of the plastidial small subunit. Plant Physiol 131:684–696
Kellogg EA (2003) What happens to genes in duplicated genomes. Proc Natl Acad Sci USA 100:4369–4371
Lal S, Choi J-H, Shaw JR, Hannah LC (1999) A splice site mutant of maize activates cryptic splice sites, elicits intron inclusion and exon exclusion, and permits branch point elucidation. Plant Physiol 121:411–418
Li H, Sullivan TD, Keegstra K (1992) Information for targeting to the chloroplastic inner envelope membrane is contained in the mature region of the maize Bt1-encoded protein. J Biol Chem 267:18999–19004
Nielsen H, Engelbrecht J, Brunak S, von Heijne G (1997) Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng 10:1–6
Ohdan T, Francisco PB Jr, Sawada T, Hirose T, Terao T, Sahto H, Nakamura Y (2005) Expression profiling of genes involved in starch synthesis in sink and source organs of rice. J Exp Bot 56:3229–3244
Patron NJ, Keeling PJ (2005) Common evolutionary origin of starch biosynthetic enzymes in green and red algae. J Phycol 41:1131–1141
Patron NJ, Greber B, Fahy BF, Laurie DA, Parker ML, Denyer K (2004) The lys5 mutations of barley reveal the nature and importance of plastidial ADP-glucose transporters for starch synthesis in cereal endosperm. Plant Physiol 135:2088–2097
Prioul J-L, Jeanette E, Reyss A, Grégory N, Giroux M, Hannah LC, Causse M (1994) Expression of ADP-glucose pyrophosphorylase in maize (Zea mays L.) grain and source leaf during grain filling. Plant Physiol 104:179–187
Rösti S, Rudi H, Rudi K, Opsahl-Sortberg H-G, Fahy B, Denyer K (2006) The gene encoding the cytosolic small subunit of ADP-glucose pyrophosphorylase in barley endosperm also encodes the major plastidial small subunit in the leaves. J Exp Bot 57:3619–3626
Sears ER (1966) Nullisomic-tetrasomic combinations in hexaploid wheat. In: Riley R, Lewis KR (eds) Chromosome manipulations and plant genetics. Oliva and Boyd, Edinburgh, pp 23–45
Shannon JC, Pien F-M, Cao H, Liu K-C (1998) Brittle-1, an adenylate translocator, facilitates transfer of extra plastidial synthesized ADP-glucose into amyloplasts of maize endosperms. Plant Physiol 117:1235–1252
Sikka VK, Choi SB, Kavakli IH, Sakulsingharoj C, Gupta S, Ito H, Okita TW (2001) Subcellular compartmentation and allosteric regulation of the rice endosperm ADPglucose pyrophosphorylase. Plant Sci 161:461–468
Small I, Peeters N, Legeai F, Lurin C (2004) Predotar: a tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics 6:1581–1590
Swigoňová Z, Lai JS, Ma JX, Ramakrishna W, Llaca M, Bennetzen JL, Messing J (2004a) On the tetraploid origin of the maize genome. Comp Funct Genomics 5:281–284
Swigoňová Z, Lai JS, Ma JX, Ramakrishna W, Llaca M, Bennetzen JL, Messing J (2004b) Close split of sorghum and maize genome progenitors. Genome Res 14:1916–1923
Taylor J S, Raes J (2004) Duplication and divergence: the evolution of new genes and old ideas. Annu Rev Genet 38:615–643
Teas HJ, Teas AN (1953) Heritable characters in maize. Description and linkage of brittle endosperm-2. J Hered 44:156–158
Tetlow IJ, Davies EJ, Vardy KA, Bowsher CG, Burrell MM, Emes MJ (2003) Subcellular localisation of ADPglucose pyrophosphorylase in developing wheat endosperm and analysis of the properties of a plastidial isoform. J Exp Bot 54:715–725
Thorbjørnsen T, Villand P, Denyer K, Olsen OA, Smith AM (1996a) Distinct isoforms of ADPglucose pyrophosphorylase occur inside and outside the amyloplasts in barley endosperm. Plant J 10:243–250
Thorbjørnsen T, Villand P, Kleczkowski LA, Olsen OA (1996b) A single gene encodes two different transcripts for the ADP-glucose pyrophosphorylase small subunit from barley (Hordeum vulgare). Biochem J 313:149–154
Tsai C, Nelson OE (1966) Starch-deficient maize mutant lacking adenosine diphosphate pyrophosphorylase activity. Science 151:341–343
Williams P, Hardeman K, Fowler J, Rivin C (2006) Divergence of duplicated genes in maize: evolution of contrasting targeting information for enzymes in the porphyrin pathway. Plant J 45:727–739
Acknowledgments
We are grateful to Prof. Alison M Smith, Prof. Peter Keeling, Dr. Duncan Stanley, and Dr. David Laurie for constructive criticism of the manuscript. This work was supported by a CASE studentship from the Biotechnology and Biological Sciences Research Council UK (BBSRC). The industrial partner for the CASE studentship was Syngenta Ltd. The John Innes Centre is supported by a core strategic grant from the BBSRC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Reviewing Editor: Dr. Patrick Keeling
Sequences have been deposited in GenBank under accession numbers AY727927, DQ118037, and DQ118038.
Rights and permissions
About this article
Cite this article
Rösti, S., Denyer, K. Two Paralogous Genes Encoding Small Subunits of ADP-glucose Pyrophosphorylase in Maize, Bt2 and L2, Replace the Single Alternatively Spliced Gene Found in Other Cereal Species. J Mol Evol 65, 316–327 (2007). https://doi.org/10.1007/s00239-007-9013-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-007-9013-0