Abstract
Ca2+/cation antiporter (CaCA) proteins are integral membrane proteins that transport Ca2+ or other cations using the H+ or Na+ gradient generated by primary transporters. The CAX (for CAtion eXchanger) family is one of the five families that make up the CaCA superfamily. CAX genes have been found in bacteria, Dictyostelium, fungi, plants, and lower vertebrates, but only a small number of CAXs have been functionally characterized. In this study, we explored the diversity of CAXs and their phylogenetic relationships. The results demonstrate that there are three major types of CAXs: type I (CAXs similar to Arabidopsis thaliana CAX1, found in plants, fungi, and bacteria), type II (CAXs with a long N-terminus hydrophilic region, found in fungi, Dictyostelium, and lower vertebrates), and type III (CAXs similar to Escherichia coli ChaA, found in bacteria). Some CAXs were found to have secondary structures that are different from the canonical six transmembrane (TM) domains–acidic motif-five TM domain structure. Our phylogenetic tree indicated no evidence to support the cyanobacterial origin of plant CAXs or the classification of Arabidopsis exchangers CAX7 to CAX11. For the first time, these results clearly define the CAX exchanger family and its subtypes in phylogenetic terms. The surprising diversity of CAXs demonstrates their potential range of biochemical properties and physiologic relevance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ca2+/cation antiporter (CaCA) superfamily proteins are integral membrane proteins with 10 to 11 transmembrane (TM) domains that transport Ca2+ or other cations using the gradient of H+ or Na+ generated by energy-coupled primary transporters (Busch and Saier 2002). CaCA proteins have been identified in animals, plants, fungi, archaea, and bacteria. The CaCA superfamily consists of five families that have been identified phylogenetically (Cai & Lytton 2004): the Na+/Ca2+ antiporters (NCX), the K+-dependent Na+/Ca2+ antiporters (NCKX and CCX), the YRBG transporters found in prokaryotes, and the cation exchangers (CAXs).
CAXs (for CAtion eXchangers) are a group of proteins that export cations of the cytosol to maintain optimal ionic concentrations in the cell. CAXs are energized by the pH gradient established by proton pumps, such as H+-ATPase or H+-pyrophosphatase (Kamiya and Maeshima 2004). CAX sequences have been deposited in public databases from bacteria, Dictyostelium, fungi, plants, lower vertebrates (fish and amphibians), and sea urchin.
The acronym “CAX” was first used to describe the cation antiporters CAX1 and CAX2 from the model plant Arabidopsis thaliana (Hirschi et al. 1996). Since then, more antiporters have been named with a CAX prefix but have no solid criteria for the classification. The lack of consistent nomenclature and classification may hinder future study regarding structure–function relationships. Although only a small number of CAXs have been functionally characterized (Table 1), there is evidence to support that CAXs have a multitude of unique biochemical properties in the CaCA superfamily. For example, Escherichia coli ChaA (a CAX) was functionally identified as a Na+/H+ antiporter (Ivey et al. 1993). ChaA suppressed the phenotype of the Na+/H+ antiporter mutant. However, it was biochemically demonstrated to have higher H+/Ca2+ activity.
All of the characterized CAXs, including bacterial open reading frames (ORFs), share some common features (Table 1). They are approximately 400 amino acids long and are predicted to have 11 TM domains, although the predicted number of TM domains may vary depending on the computer program used. The polypeptide is divided into two “half proteins” by a short, negatively charged loop between TM6 and TM7, termed the “acidic motif” (Ivey et al. 1993).
In eukaryotes, cellular localization is a factor that is critical for the function of the protein. For example, CAXs are thought to play important roles in signal transduction. In signaling events, the basal Ca2+ concentration in the cytosol is restored by sequestering the transient increase of free calcium into the vacuole (Sanders et al. 2002). So far, most of the characterized eukaryotic CAXs, such as VCX1 from yeast (Cunningham and Fink 1996) and VCAX1 from mung bean (Ueoka-Nakanishi et al. 2000), are localized to the vacuole. However, localization of most plant CAXs are still unknown, and there is no report on the localization of CAXs from filamentous fungi species. Different isoforms of Arabidopsis Ca2+ ATPases (ACA) and Na+/H+ (NHX) antiporters are localized to different cellular locations (Yokoi et al. 2002; Schiott et al. 2004). CAXs are also a multigene family in Arabidopsis (Mäser et al. 2001) and rice (Kamiya et al. 2005). By analogy to ACA and NHX transporters, it is possible that some CAXs are located on membranes other than the vacuolar membrane. In fact, biochemical experiments suggest that H+/Ca2+ antiport activity is present in the plasma membrane and chloroplast thylakoid membrane (Kasai and Muto 1990; Ettinger et al. 1999). Recently, two plant CAXs from rice and soybean were reported to be localized to the plasma membrane (Luo et al., 2005; Qi et al., 2005).
Substrate specificity of transporters is also an important determinant of their physiological functions. In this regard, it is of note that the substrate range of CAXs may extend beyond calcium and sodium. It has been reported that CAX2 from A. thaliana and OsCAX1a from rice transport both calcium and manganese (Hirschi et al. 2000; Kamiya and Maeshima 2004). A naturally occurring mutant of the yeast vacuolar Ca2+/H+ exchanger VCX1, termed “MNR1,” can transport manganese (del Pozo et al. 1999; Pittman et al. 2004). CAX2 also transports cadmium (Hirschi et al. 2000), and this may be true for CAX1 as well because in the presence of excess cadmium, calcium transport by CAX1 is significantly inhibited (Shigaki et al. 2003). Overall, these results may be only representative of the large number of CAXs that transport multiple cations. Further phylogeny studies may aid rational initial experimentation regarding CAX transport function.
Some determinants of cation specificity in CAXs have been characterized. For example, the nine-amino-acid-long region of CAX1 has been shown to confer calcium transport ability to its close non-calcium-transporting homologue CAX3 (Shigaki et al. 2001). The three-amino-acid-long manganese specificity determinant of CAX2 has also been identified (Shigaki et al. 2003). In rice OsCAX1a, substrate selectivity has been shown to be specified by residues in its repeat regions (Kamiya and Maeshima 2004). Phylogenetic analyses should be useful to predict the cellular localization, transport mechanism, and substrate specificities of CAXs.
CAXs are now recognized as tools to engineer plants for nutritional enrichment of food and for phytoremediation to clean up heavy-metal contaminations in soils. For example, we have demonstrated that carrots, potatoes, and tomatoes expressing the Arabidopsis Ca2+/H+ antiporter CAX1 contain higher calcium than vector controls (Park et al. 2004; Park et al. 2005a, 2005b). In terms of altering metal uptake, plants expressing the Arabidopsis metal/H+ antiporter CAX2 appear to be slightly more tolerant to some metal stresses and accumulate higher levels of particular metals compared with controls (Hirschi et al. 2000). To maximize the efficiency and specificity for plants to accumulate cations of interest, and to tap into the potentially useful functions of the proteins, the knowledge of CAXs from a wide range of organisms will be a valuable asset.
Overall, the information accumulated so far on the characterized CAXs points to a potential diversity in their substrate range and physiological functions. In plants, multiple isoforms are recognized in the Arabidopsis genome (Mäser et al. 2001), and in rice, diverse expression patterns among CAX isoforms have been reported (Kamiya et al. 2005). Evolutional relationships among the characterized and uncharacterized CAXs will lead to a better understanding of their potential roles. In this study, we wanted to explore the diversity of CAXs, to define the CAX family, and to identify subgroups within the family. Our study is the first comprehensive phylogenetic analysis of the CAX family, and it defines a framework for future molecular and biochemical research.
Methods
BLAST Search and Sequence Alignment
Protein BLAST searches were performed in August, 2005, using the National Center for Biotechnology Information Web site (http://www.ncbi.nlm.nih.gov/BLAST/) on the nonredundant protein database. As reference sequences for BLAST, two representative CAXs, A. thaliana CAX1 and E. coli ChaA, were used. We identified 138 sequences (Supplementary Material) with an E value ≤0.01, and these sequences were considered as phylogenetically related and used for further analyses. The cutoff value 0.01 was used because lowering the stringency to >0.01 returns homologues of Ca2+/Na+ antiporters, which belong to a separate family. Multiple entries for identical proteins were omitted. Multiple sequence alignments (Supplementary Material)were performed using ClustalW, available on the European Bioinformatics Institute Web site (http://www.ebi.ac.uk/clustalw/), with additional manual adjustments. Only the homologous regions were used for the multiple sequence alignments. PSI-BLAST was performed to identify weak but biologically relevant sequence similarities (Altschul et al. 1997) to Arabidopsis CAXs and the second TM domain of the yeast CAX YNL321W. The general absence of CAX exchanger ORFs from archaebacterial genomes, except for one from Methanosarcina, was confirmed using the data available from the TransportDB Web site (http://www.66.93.129.133/transporter/wb/index2.html) at the Institute for Genomic Research.
Phylogenetic Analyses and Tree Construction
The phylogenetic trees on the data sets were constructed using heuristic searches for maximum likelihood and maximum parsimony. For maximum likelihood reconstruction of trees, the RAxML tool (version 3.0) with the Jones, Taylor, and Thornton (JTT) model of amino-acid substitution (and default settings of the program) was used (Stamatakis et al. 2005). For maximum parsimony reconstruction of trees, the PAUP* tool (version 4.0; Swafford 1998) was used as follows. First, a phylogenetic tree was obtained using a fast heuristic search of random stepwise addition of sequences. Then, the resulting tree was used as the initial tree in an heuristic search with tree bisection reconnection (TBR) moves. Finally, the strict consensus tree of all optimal trees found by the heuristic search was computed.
Topologic Analysis
Membrane topology prediction was performed using TMHMM 2.0 (Krogh et al. 2001) from the Center for Biologic Sequence Analysis at Technical University of Denmark (available at http://www.cbs.dtu.dk/services/TMHMM/). Möller et al. (2001) evaluated various membrane topology prediction programs using the protein sequences with an experimentally determined topology. The topology prediction by TMHMM was more accurate than other popular methods, such as the program by Kyte and Doolittle (1982) and TopPred II (Claros and von Heijne 1994).
Results and Discussion
The CAX Family Consists of Three Phylogenetic Subgroups
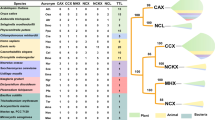
CAXs are one of the five families that belong to the CaCA) superfamily (Cai and Lytton 2004). We constructed phylogenetic trees based on maximum likelihood and maximum parsimony. These two methods produced essentially identical results. In both methods, the analysis of 138 full-length CAX polypeptides identified three major groups within this family (Fig. 1). A distant relationship between Arabidopsis CAX1 and E. coli ChaA has been previously recognized (Hirschi et al. 1996). The identification of the third group is due primarily to the recent deposition of new sequences in the databases. This third group is phylogenetically closer to plant CAXs than bacterial CAXs, such as ChaA. However, their polypeptide is approximately twice as long as that of CAX1 or ChaA. The most striking feature of the CAXs in the new group is the presence of two unique TM domains located in their N-terminus half. Therefore, we regard this group as independent from the other two.
A maximum likelihood phylogenetic tree made from 138 full-length CAXs in the public database. Three major groups and their subgroups are indicated. The tree branches are labeled with genera. When there are multiple CAXs from the same genus, they are distinguished by hyphenated numbers. The complete alignment, species and strain names, and the GI numbers of all 138 sequences are available as supplementary materials online.
We propose to name these three subfamilies types I (CAXs similar to A. thaliana CAX1), II (the new group), and III (CAXs similar to E. coli ChaA) CAXs (Fig. 1). The type I CAXs include all of the plant CAXs but also include CAXs from other organisms. The type II CAXs are found in fungi, Dictyostelium, and lower vertebrates (fish and amphibians). Type III CAXs are found only in bacteria. Within the type I CAX category, eight groups can be recognized (A through H). Type III CAXs are further divided into four (A through D) subgroups.
Conservation of certain residues of the CaCA superfamily polypeptides was reported earlier (indicated by stars in Fig. 2; Cai & Lytton 2004). The alignment of 12 representative CAXs confirmed that some of these residues are also conserved in CAX polypeptides. However, many other residues are not conserved in CAXs (Fig. 2), whereas many residues are specifically conserved in CAXs, suggesting unique protein conformations.
Multiple sequence alignment of 12 representative CAXs. Alignments were performed using ClustalW from the European Bioinformatics Institute Web site. Consensus amino-acid residues are boxed in black (identical) or gray (similar). Gaps introduced into sequences to increase their similarity are denoted by hyphens. The N-terminus regions of some sequences are not shown because their alignment is poor. The predicted 11 putative TM spans (TM1 to TM11) are overlined. The two α-repeats are indicated by gray lines, and the signature motif regions for CAXs within these repeats, as reported by both Cai and Lytton (2004) and Kamiya and Maeshima (2004), are indicated by double gray lines. The two conserved glutamic-acid residues are indicated by an arrow. Stars indicate the conserved residues reported for the CaCA superfamily polypeptides (Cai and Lytton 2004).
There is striking conservation of many glycine residues among CAXs (G125, G129, G174, G180, G181, G359, G409, and G412 for CAX1). Glycine is the only achiral amino acid that occurs in natural proteins, and therefore it allows a polypeptide many possible conformations. Yan and Sun (1997) proposed a role of glycine residues as providing flexibility for active enzyme sites. Some of the conserved glycine residues may be important in conferring structural flexibility to CAX exchangers.
Two internally repeated regions, designated as α-1 and α-2, are known in Na+/Ca2+ exchangers and may be the sites of ion binding (Philipson & Nicoll 2000). The two highly conserved acidic residues that may be involved in neutralizing the positive charges of the cation substrate (Cai & Lytton 2004a) are also conserved in the CAXs (indicated by arrows in Fig. 2).
CAXs From Plants Form Two Distinct Clades (Types I-A and -B)
Currently, there are 17 full-length CAXs from plants in the public databases. Two plant species with sequenced genomes, A. thaliana and rice, have multiple CAXs. Phylogenetic analysis divides these CAXs into 2 distinct clades. Three Arabidopsis CAXs (CAX1, CAX3, and CAX4) and 3 rice CAXs (OsCAX1a, b, and c) belong to type I-A, and 3 Arabidopsis CAXs (CAX2, CAX5, and CAX6) and 3 rice CAXs (OsCAX2, 3, and 4) belong to type I-B (Fig. 1). It is likely that other monocot and dicot species also have CAXs in both groups. All plant CAXs, regardless of the group to which they are assigned, have 11 predicted hydrophobic TM domains with an acidic motif after TM span 6 (Fig. 3). Although there are few differences in the α-repeats of each clade, type I-A transporters appear to have a highly hydrophilic segment after TM span 4. It contains multiple positively charged residues, and therefore is distinct from the acidic motif.
Membrane topology of selected CAXs. The topologic models were generated by the TMHMM program (Krogh et al. 2001). The red areas indicate predicted TM domains, and the blue and magenta lines indicate the regions that are predicted to be inside or outside of the membrane, respectively.
It is possible that the reason for the dichotomy of plant CAXs may be different substrate specificities. For example, CAX1 (type I-A) has been considered to be a specialized calcium transporter, whereas CAX2 (type I-B) transports multiple cations such as Ca2+, Cd2+, and Mn2+ (Hirschi et al. 2000). However, current substrate status may simply reflect the fact that exhaustive tests have not been done. In fact, OsCAX1a (type I-A) has recently been shown to transport Mn2+ as well as Ca2+, and competition assays suggest that other plant CAXs also transport multiple cations (Shigaki et al. 2003). Further experimental work will clarify the substrate specificities of the enzymes in the two groups.
Arabidopsis CAX7-11 Are Not CAXs
In the Arabidopsis genome, five additional putative antiporters exist that have been named CAX7 to CAX11 (Mäser et al. 2001). These antiporters display limited primary amino-acid sequence homology with any CAX. In fact, they have a striking sequence similarity to the mammalian K+-dependent Na+/Ca2+ antiporter NCKX6 (Cai and Lytton, 2004b), which is also termed “NCLX” and was reported to be K+-independent by Palty et al. (2004). The phylogenetic tree of the 13 representative CAXs, CAX7 to CAX11, and the human K+-dependent Na+/Ca2+ antiporter NCKX6 clearly indicates that CAX7 to CAX11 are closer to the K+-dependent Na+/Ca2+ antiporter than to any of the CAXs (Fig. 4). Specifically, CAX 7 to CAX11 share the characteristic α-repeats GNG(A/S)PD in α-1 and (G/S)(N/D)SxGD in α-2 with NCKX6 (Cai and Lytton 2004); as well as a high degree of homology immediately surrounding this region, particularly on the N-terminus. It is therefore most likely that this group of Arabidopsis transporters have different biochemical properties from those of other CAX proteins. CAXs and mammalian NCKX6, among others, are members of the CaCA superfamily (Cai and Lytton 2004). Arabidopsis CAX7 to CAX11 belong to this superfamily, but there is no available information on function. Recently, Cai and Lytton (2004) proposed a new group that separates the mammalian NCKX6 from NCKX1-5 and tentatively termed the group the “Cation Calcium eXchanger” (CCX) family. Based on sequence homology and characteristic α-repeats, Arabidopsis CAX7 through CAX11 belong to this new family. Therefore, we propose to rename these genes CCX1 (CAX7) to CCX5 (CAX11). In our classification scheme, there are only six Arabidopsis CAX transporters (CAX1 to CAX6).
Plant Genomes Contain Close Relatives of CAXs
A PSI-BLAST search with CAX1 as the reference sequence identified a homologous region in the C-terminus of a putative transporter (At1g53210). This deduced antiporter has a plant CAX-type secondary structure with 10 to 11 TM domains. Interestingly, At1g53210 has two EF-hand calcium-binding motifs, suggesting its role in regulation by calcium. According to publicly available microarray data, this gene is highly expressed in all tissues and was recently reported to be localized to the vacuole (Carter et al. 2005). Three additional putative antiporters are highly homologous to At1g53210 in the Arabidopsis genome. Homologues also exist in other plant genomes.
CAXs From Protozoa Form a Separate Clade (Type I-C)
The public databases contain only four CAXs from protozoa (from Plasmodium, Cryptosporidium, and Leishmania), and three of them form a tight cluster within the type-I CAX (type I-C; Fig. 1). The protozoan CAXs are similar to plant CAXs in size and proposed membrane topology. The CAX from Leishmania appears to be most closely related to fungal type I-F CAXs.
Fungi Have Multiple Groups of CAXs That Are Diverse in Structure (Types I-D, -E, and -F)
Like Arabidopsis and rice, fungi have multiple CAX ORFs in their genome. Phylogenetic analysis revealed two distinct groups in these proteins (Fig. 1). Of these two, one clade is distant enough from plant or prokaryotic CAXs and appears to be an independent phylogenetic group (type II). CAXs that form the other clade are similar enough to plant CAXs to be classified as type I, and we subdivide this group into types I-D, I-E, and I-F.
The three groups of fungal type I CAXs are clearly distinguished by their secondary structures (Fig. 3). Type I-D CAXs are approximately 500 amino acids long and are predicted to have 9 TMs. The size of type I-E CAXs varies from 600 to 800 amino acids. They have 11 predicted TMs with a long N-terminus tail and a middle loop region after TM6. Type I-F CAXs are approximately 400-aa long and have 11 predicted TMs. However, they lack the N-terminus tail and middle loop, which makes them structurally similar to plant CAXs. Many fungal species have multiple type I CAXs. For example, Neurospora has one type I-D and I-F CAX and appears to have two type I-E CAXs.
Type I CAXs in Bacteria Are Classified into Two Groups (Types I-H and -G)
Many, but not all bacterial genomes have CAXs that belong to type I. Among these sequences, CAXs from cyanobacteria form a tight cluster (type I-G), and no other types of CAXs are present in cyanobacterial genomes except for a Synechococcus CAX, which is classified in type III-B. All other bacterial type I CAXs form a relatively loose cluster (type I-H), and these CAXs appear to be phylogenetically closer than other type I CAXs to type III CAXs.
Amino-acid sequences of these proteins are highly homologous to all other members of type I CAXs. Recently, an attractive hypothesis was proposed on the evolution of some plant cation transporters. Waditee et al. (2004) reported high homologies between CAXs from cyanobacteria and plants. Song et al. (2004) reported a similar finding between Na+/H+ exchangers from cyanobacteria and Arabidopsis. Both reports suggested that these plant transporters were derived from cyanobacteria. However, CAXs from other bacteria and fungi are similarly related to cyanobacterial CAXs (Fig. 1). Therefore, the close phylogenetic relationship is not specific to CAXs from plants and cyanobacteria. We thus propose that CAX genes already existed before the divergence of the major kingdoms or domains.
Both groups of CAXs have 11 TM domains separated into two segments by an acidic motif (Fig. 3). It appears that some of the bacteria having a type I CAX also have a putative type III CAX. It is interesting to note that an archaeal CAX from Methanosarcina also belongs to type I-H (Fig. 1). The origin of Methanosarcina CAX is difficult to resolve, but recent findings emphasize the frequent occurrence of horizontal transfer in prokaryotic evolution (Martin & Embley 2004).
The Type II CAXs Are Characteristic in Secondary Structure
This group of CAXs is present in fungi, Dictyostelium, and Xenopus (Fig. 1). There are also type II CAX expressed sequence tags (ESTs) from fish species and the sea urchin. However, they are absent in higher vertebrates and other animal species, including insects and nematodes. Most genomes appear to have only one copy of this exchanger. The proteins in this group consist of approximately 900 to 1150 amino acids and are more than twice as long as E. coli ChaA or Arabidopsis CAX1. The C-terminus half is highly similar in primary amino-acid sequence and topology to the type I or III CAXs that contains 11 TM domains (Fig. 3). The N-terminus half is unique to type II CAXs and mostly hydrophilic (Fig. 3). However, there are two putative TM domains in this region, making the total number of TM domains 13 (Fig. 3).
The first TM span is homologous to a conserved domain (DUF307) found in small membrane proteins in bacteria and fungi. The function of this domain is unknown. A PSI-BLAST search identified the homology of the second TM span to the β subunit proteins of the Na+, K+-ATPase (“sodium pump”). This subunit of the sodium pump is not part of the catalytic domain and possibly plays a role in its plasma membrane delivery (McDonough et al. 1990; Laughery et al. 2003). Therefore, it is possible that the type II CAXs are localized to the plasma membrane and that the localization signal is located in this second TM region, although this must be tested experimentally.
The type II CAXs appear to be either approximately 900 or 1100 amino acids long (Fig. 3). The difference is in the length of the middle loop after the sixth TM domain. Saccharomyces cerevisiae YNL321W lacks such a loop (908 amino acids; Fig. 3). However, a type II CAX from another yeast species, Yarrowia lipolytica, has a long loop of approximately 200 amino acids, making the total length 1145 amino acids (Fig. 3). The loops are heterogeneous. The N-terminus of the Saccharomyces YNL321W exchanger appears to be located inside the cytosol, whereas the Yarrowia exchanger is predicted to have its N-terminus outside of the cytosol (Fig. 3).
Additionally, type II CAXs have significantly different α-repeats from those of type I CAXs. Very few of the residues Kamiya and Maeshima (2004) demonstrated to be essential for Ca2+ or Mn2+ transport in OsCAX1a are conserved.
Type III CAXs Are Found Only in Bacteria
The Ca2+/H+ antiporter ChaA from E. coli and related proteins (type III CAXs) show homology to CAXs from plants and fungi. However, it is clear that these exchangers form a separate clade from type I or type II CAXs (Fig. 1). Type III CAX exchangers are found only in bacteria. Some bacterial species were found to have both type I and type III CAXs.
Four clear clades are recognized within type III CAXs (Fig. 1). Type III-A consists of CAXs mainly from plant pathogenic or symbiotic bacteria but also includes CAXs from Bordetella. Type III-A CAXs are the phylogenetic group closest to type I CAXs. Type III-B consists of CAXs from a diverse range of bacterial species including Gram-negative and -positive bacteria and one cyanobacterium. Type III-C includes ChaA from E. coli, which is the only well-characterized type III CAX to date. Type III-D includes CAXs from diverse bacterial species, including animal and plant pathogens.
Type III CAXs are also predicted to have 11 TM domains and approximately 400 amino acids. Types III-B, III-C, and III-D CAXs have an acidic motif after TM span 6. However, type III-A CAXs contain many positively charged residues in this region and therefore lack an acidic motif. The N-terminus of type III CAXs is predicted to be inside the membrane, except for type III-A CAXs, which appear to have the N-terminus outside the membrane (Fig. 3). Some bacterial species have multiple type III CAXs classified into different subgroups.
CAXs Are Absent in Higher Animal and Archaebacterial Genomes
Database searches failed to identify CAX homologues in most animal species, including higher vertebrates, insects, and nematodes. However, CAXs do exist in lower vertebrates and sea urchin. The existence of CAXs in isolated groups of animals suggests the disappearance of CAXs from other animal species because of selection pressure. Future functional studies of CAXs in lower animal species may identify a fundamental role of all CAXs.
Many archaebacterial genomes have been sequenced and annotated completely. Archaebacterial genomes contain 0–3 open reading frames that belong to the CaCA superfamily. Except for the Methanosarcina CAX, all of these antiporters show homology to the mammalian NCKX6/NCLX-like Na+/Ca2+ antiporters rather than CAXs. The absence of CAXs from archaebacterial genomes implies that CAXs branched off from the ancestral Ca2+/cation antiporters after the divergence of eubacteria and archaebacteria.
Conclusion
In conclusion, this study has defined the CAX family, and some former plant CAXs have been reassigned to the CCX family. The three types of CAXs show remarkable diversity, suggesting multiple physiologic roles.
References
Allaway D, Calvaco L, Saini S, Hocking P, Lodwig EM, Leonard ME, Poole PS (2000) Identification of a putative LPS-associated cation exporter form Rhizobium leguminosarum bv. viciae. FEMS Microbiol Lett 186:47–53
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Busch W, Saier MH (2002) The transporter classification (TC) system, 2002. Crit Rev Biochem Mol Biol 37:287–337
Cai X, Lytton J (2004a) The cation/Ca2+ exchanger superfamily: Phylogenetic analysis and structural implications. Mol Biol Evol 21:1692–1703
Cai X, Lytton J (2004b) Molecular cloning of a sixth member of the K+-dependent Na+/Ca2+ exchanger gene family, NCKX6. J Biol Chem 279:5867–5876
Carter C, Pan S, Zouhar J, Avila EL, Girke T, Raikhel NV (2005) The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected protein. Plant Cell 16:3285–3303
Catala R, Santos E, Alonso JM, Ecker JR, Martinez-Zapater JM, Salinas J (2004) Mutations in the Ca2+/H+ transporter CAX1 increase CBF/DREB1 expression and the cold-acclimation response in Arabidopsis. Plant Cell 15:2940–2951
Cheng NH, Pittman JK, Barkla BJ, Shigaki T, Hirschi KD (2003) The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell 15:347–364
Claros MG, von Heijne G (1994) TopPred II: An improved software for membrane protein structure predictions. Comput Appl Biosci 10:685–686
Cunningham KW, Fink GR (1996) Calcineurin inhibits VCX1-dayependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol 16:2226–2237
del Pozo L, Osaba L, Corchero J, Jimenez A (1999) A single nucleotide change in the MNR1 (VCX1/HUM1) gene determines resistance to manganese in Saccharomyces cerevisiae. Yeast 15:371–375
Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R (1998) The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 280:69–77
Ettinger WF, Clear AM, Fanning KJ, Peck ML (1999) Identification of a Ca2+/H+ antiport in the plant chloroplast thylakoid membrane. Plant Physiol 119:1379–1385
Felsenstein J (1996) Inferring phylogenies from protein sequences by parsimony, distance, and likelihood methods. Methods Enzymol 266:418–427
Hirschi K (2001) Vacuolar H+/Ca2+ transport: Who’s directing the traffic? Trends Plant Sci 6:100–104
Hirschi KD, Korenkov VD, Wilganowski NL, Wagner GJ (2000) Expression of Arabidopsis CAX2 in tobacco. Altered metal accumulation and increased manganese tolerance. Plant Physiol 124:125–133
Hirschi K, Zhen R, Cunningham KW, Rea PA, Fink GR (1996) CAX1, a H+/Ca2+ antiporter from Arabidopsis. Proc Natl Acad Sci USA 93:8782–8786
Ivey DM, Guffanti AA, Zemsky J, Pinner E, Karpel R, Padan E, Schuldiner S, Krulwich TA (1993) Cloning and characterization of a putative Ca2+/H+ antiporter gene from Escherichia coli upon functional complementation of Na+/H+ antiporter-deficient strains by the overexpressed gene. J Biol Chem 268:11296–11303
Kamiya T, Akahori T, Maeshima M (2005) Expression profile of the genes for rice cation/H+ exchanger family and functional analysis in yeast. Plant Cell Physiol 46:1735–1740
Kamiya T, Maeshima M (2004) Residues in internal repeats of the rice cation/H+ exchanger are involved in the transport and selection of cations. J Biol Chem 279:812–819
Kasai M, Muto S (1990) Ca2+ pump and Ca2+/H+ antiporter in plasma membrane vesicles isolated by aqueous two-phase partitioning from maize leaves. J Membr Biol 114:133–142
Krogh A, Larsson B, von Heijne G, Sonnhammer ELL (2001) Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J Mol Biol 305:567–580
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132
Laughery MD, Todd ML, Kaplan JH (2003) Mutational analysis of α-β subunit interactions in the delivery of Na, K-ATPase heterodimers to the plasma membrane. J Biol Chem 278:34794–34803
Luo GZ, Wang HW, Huang J, Tian AG, Wang YJ, Zhang JS, Chen SY (2005) A putative plasma membrane cation/proton antiporter from soybean confers salt tolerance in Arabidopsis. Plant Mol Biol 59:809–820
Martin W, Embley TM (2004) Early evolution comes full circle. Nature 431:134–137
Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJ, Sanders D, Harper JF, Tchieu J, Gribskov M, Persans MW, Salt DE, Kim SA, Guerinot ML (2001) Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol 126:1646–1667
McDonough AA, Geering K, Farley RA (1990) The sodium pump needs its beta subunit. FASEB J 4:1598–1605
Möller S, Croning MDR, Apweiler R (2001) Evaluation of methods for the prediction of membrane spanning regions. Bioinformatics 17:646–653
Page RD (1996) TREEVIEW: An application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Palty R, Ohana E, Hershfinkel M, Volokita M, Elgazar V, Beharier O, Silverman WF, Argaman M, Sekler I (2004) Lithium-calcium exchange is mediated by a distinct potassium-independent sodium-calcium exchanger. J Biol Chem 279:25234–25240
Park S, Cheng NH, Pittman JK, Yoo KS, Park J, Smith RH, Hirschi KD (2005a) Increased calcium levels and prolonged shelf life in tomatoes expressing Arabidopsis H+/Ca2+ transporters. Plant Physiol 139:1194–1206
Park S, Kang TS, Kim CK, Han JS, Kim S, Smith RH, Pike LM, Hirschi KD (2005b) Genetic manipulation for enhancing calcium content in potato tuber. J Agric Food Chem 53:5598–5603
Park S, Kim CK, Pike LM, Smith RH, Hirschi KD (2004) Increased calcium in carrots by expression of an Arabidopsis H+/Ca2+ transporter. Mol Breed 14:275–282
Philipson KD, Nicoll DA (2000) Sodium-calcium exchange: A molecular perspective. Annu Rev Physiol 62:111–133
Pittman JK, Cheng NH, Shigaki T, Kunta M, Hirschi KD (2004) Functional dependence on calcineurin by variants of the Saccharomyces cerevisiae vacuolar Ca2+/H+ exchanger Vcx1p. Mol Microbiol 54:1104–1116
Qi BS, Li CG, Chen YM, Lu PL, Hao FS, Shen GM, Chen J, Wang XC (2005) Functional analysis of rice Ca2+/H+ antiporter OsCAX3 in yeast and its subcellular localization in plant. Prog Biochem Biophys 32:876–881 (in Chinese)
Ruknudin A, Schulze DH (2002) Proteomics approach to Na+/Ca2+ exchangers in prokaryotes. Ann N Y Acad Sci 976:103–108
Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell 14:S401–S417
Schiott M, Romanowsky SM, Baekgaard L, Jakobsen MK, Palmgren MG, Harper JF (2004) A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc Natl Acad Sci USA 101:9502–9507
Shigaki T, Cheng NH, Pittman JK, Hirschi K (2001) Structural determinants of Ca2+ transport in the Arabidopsis H+/Ca2+ antiporter CAX1. J Biol Chem 276:43152–43159
Shigaki T, Hirschi K (2000) Characterization of CAX-like genes in plants: Implications for functional diversity. Gene 257:291–298
Shigaki T, Pittman JK, Hirschi KD (2003) Manganese specificity determinants in the Arabidopsis metal/H+ antiporter CAX2. J Biol Chem 278:6610–6617
Song CP, Guo Y, Lambert G, Galbraith DW, Jagendorf A, Zhu JK (2004) A probable Na+(K+)/H+ exchanger on the chloroplast envelope functions in pH homeostasis and chloroplast development in Arabidopsis thaliana. Proc Natl Acad Sci USA 101:10211–10216
Stamatakis A, Ludwig T, Meier H (2005) RAxML-III: A fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics 21:456–463
Swafford DL (1998) PAUP*: Phylogenetic analysis using parsimony (*and other materials). Sinauer, Sunderland, MA
Ueoka-Nakanishi H, Nakanishi Y, Tanaka Y, Maeshima M (1999) Properties and molecular cloning of Ca2+/H+ antiporter in the vacuolar membrane of mung bean. Eur J Biochem 262:417–425
Ueoka-Nakanishi H, Tsuchiya T, Sasaki M, Nakanishi Y, Cunningham KW, Maeshima M (2000) Functional expression of mung bean Ca2+/H+ antiporter in yeast and its intracellular localization in the hypocotyl and tobacco cells. Eur J Biochem 267:3090–3098
Waditee R, Hossain GS, Tanaka Y, Nakamura T, Shikata M, Takano J, Takabe T, Takabe T (2004) Isolation and functional characterization of Ca2+/H+ antiporters from cyanobacteria. J Biol Chem 279:4330–4338
Yan BX, Sun YQ (1997) Glycine residues provide flexibility for enzyme active sites. J Biol Chem 272:3190–3194
Yokoi S, Quintero FJ, Cubero B, Ruiz MT, Bressan RA, Hasegawa PM (2002) Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J 30:529–539
Acknowledgments
We are grateful for John M. Ward, Heven Sze, and the members of the Hirschi laboratory for critical reading of the manuscript and helpful suggestions. This work was funded by the National Science Foundation (Grant No. 0209777 to K. H.).
Author information
Authors and Affiliations
Corresponding author
Additional information
[Reviewing Editor: David Guttman]
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Shigaki, T., Rees, I., Nakhleh, L. et al. Identification of Three Distinct Phylogenetic Groups of CAX Cation/Proton Antiporters. J Mol Evol 63, 815–825 (2006). https://doi.org/10.1007/s00239-006-0048-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-006-0048-4