Abstract
The incomplete correlation between the organismal complexities and the number of genes among eukaryotic organisms can be partially explained by multiple protein products of a gene created by alternative splicing. One type of alternative splicing involves alternative selection of mutually exclusive exons and creates protein products with substitution of one segment of the amino acid sequence for another. To elucidate the evolution of the mutually exclusive 115-bp exons, designated flip and flop, of vertebrate AMPA receptor genes, the gene structures of chordate (tunicate, cephalochordate, and vertebrate) and protostome (Drosophila and Caenorhabditis elegans) AMPA receptor subunits were compared. Phylogenetic analysis supports that the vertebrate flip and flop exons evolved from a common sequence. Flip and flop exons exist in all vertebrate AMPA receptor genes but only one 115-bp exon is present in the genes of tunicates and cephalochordates, suggesting that the exon duplication event occurred at the ancestral vertebrate AMPA receptor gene after the separation of vertebrates from primitive chordates. The structures of animal AMPA receptor genes also suggest that an intron insertion to separate the primordial flip/flop exon from the M4-coding exon occurred before the exon duplication event and probably at the chordate lineage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Creations of transcriptome diversification, by mechanisms such as alternative splicing and RNA editing, gene duplication, and formation of chimeric genes, are major evolutionary processes to generate functional complexity of genomes (Modrek and Lee 2002; Bass 2002; Long et al. 2003). The significance of alternative splicing in the formation of functional complexity was partially elucidated by genomewide analysis showing that 35–60% of human genes express multiple splicing isoforms (Mironov et al. 1999; Modrek et al. 2001; Modrek and Lee 2002). Interestingly, nerve-specific genes and genes of the signaling system are highly represented among the alternatively spliced genes, suggesting a general adaptive benefit of acquiring alternative splicing for gene activities required in complex systems (Modrek et al. 2001). The existence of splicing isoforms has been documented in many ion-channel genes (Sommer et al. 1990; Snutch et al. 1991; Kuhse et al. 1991; Copley 2004). With complete genome information for many organisms, clarification of the evolution of alternative splicing in ion-channel genes has become feasible (Copley 2004). The vertebrate α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPA receptor [AMPAR]) gene family, whose transcripts are modified by alternative splicing and RNA editing, provides an interesting case for studying the evolution of new gene functions (Hollmann and Heinemann 1994; Seeburg et al. 1998; Kung et al. 2001).
The AMPA receptor family belongs to the ionotropic glutamate receptor (iGluR) superfamily, which also includes the kainate and N-methyl-D-aspartate (NMDA) receptor families, and mediates fast synaptic transmission in the central nervous system. While NMDA receptors display a distinctly different pharmacological profile, AMPAR and kainate receptors, together designated non-NMDA receptors, can be activated by a common group of agonists. Sequence similarities among vertebrate non-NMDA receptors are higher than those between non-NMDA and NMDA receptors. Sixteen mammalian genes are known to encode subunits of NMDA and non-NMDA receptors. In addition, two orphan iGluR subunits (δ1 and δ2) constitute the δ family of the mammalian iGluR (Hollmann 1999). Subunits of iGluR presumably share a common topology, consisting of an extracellular N terminal domain, four membrane segments, M1–M4, and a large extracellular loop (L3) between M3 and M4 (Hollmann et al. 1994; Hollmann 1999).
Mammalian AMPARs are assembled from the products of GRIA1–GRIA4 genes (also known as GluR1–GluR4 and GluRA–GluRD) in homomeric and heteromeric forms with slightly divergent properties (Keinänen et al. 1990; Hollmann and Heinemann 1994). In general, the properties of nonmammalian gnathostome AMPARs are similar to those of their mammalian counterparts (Chang et al. 1998). Phylogenetic analyses of vertebrate AMPAR genes showed that the members of the AMPAR gene family increased from two in the jawless hagfish, an agnathan, to four in tetrapods and cartilaginous fish. And as a result of teleost-specific duplication, the number of teleost AMPAR genes is nearly double that of tetrapods (Chen et al. 2001). iGluR subunits have also been identified from invertebrates, however, sequence divergences among invertebrate iGluR subunits are much more frequent than those among vertebrate ones (Hollmann 1999; Mellem et al. 2002). Moreover, none of the invertebrate iGluRs is known to be activated by AMPA, the preferred agonist of mammalian AMPARs. Nevertheless, the phylogenetic relationship inferred by the sequence similarities of the M2 and M3 segments suggests that Drosophila melanogaster and Caenorhabditis elegans genomes contain homologues of the mammalian AMPAR genes (Sprengel et al. 2001).
All the primary transcripts of gnathostome GRIA1–GRIA4 studied so far are alternatively spliced at two mutually exclusive exons, termed flop and flip, situated between the L3- and the M4-coding exons (Sommer et al. 1990; Kung et al. 1996; Paperna et al. 1996; Wu et al. 1996; Chen et al. 1999), whereas no invertebrate iGluR transcripts are alternatively spliced in the corresponding region (Hollmann 1999). Flip and flop are two adjacent exons of identical size, 115 bp, and their translated sequences are very similar, suggesting that these two exons may derive from an intragenic duplication. To explore the possibility of intragenic duplication and to determine the date of duplication, we identified AMPAR-like genes of invertebrate by phylogenetic analysis and by analysis of the gene structures. Our evidence suggests that a split of the 115-bp primordial exon from the M4-coding exon occurred at the lineage evolved to chordate, and an intragenic duplication event took place at a single ancestral vertebrate AMPAR gene to give rise to the present flop and flip exons of all vertebrate AMPAR genes.
Materials and Methods
Searches of Animal Ionotropic Glutamate Receptor Genes from Databases
The classification and accession numbers of human ionotropic glutamate receptors have been summarized by Hollmann (1999). Sequences of protostome (Drosophila melanogaster and Caenorhabditis elegans) iGluR subunits were obtained from flybase (http://www.flybase.org/) and wormbase (http://www.wormbase.org/), where the genes have been annotated and their phylogenetic relationships have been inferred (Sprengel et al. 2001). Genes encoding ionotropic glutamate receptor or AMPA receptor (AMPAR) subunits of pufferfish (Fugu rubripes) and sea squirt (Ciona intestinalis) were obtained by using the M1–M4 amino acid sequence of human GRIA2 as query to search nucleotide databases (tblstn [Altschul et al. 1997]). Twelve sequence entries, including two repetitious ones, were retrieved from the Ciona database v1 (DOE Joint Genome Institute; http://www.jgi.doe.gov/). The sequences of these entries were individually compared to the protein database (http://www.ncbi.nlm.nih.gov/) by blastx (Altschul et al. 1997) to find putative exonic sequences. Similar predictions of exons were also obtained by comparing the Ciona sequences to the protein sequences by Wise2 (http://www.sanger.ac.uk/software/wise2). The result of gene assembly is summarized in Table 1, where the names of genes were designated according to the inferred phylogenetic relationship established in this study.
Seven genomic sequences, which corresponded to the seven AMPAR subunits previously identified by an RT-PCR survey (Chen et al. 2001) of pufferfish, were obtained from the pufferfish genome project (http://www.fugu.hgmp.mrc.ac.uk/). Exonic sequences, including the flip and flop sequences, were predicted by GENSCAN (Burge and Karlin 1997; http://www.genes.mit.edu/GEMESCAM.html). The sequences derived from the putative pufferfish exons were also compared to the mammalian AMPAR sequences to confirm the prediction of exons.
Analysis of the Hagfish AMPA Receptor GeneStructure
Complementary DNA sequences coding for the first three membrane domains, M1–M3, and a small portion of the L3 of the two hagfish (Paramyxine nelsoni) AMPAR subunits have been reported (Chen et al. 2001). The C terminus-coding sequences of these genes were obtained by 3′’-RACE (rapid amplification of cDNA ends). The sequences of PCR primers and their annealing sites to the hagfish AMPAR genes are listed in Table 2 and depicted in Figure 1, respectively. Total RNA (1 μg) extracted from hagfish brain was reverse transcribed using the CDS primer of the SMART kit (Clontech). One tenth of the resulting cDNA was used as the template for PCR with UPM (Clontech), and either #293 (hfGRIA1) or #291 (hfGRIA2) as primer. A nested PCR was performed by using one twentieth of the first-round PCR product with the NUP (Clontech) and either #289 (hfGRIA1) or #287 (hfGRIA2) as primer. RACE products were cloned to pGEMTeasy vector (Promega) and sequenced using the Bigdye terminator system (Applied Biosystems).
Annealing sites of PCR primers. A vertebrate AMPAR gene is depicted. Boxes, lines, and arrows represent exons, introns, and primer annealing sites, respectively. Arrows point to the 3′ end of oligonucleotides. Filled boxes represent the coding regions of the four membrane segments (starting from the left, M1–M4) and hatched boxes are the flip and flop exons. The sequences of the primers are listed in Table 2.
Identification of animal AMPA receptor subunit by phylogenetic analysis. A Alignment of the M1-M4 sequences of animal AMPAR subunits. Accession numbers of these genes are listed in Table 1. Only the sequences of those subunits inferred as the animal AMPAR and AMPAR-like subunits were aligned by the Pileup program of the GCG package using a default setting. Sequences of the amphioxus AMPAR genes, amGRIA1a, amGRIA1b, and amGRIA2, are incomplete. Gaps were then manually removed or adjusted to obtain the alignment shown here. Shadowed amino acids indicate matches to the consensus sequence (more than 50%). M1, M2, M3, and M4 represent the four membrane spanning segments. Arrowheads indicate the conserved locations of intron insertion sites, and the phases of intron insertion are shown above. B Phylogeny of the animal ionotropic glutamate receptors. Those subunits with only partial sequence information are marked with asterisks. Horizontal lines indicate genetic distance. Numbers are bootstrap values based on 1000 runs. Only the values of important nodes or values >50% are shown. The five families are marked by vertical lines. A summary of the phylogenetic classifications is listed in Table 1. Prefixes pf, hf, ci, D, and ce, respectively, indicate pufferfish, hagfish, Ciona, Drosophila, and C. elegans genes. Protein IDs were used for those subunits whose classifications have not been fully confirmed.
The genomic DNA fragments carrying the L3-, flop-, and M4-coding exons of hfGRIA1 and hfGRIA2 were amplified by the Expand long template system (Roche) using DNA (300 ng) extracted from hagfish liver as the template and gene-specific primer pairs, either #455–#487 (hfGRIA1) or #461–#463 (hfGRIA2). Nested PCR was then performed with #456–#488 (hfGRIA1-specific pair) or #430–#449 (hfGRIA2-specific pair) to further enrich the two genes. Restriction endonuclease maps of the resultant PCR amplicons, 8 kb (hfGRIA1) and 5 kb (hfGRIA2) in length, were determined after cloning. The exon-containing fragments were identified by hybridization to the respective hagfish 3′’-RACE products, cloned, and sequenced. Furthermore, restriction endonuclease fragments carrying the flip exons of hfGRIA1 and hfGRIA2 were also identified by Southern hybridization, using cDNA molecules encoding the flip isoforms of tilapia (Oreochromis massambicus) tfGRIA1α and tfGRIA2β as probes, and sequenced (Kung et al. 1996; Wu et al. 1996). These genomic DNA sequences were compared to the 3′-RACE products and fitted to the splicing consensus sequence to mark the intron–exon boundaries.
Analysis of the Amphioxus AMPA Receptor Gene Structure
Genomic DNA of amphioxus (Epigonichthys lucayanus) was extracted from two ethanol-preserved animals. Genomic DNA fragments carrying the coding region of M1–M3 of the amphioxus AMPAR were amplified by the degenerate primer pair, #104 and #105 (Table 2) (Chen et al. 2001), and Expand long template system (Roche). One fifth of the resultant DNA was subjected to a second PCR amplification by the same primer pair. Southern blot hybridization showed that three amplified amphioxus genomic DNA fragments were homologous to the zebrafish AMPAR cDNA (data not shown). DNA sequences of these amphioxus PCR amplicons, 1.1 kb (amGRIA1a), 0.95 kb (amGRIA1b), and 0.7 kb (amGRIA2) in length, were determined after cloning. These DNA sequences were compared to the protein database (http://www.ncbi.nlm.hih.gov/) by blastx to confirm that the translated sequences of the amphioxus DNA are homologous to the vertebrate AMPAR subunits (Fig. 2A). The exons were identified by the regions which encoded protein sequences displaying high similarities to the vertebrate AMPAR subunits and matched the splicing consensus.
Cloning of the L3-coding region of amphioxus AMPAR genes was achieved by two sequential walking steps. First, two rounds of PCR using the degenerate primer pair, #113 and #114 (Table 2 and Fig. 1), were performed to amplify the M3- and L3-coding regions. Only one 0.7-kb amplicon specifically hybridized to the AMPAR probes, and the sequence of this 0.7-kb DNA partially overlapped with that of the previously cloned amGRIA2. The exon–intron boundaries were determined by blastx analysis as previously described. Next, the L3- and M4-coding regions were obtained by PCR using the amGRIA2-specific primer (#542) and the degenerate primer (#544), followed by another amplification using nested amGRIA2 primers (#543 and #544; Table 2 and Fig. 1). The resultant 1.5-kb amplicon partially overlapped with the 0.7-kb amplicon. By stepwise PCR amplifications, the sequence and gene structure of the M2-, M3-, L3-, and M4-coding regions of amGRIA2 were determined. A similar strategy failed to clone the 3′ halves of amGRIA1a and amGRIA1b.
Phylogenetic Analyses
To construct phylogenetic trees of animal iGluR subunits, comparisons of M1–M4 amino acid sequences were performed with CLUSTAL_X (Thompson et al. 1997) using default parameters (data not shown). Unless specified, only animal genes with known M1–M4 sequences were included for comparisons. Phylogenetic trees were constructed by neighbor-joining (NJ) analysis as implemented in the program MEGA version 2.1 (Kumar et al. 2001). Phylogenetic analyses of the flip and flop sequences were performed with the alignment of animal AMPAR subunits and the corresponding regions of a few selected kainate receptor subunits. Distances among these sequences were calculated by Poisson distance and phylogenetic trees were constructed without outgroups or using kainate receptors as outgroups. The resulting topologies were essentially the same. The robustness of NJ trees was assessed by bootstrap analysis with 1000 replications.
Results
Amphioxus and Hagfish AMPAR Genes
To study the evolution of animal AMPAR genes, genes of selected animals from protostomia and Chordata, including amphioxus (Cephalochordata), Ciona intestinalis (Tunicata), and Vertebrata, were obtained either by PCR or by searching databases. The AMPAR of jawless vertebrate (Agnatha; hagfish) was also cloned. Using a pair of degenerate primers pairing to the M1- and L3-coding region of the gnathostome AMPAR genes, three genomic DNA amplicons of amphioxus (Epigonichthys lucayanus) were obtained by PCR and designated amGRIA1a, amGRIA1b, and amGRIA2 (Materials and Methods). Exons of these genes were predicted by comparing all putative translated regions to the protein database. The predicted sequences display the highest sequence identities to animal AMPAR subunits (Fig. 2A). The translated sequences of amGRIA1a and amGRIA1b are 99% identical (Fig. 2A and data not shown). On the other hand, the amino acid sequence of amGRIA2 shares only 88 and 87% identity, respectively, to that of amGRIA1a and amGRIA1b. The degrees of homology between the exonic sequences of amGRIA1a/b and amGRIA2 are about 88%, which is less than that between amGRIA1a and amGRIA1b (99%). Excluding an insertion/deletion sequence, the intronic sequences of amGRIA1a and amGRIA1b are well conserved (87% degrees of homology), whereas the intronic sequences between amGRIA1a/b and amGRIA2 share only low degrees of homology (40–44%). Since the nucleotide sequences of amGRIA1a and amGRIA1b are highly conserved, the possibility that amGRIA1a and amGRIA1b are allelic cannot be ruled out. In summary, these results suggest that amphioxus possesses at least two AMPAR genes. Next, the L3- and M4-coding exons of amGRIA2 were determined after sequence analysis of the PCR amplicons (Materials and Methods). The translated sequences of these exons are shown in Fig. 2A.
Hagfish brains express two AMPAR subunits, hfGRIA1 and hfGRIA2 (Chen et al. 2001). The C-terminal coding sequences of hfGRIA1 and hfGRIA2 were obtained by 3′-RACE (Materials and Methods) and the translated sequences are shown in Figure 2A. Nine of 10 sequenced hfGRIA1 3′-RACE products encoded a protein sequence similar to the mammalian flop isoforms and the remaining product encoded a protein similar to the mammalian flip isoforms (Fig. 3A). However, all 10 sequenced hfGRIA2 RACE products encoded the flop isoform.
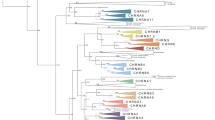
Phylogenetic analysis of the flip/flop region of AMPA receptor subunits. A Alignment of the amino acid sequence of non-NMDA receptor subunits. Shadowed amino acids indicate matches to the consensus sequence (>50%). Asterisks indicate invariant amino acid residues unique to the mammalian flip and flop isoforms (Sommer et al, 1990). Flip and flop isoforms are designated by postfixes i and o, respectively. B Phylogeny of the flip/flop region of animal AMPA receptor subunits. The alignment shown in A was used to construct the tree. Human kainate receptor subunits (GRIK1 and GRIK4) were chosen as outgroups to construct the tree. Numbers are bootstrap values based on 1000 runs. Only values >50% are shown.
Identification of Animal AMPAR and AMPAR-like Genes by Phylogenetic Analyses
The accession numbers of genes studied here are listed in Table 1. New searches by definition or by sequence similarities of human, C. elegans, and Drosophila databases retrieved sets of iGluR genes similar to those reported by Hollmann (1999) and Sprengel et al. (2001). The M1–M4 region, which includes the four membrane segments and the large extracellular L3, is the most conserved region among various iGluR subunits and was used to search the tunicate database. Exonic sequences of the Ciona genes were predicted by matching to the protein database and were then matched to the splicing consensus sequences (data not shown). The exons thus predicted are identical to those annotated in the Ciona database. Nine of the 10 Ciona scaffolds encode the M1–M3 sequence, and 7 of these scaffolds also include the L3- and M4-coding sequences (Table 1, Fig. 2A, and data not shown).
The phylogenetic relationships of the putative animal iGluRs were established to identify Ciona and protostome homologues of mammalian AMPAR subunits. Three similar phylogenetic trees were established, respectively, on the sequence similarities of M1–M4 (Fig. 2B), of M1–M3 (data not shown), and of M1–M4 removing the extra L1 sequences found in some of the animal iGluR subunits (Chen et al. 2001) (Fig. 2 and data not shown). Similar to the phylogenetic relationships previously inferred by analysis of M2–M3 sequences among vertebrate and protostome AMPAR subunits (Sprengel et al. 2001), the tunicate and protostome genomes contain at least one gene encoding analogous proteins of the four mammalian iGluR families (Fig. 2B). Furthermore, several iGluR subunits of insect and worm with no vertebrate homologue were also found (protostomia-specific receptor; Fig. 2B). However, so far there is no evidence to suggest that Ciona possesses an iGluR subunit homologous to the protostomia-specific iGluR subunits (Fig. 2B and data not shown). The two Ciona non-NMDA receptor genes, ciGRIA1 and ciGRIK1, are clustered with the vertebrate AMPAR and kainate receptor genes, respectively (Fig. 2B). The phylogenetic analysis also confirms that the newly cloned amphioxus genes, amGRIA1a/b and amGRIA2, are homologues of vertebrate AMPAR genes. Supported by a very strong bootstrap value (99%), the animal AMPAR/AMPAR-like gene family should contain the Drosophila DGluRIA and DGluRIB, the C. elegans ceglr1 and ceglr2, and Ciona ciGRIA1 (Fig. 2B). Consistent with the phylogenetic relationships inferred here, the ceglr1 and ceglr2 have been suggested as components of a functional non-NMDA receptor (Mellem et al. 2002). Similar phylogenetic relationships of these animal AMPAR and AMPAR-like genes were consistently observed regardless of the regions selected for comparisons and methods used to construct the trees (data not shown; Hollmann 1999; Sprengel et al. 2001). On the other hand, the previous assignments (Sprengel et al. 2001) of Drosophila AAF59382 and AAF46470 to the AMPAR-like gene family cannot be supported by the phylogenetic tree established on the similarities of M1–M4 sequences in this study.
Agnathan AMPAR Genes Contain Flip and FlopExons
Transcripts of both flip and flop isoforms were present in the hagfish hfGRIA1 3’-RACE products. However, a flip transcript was not found in the hfGRIA2 RACE product. The flop exon of gnathostome GRIA1–GRIA4 is upstream to the flip exon (Fig. 4) (Sommer et al. 1990; Köhler et al. 1994; Chen et al. 1999). The genomic fragments carrying the flop and M4-coding exons of hfGRIA1 and hfGRIA2 were amplified by PCR (Materials and Methods) to search for the presence of the flip exon in hfGRIA2 and to determine the gene structures. Each of the hfGRIA1 and hfGRIA2 amplicons contained a putative coding region encoding a peptide similar to the gnathostome flip sequences (Fig. 3A), supporting that the flip exon is situated between the flop and the M4-coding exons (Fig. 4). Expression of the newly identified hfGRIA2 flip exon was confirmed by RT-PCR analysis using the primer pair (#449–#538; Table 1 and Fig. 1) complementary to the predicted flip and M4-coding exons (data not shown). In summary, the 3’-RACE and RT-PCR analyses support that the flop and flip exons of both hagfish AMPAR genes are expressed and are alternatively spliced by mutual exclusion.
Partial gene structures of animal non-NMDA receptor genes. Exons (open boxes) were drawn to scale, while introns (lines) were not. Numbers in the boxes are the lengths of exons (base pairs). A dashed line indicates where the intron has not been fully cloned. Filled boxes are the coding regions of the membrane segments. Hatched boxes are exons coding for the alternatively spliced exons flip ( ) and flop (
) and flop ( ) of the vertebrate AMPAR subunits as well as the corresponding region of the invertebrate non-NMDA receptor subunits (
) of the vertebrate AMPAR subunits as well as the corresponding region of the invertebrate non-NMDA receptor subunits ( ). P0, P1, and P3 are the three phases of intron insertion. Gnathostome and agnathan AMPA receptor genes are represented by mouse GRIA2 (Köhler et al., 1994) and hfGRIA1, respectively. Mouse GRIK1 and GRIK4 as well as Ciona GRIK1 (ciGRIK1) are selected to represent the chordate kainate receptor genes.
). P0, P1, and P3 are the three phases of intron insertion. Gnathostome and agnathan AMPA receptor genes are represented by mouse GRIA2 (Köhler et al., 1994) and hfGRIA1, respectively. Mouse GRIK1 and GRIK4 as well as Ciona GRIK1 (ciGRIK1) are selected to represent the chordate kainate receptor genes.
Primitive Chordate AMPAR Genes Have Only One Exon as the Primordial Vertebrate Flip/Flop Exon
The gene structures of invertebrate AMPAR and AMPAR-like genes were predicted from sequences deposited in the database and from PCR amplicons (Materials and Methods). Figure 4 shows the gene structures of representative AMPAR genes. The structure of the L3-coding exon, which is flanked by introns inserted at homologous sites and in the same phases (PO and P2), is well conserved in the animal AMPAR genes (Figs. 2A and 4). Although the 3’ intron insertion site and phase are not preserved in the C. elegans ceglr1, the 5’ intron–exon boundary of the L3-coding exon is conserved in ceglr1. The L3-coding exons of Ciona ciGRIA1 and C. elegans ceglr2 preserve the consensus intron insertion sites but theses exons are split (Fig. 4). In addition, the structure of M3-coding exon is conserved among gnathostome and C. elegans AMPAR genes. On the other hand, the structures of the M3- and L3-coding exons are different between AMPAR and the kainate receptor genes (Fig. 4). Moreover, the gene structures of AAF59382 and AAF46470 are different from that of the AMPAR genes, further supporting that these genes are unlikely AMPAR homologues (data not shown).
Other than the already identified 115-bp exons, the sequences between the L3- and the M4-coding exons of ciGRIA1 and amGRIA2 are devoid of coding sequence related to the iGluR subunits, indicating that ciGRIA1 and amGRIA2 are unlikely to possess an alternate translated region between L3 and M4. Interestingly, the 115-bp exon is fused to the M4-coding exon in the Drosophila and C. elegans AMPAR genes, indicating that the 115-bp exon split from the M4-coding exon in the chordate lineage. The translated sequences of the chordate 115-bp exon and the equivalent regions of protostome AMPAR-like, two human kainate receptor subunits (GRIK1 and GRIK4), and Ciona GRIK1 were aligned (Fig. 3A). Five residues, -S-KDSG- and -N-GGGD, have been shown to be respectively conserved in the mammalian flip and flop sequences (Sommer et al. 1990) (Fig. 3A). The mammalian signature sequences are preserved in almost all of the vertebrate AMPAR subunits, whereas they are not preserved in the invertebrate AMPAR or in the kainate receptor subunits.
The phylogenetic relationship of flip and flop sequences was constructed (Fig. 3B). Supported by strong bootstrap values, the vertebrate flip and flop sequences separate into two clusters. The tunicate, cephalocordate, and protostome sequences are on the base of the vertebrate flip and flop sequences, confirming that a single ancestral exon gives rise to the flip and flop exons of vertebrates. Interestingly, vertebrate flip sequences are more diverged among themselves than the flop sequences are, implying a functional constraint of the relatively fast desensitizing flop channels in vertebrates.
Discussion
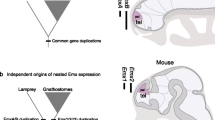
Ten scaffolds from the Ciona genome were discovered to encode proteins homologous to subunits of the four mammalian families of iGluRs (Table 1 and Fig. 2B). Phylogenetic analysis indicates the all 10 known Ciona iGluR genes grouped with the vertebrate genes, and hence, there is no evidence to suggest that the Ciona genome encodes a protostomia-specific receptor (Fig. 2B). Our analyses of phylogenetic relationships and of the gene structures confirmed that only four of the seven protostome AMPAR-like genes identified by Sptrengel et al. (2001), two each of Drosophila and C. elegans, are homologues of the chordate AMPAR genes (Table 1, Figs. 2B and 4). The structure of the remaining three protostome genes (i.e., Drosophila AAF46470 and AAF50976) do not preserve that of the vertebrate AMPAR genes (data not shown). This study demonstrates that, like the gnathostome AMPAR genes, the agnathan genes contain flip and flop exons, whereas the primitive chordate AMPAR genes possess only a single exon, homologous to the flip and flop exons, residing between the L3- and the M4-coding exons (Figs. 3 and 4). The phylogenetic analysis shows that the vertebrate flip and flop sequences are equally diverged from the invertebrate sequences, supporting that the mutually exclusive exons of vertebrate AMPAR genes evolve from a common ancestral exon (Fig. 3).
Independent duplication events giving rise to mutually exclusive exons of other ion channels have been demonstrated in various animal lineages (Copley 2004). This study demonstrates that the duplication event creating the flip and flop isoforms of AMPAR is unique to the vertebrate lineage. In addition, comparisons of the chordate and protostome AMPAR gene structures suggest that the splitting of the ancestral exon from the M4-coding exon occurs in the chordate lineage and thus antedates the exon duplication event. This observation is consistent with the notion that complete exon duplication is the most plausible mechanism responsible for the evolution of alternative splicing of tandem exons (Kondrashov and Koonin 2001). Since all vertebrate AMPAR genes possess tandem exons, the duplication event takes place before the radial duplication of AMPAR genes in the vertebrates.
Two additional evolutionary processes of the vertebrate AMPAR gene can be expected after the exon duplication event: functional diversifications of the duplicated exons and adoption of a mutually exclusive usage of one exon. In theory, the evolutionary fates of the duplicated exons should be similar to those of the duplicated genes: nonfunctionalization, neofunctionalization, and, possibly also, subfunctionalization (Kondrashov and Koonin 2001; Prince and Pickett 2002; Long et al. 2003). Both the flip and the flop exons of each AMPAR gene are expressed in vertebrate brains, showing that both duplicated exons are functional. Adaptive evolution to acquire a new or a subtly different function may occur to avoid nonfunctionalization. The flip/flop region is part of the ligand-binding domain labeled S2 of the AMPAR and affects the gating properties (Sommer et al. 1990; Stern-Bach et al. 1994; Partin et al. 1996). In general, mammalian AMPAR channels composed of flop isoforms desensitize more rapidly than those composed of the flip isoforms (Sommer et al. 1990; Partin et al. 1996). Gating properties, such as activation, desensitization, inactivation, and recovery, of the AMPAR affect the efficiency of transsynaptic signaling, chance of coincidence detection, and integration of synaptic events. Detailed studies of the kinetic properties of invertebrate non-NMDA receptors have been hampered by small and sometimes nondetectable channel activities when exogenously expressed. A very fast desensitization rate of the protostome iGluRs is one of the plausible reasons for the difficulties of measuring these channels (Schuster et al. 1991; Strutz-Seebohm et al. 2003). It is yet to be determined if the more efficient AMPAR channels, i.e., those composed of the flip isoforms, are novel to the vertebrate central nervous system. Nevertheless, the duplication and subsequent functional diversifications of the flip and flop sequences provide ways to modify the gating properties of AMPAR vertebrate channels without changing the other properties such as subcellular localizations and subunit interactions, respectively, determined by the C- and N-terminal portions of the receptors (Ayalon and Stern-Bach 2001; Bredt and Nicoll 2003). Since the expression of both splicing variants is under the same regulatory control, a family of heterogeneous channels, composed of various splicing isoforms and with different desensitization kinetics, may be formed in a cell. Hence, the evolution of flip and flop exons increases the dynamics of postsynaptic AMPAR signals (Jones and Westbrook 1996).
Since the size of the duplicated exon is not a multiple of three, evolution of an alternative splicing is required to maintain a correct reading frame. Moreover, utilization of alternative splicing can be an advantageous trait, for it allows tissue- and development-specific expression of isoforms (subfunctionalization) as demonstrated in mouse development (Monyer et al. 1991). It is yet to be determined how alternative splicing is controlled in different cells. Both sequence-based mutations of the splicing sites and trans-factor-based alternations can be expected to modify the splicing efficiency and thus may contribute to the establishment and control of the alternate uses of flip and flip exons (Ast 2004).
Abbreviations
- AMPAR:
-
AMPA receptor
- GluR:
-
glutamate receptor
- iGluR:
-
ionotropic glutamate receptor
- M:
-
membrane domain
- L3:
-
loop between M3 and M4
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs Nucleic Acids Res 25:3389–3402
Ast G (2004) How did alternative splicing evolve? Nature Rev Genet 5:773–782
Ayalon G, Stern-Bach Y (2001) Functional assembly of AMPA and kainate receptors is mediated by several discrete protein–protein interactions Neuron 31:103–113
Bass BL (2002) RNA editing by adenosine deaminases that act on RNA Annu Rev Biochem 71:817–846
Bredt DS, Nicoll RA (2003) AMPA receptor trafficking at excitatory synapses Neuron 40:361–379
Burge C, Karlin S (1997) Prediction of complete gene structures in human genomic DNA J Mol Biol 268:78–94
Chang H-M, Wu Y-M, Chang Y-C, Hsu Y-C, Hsu H-Y, Chen Y-C, Chow W-Y (1998) Molecular and electrophysiological characterizations of fGluR3α, an ionotropic glutamate receptor subunit of a teleost fish Brain Res Mol Brain Res 57:211–20
Chen Y-C, Kung S-S, Chen B-Y, Hung C-C, Chen C-C, Wang T-Y, Wu Y-M, Lin W-H, Tzen C-S, Chow W-Y (2001) Identifications, classification and evolution of the vertebrate α–amino–3–hydroxy–5–methyl–4–isoxazole propionic acid (AMPA) receptor subunit genes J. Mol Evol 53:690–02
Chen Y-C, Kung S-S, Wu Y-M, Huang C-J, Chow W-Y (1999) Genomic organization of the Oreochromis mossambicus glutamate receptor subunit 2β gene (fGluR2β): presence of two different 5’-untranslated regions Gene 237:241–51
Copley RR (2004) Evolutionary convergence of alternative splicing in ion channels Trends Genet 20:171–76
Hollmann M (1999) Structure of ionotropic glutamate receptors. In: Jonas P, Monyer H (eds) Ionotropic glutamate receptors in the CNS. Springer-Verlag, Berlin/Heidelberg, pp 1–98
Hollmann M, Heinemann S (1994) Cloned glutamate receptors Annu Rev Neurosci 17:31–108
Hollmann M, Maron C, Heinemann S (1994) N-Glycosylation site tagging suggests a three transmembrane domain topology of the glutamate receptor GluR1 Neuron 13:1331–1343
Jones MV, Westbrook GL (1996) The impact of receptor desensitization on fast synaptic transmission Trends Neurosci 19:96–101
Keinänen K, Wisden W, Sommer B, Werner P, Herb A, Verdoorn TA, Sakmann B, Seeburg PH (1990) A family of AMPA-selective glutamate receptors Science 249:556–560
Köhler M, Kornau H-C, Seeburg PH (1994) The organization of the gene for the functionally dominant α-amino 3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunit GluR-B J Biol Chem 269:17367–17370
Kondrashov FA, Koonin EV (2001) Origin of alternative splicing by tandem exon duplication Hum Mol Genet 10:2661–2669
Kuhse J, Kuryatov A, Maulet Y, Malosio ML, Schmieden V, Betz H (1991) Alternative splicing generates two isoforms of the a2 subunit of the inhibitory glycine receptor FEBS Lett 283:73–77
Kumar S, Tamura K, Jakobsen IB, Nei M (2001) MEGA2: molecular evolutionary genetics analysis software Bioinformatics 17:1244–1245
Kung S-S, Chen Y-C, Lin W-H, Chen C-C, Chow W-Y (2001) Q/R RNA editing of the AMPA receptor subunit 2 (GRIA2) transcript evolves no later than the appearance of cartilaginous fishes FEBS Lett 509:277–281
Kung S-S, Wu Y-M, Chow W-Y (1996) Characterization of two fish glutamate receptor cDNA molecules: absence of RNA editing at the Q/R site Mol Brain Res 35:119–130
Long M, Betrán E, Thornton K, Wang W (2003) The origin of new genes: glimpses from the young and old Nature Rev Genet 4:865–875
Mellem JE, Brockie PJ, Zheng Y, Madsen DM, Maricq AV (2002) Decoding of polymodal sensory stimuli by postsynaptic glutamate receptors in C. elegans Neuron 36:933–944
Mironov AA, Fickett JW, Gelfand MS (1999) Frequent alternative splicing of human genes Genome Res 9:1288–1293
Modrek B, Lee C (2002) A genomic view of alternative spicing Nature Genet 30:13–19
Modrek B, Resch A, Grasso C, Lee C (2001) Genome-wide detection of alternative splicing in expressed sequences of human gene. Nucleic Acids Res 29:2850–2859
Monyer H, Seeburg PH, Wisden W (1991) Glutamate-operated channels: developmentally early and mature forms arise by alternative splicing Neuron 6:799–810
Paperna T, Lamed Y, Teichberg VI (1996) cDNA cloning of chick brain α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors reveals conservation of structure, function and post-transcriptional processes with mammalian receptors Mol Brain Res 36:101–113
Partin KM, Fleck NW, Mayer ML (1996) AMPA receptor flip/flop mutants affecting deactivation, desensitization, and modulation by cyclothiazide, aniracetam, and thiocyanate J Neurosci 16:6634–6647
Prince VE, Pickett FB (2002) Splitting pairs: the diverging fates of duplicated genes Nature Rev Genet 3:827–837
Schuster CM, Ultsch A, Schloss P, Cox JA, Schmitt B, Betz H (1991) Molecular cloning of an invertebrate glutamate receptor subunit expressed in Drosophila muscle Science 254:112–114
Seeburg PH (1998) RNA editing of brain glutamate receptor channels: mechanism and physiology Brain Res. Rev. 26:217–229
Snutch TP, Tomlinson J, Leonard JP, Gilbert MM (1991) Distinct calcium channels are generated by alternative splicing and are differentially expressed in the mammalian CNS Neuron 7:45–57
Sommer B, Keinänen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Köhler M, Takagi T, Sakmann B, Seeburg PH (1990) Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS Science 249:1580–1585
Sprengel R, Aronoff R, Völkner M, Schmitt B, Mosbach R, Kuner T (2001) Glutamate receptor channel signatures Trends Phamacol Sci 22:7–10
Stern-Bach Y, Russo S, Neuman M, Rosenmund C (1998) A point mutation in the glutamate binding site blocks desensitization of AMPA receptors Neuron 21:907–918
Strutz-Seebohm N, Werner M, Madsen DM, Seebohm G, Zheng Y, Walker CS, Maricq AV, Hollmann M (2003) Functional analysis of Caenorhabditis elegans glutamate receptor subunits by domain transplantation J Biol Chem 278:44691–44701
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools Nucleic Acids Res 25:4876–4882
Wu Y-M, Kung S-S, Chow W-Y (1996) Determination of relative abundance of splicing variants of Oreochromis glutamate receptors by quantitative reverse-transcriptase PCR FEBS Lett 390:157–160
Acknowledgments
We are grateful to Dr. T.-C. Mok of National Sun Yat-sen University, Kaoshung, and Dr. K.-C. Shao of Academia Sinica, Taipei, for providing hagfish and amphioxus. This work was supported by the National Science Council of the Republic of China (NSC92-2311-B-007-010 and NSC 92-3112-B-007-002).
Author information
Authors and Affiliations
Corresponding author
Additional information
[Reviewing Editor: Dr. Manyuan Long]
Rights and permissions
About this article
Cite this article
Chen, YC., Lin, WH., Tzeng, DW. et al. The Mutually Exclusive Flip and Flop Exons of AMPA Receptor Genes Were Derived from an Intragenic Duplication in the Vertebrate Lineage. J Mol Evol 62, 121–131 (2006). https://doi.org/10.1007/s00239-004-0291-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-004-0291-5