Abstract
We investigated the effect of generation time (as controlled by chemostat flow rate) and temporal variability in nutrient (arginine) availability on selection at a regulatory locus in Escherichia coli. We first determined the fitness conferred by argRK12 (which regulates the arginine regulon) relative to argRB (a weak constitutive) in constant environments at several generation times across a range of concentrations of arginine. The relative fitness of argRK12 with respect to argRB declines with longer generation times in the absence of arginine yet becomes independent of generation time in the presence of excess arginine. Control experiments show this differential response in selection is entirely attributable to transcriptional regulation by argRK12. Temporal variability in the supply of arginine generates fluctuations in selection. A simple model, based on the assumption that relative fitness tracks changes in arginine availability instantaneously, captures many of the features of the oscillating allele frequencies and accurately predicts the direction and intensity of selection in environments where arginine concentrations fluctuate frequently or infrequently. However, the model fails to predict the direction and intensity of selection in environments that fluctuate at moderate frequencies. This suggests that phenotypic lag, wherein cellular physiology changes more slowly than the environment, may be influencing the outcome of competition in this experimental system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All environments vary, so it is hardly surprising that the effect on selection of environmental heterogeneity has long been of interest to evolutionary biologists. Indeed, a considerable body of theory and experiment exists regarding the impact of spatial and temporal variation on selection and polymorphism (Dempster 1955; Haldane Jayakar 1963; Felsenstein 1976; Chesson 1985; Hedrick 1986; Gillespie 1991; Chesson 2000; Meyers and Bull 2002). Both theory and interpretation of experimental results assume that a given genotype in a given environment has a given phenotype and, thus, a unique fitness. However, little attention has been paid to the impact on fitness of physiological transitions from one state to another, how they might affect the outcome of competition, and whether they can be predicted from studies of fitness in constant environments. In this paper we investigate the impact on fitness of changes in gene expression generated when cycling between two environmental extremes.
Building on the work of Suiter et al. (2003), we investigate the impact of oscillating concentrations of environmental arginine on fitness (relative growth rate) of clones of Escherichia coli that differ at argR. This is an ideal model system to investigate selection in a temporally variable environment. The role of argR in transcriptional control of the arginine regulon has long served as a paradigm of gene regulation (Lim et al. 1988). Computer controlled peristaltic pumps deliver alternate media into chemostat growth chambers to provide highly reproducible cyclic environments suitable for competition studies. Changes in allele frequency can be rapidly and accurately determined, and the abundance of arginine in the environment readily ascertained. Competitors can be accurately censused and the key environmental variable (the concentration of arginine) is easily monitored.
ArgR regulates the biosynthetic arg regulon of Escherichia coli (Ennis and Gorini 1961). E. coli strains K12 and B carry alleles that differ by a single amino acid replacement (Jacoby and Gorini 1969; Lim et al. 1988; Van Duyne et al. 1996; Sunnerhagen et al. 1997). Replacing proline by leucine at site 70 converts arginine, a potent corepressor with ArgRK12, into a weak coinducer with ArgRB. So whereas ArgRK12 maintains tight control of gene expression, allowing full induction in the absence of environmental arginine and strong repression in its presence, ArgRB maintains weak constitutive expression that increases slightly in the presence of exogenous arginine (Ennis and Gorini 1961; Tian et al. 1988). Note that even in the absence of arginine, ArgRK12 and ArgRB will each bind the operator to modulate arg regulon expression. Unlike these natural alleles, a completely inactive laboratory mutant, ArgR38 (Tian et al. 1988), produces strong constitutive expression under all conditions and serves as a control for the fitness effects of transcriptional regulation.
Suiter et al. (2003) showed that argRK12 is selectively favored in the presence of arginine whereas argRB is favored in its absence. The fitness (relative growth rate) of argRK12 relative to argB (w K12 B ) rises quickly in response to arginine until a broad fitness plateau is reached (Fig. 1A). By contrast, the fitness of argR38 (encoding an inactive repressor) relative to argRB(encoding an unresponsive repressor) remains low and constant regardless of arginine availability. Hence, the changes in fitness seen in competitions with argRK12 are attributable to regulation by the active ArgRK12 repressor in response to arginine availability, and to no other cause. Despite being able to attribute changes in fitness to regulatory action by the ArgRK12 repressor in response to arginine availability, the physiological basis of the selection is, as yet, not understood.
A The fitness of argRK12 relative to argRB as a function of arginine availability. Concave fitness functions of this kind are predicted to make fitness sensitive to the length of the environmental cycle. In this example, infrequent cycling between 1 and 0 mM arginine produces an expected fitness of \( \overline {w_B^K }=(w_B^K (1) + w_B^K (0))/2=0.966 \) and argRB wins the competition. With frequent cycling the expected fitness is \( w_B^K (0.5)=1.032 \) and argRK12 wins the competition. B Cycling between media with 1 and 0 mM arginine need not produce an instantaneous change in the arginine concentration in the chemostat growth chamber. In this simulation 20 time units (thin line; cycle time, 40 units) is needed before the steady-state value of 0.9 mM arginine is approached (0.1 mM is consumed by the population). As cycle frequency increases the steady-state ceases to be approached (thick line; cycle time, 20 units), although there may remain sufficient time for all the arginine to be consumed when the 0 mM medium is pumped into the chamber. At higher frequencies (dashes; cycle time, 2.5 units) there is insufficient time for arginine concentrations to deviate very far from the mean value, \( \bar R \). Selection in a cyclic environment can be predicted if it is assumed that fitness (A) instantaneously tracks cycling arginine concentrations (B).
Suiter et al. (2003) cycled between media with high and low arginine to produce a variable environment. The length of a cycle is defined as the time taken to sequentially deliver the two media (one high, the other low in arginine) into the chemostat growth chamber. A half-cycle is defined as the time over which one medium is pumped into the growth chamber. Each half-cycle can be divided into two periods: the first is a transitional phase where the arginine level rises or falls to its new steady-state value, and the second is the length of time spent at the new steady-state value.
Fig. 1B simulates the effect changing cycle times on the kinetics of arginine in the chemostat growth chamber. With long half-cycles (20 time units; thin line) the arginine level closely approaches its steady-state value (0.9 units). A switch to medium without arginine produces an abrupt drop and by 27 time units no arginine is left in the chemostat growth chamber. The cycle restarts at 40 time units. With short half-cycles (1.25 time units; dashed line) there is insufficient time to displace the arginine concentration in the growth chamber very far from its mean value and steady-state values are never approached. These simulations illustrate several important facets of arginine dynamics in the chemostat growth chamber: (1) long half-cycles of equal duration do not imply that the times spent at the two steady states are of equal duration (half-cycles of 20 time units); (2) in fact, one steady state need never be approached (half-cycles of 10 time units, thick line); (3) short half-cycles prevent arginine levels approaching steady-state (half-cycles of 1.25 time units); and (4) very short half-cycles (<0.1 time units; not shown) force the arginine levels to converge to a near-steady-state mean value.
Suiter et al. (2003) showed that the outcome of competition in environments that cycle between high and low arginine depends on the length of the cycles. Short cycles favor argRK12, whereas long cycles favor argRB. This dependence arises as a consequence of the concave relationship between fitness and arginine concentrations (Fig. 1A). For such a concave fitness function, the mean fitness across environments is less than the fitness in the mean environment; i.e., \( \overline {w_B^{K12} (R_i )} \le w_B^{K12} (\overline R ), \) where \( {w_B^{K12} (R_i )} \) is the fitness function and R i is the concentration of arginine in environment i. For example, with long cycles the proportion of time spent in transit between high (1 mM) and low (0 mM) arginine is small compared to the time spent at either steady-state (high or low arginine). Assuming half-cycles of equal length, the fitness of argRK12 relative to argRB converges on a simple arithmetic mean, \( \overline {w_B^{K12} (R_i )}=\left( {w_B^{K12} (1) + w_B^{K12} (0)} \right)/2 < 1\). With very short cycles there is insufficient time for arginine to be displaced far from its mean value and fitness converges on \(w_B^{K12} (\overline R ) > 1 \). Hence, the outcome of competition depends on the length of the cycles.
There are five outstanding questions regarding this experimental system: (1) the kinetics of arginine in the chemostat growth chamber, (2) the relationship between relative fitness and the chemostat dilution rate (cell generation time), (3) exploration of the physiological basis of selection in variable environments, and (4) the biochemical basis of selection.
(1) Our first goal is to investigate the kinetics of arginine following a switch in medium delivered to the chemostat growth chamber. The ability to monitor variable arginine concentrations is key to understanding competitive outcomes in dynamic environments.
(2) Our second goal is to determine the impact on fitness of changes in generation time. The generation time of a chemostat population is under the direct control of the experimenter (Kubitschek 1970). The growth rate, μ i , of a pure population i inhabiting a chemostat at steady-state equals the dilution rate, D, the fractional rate of replacement of medium in the growth chamber. E. coli has a negligible death rate in chemostat culture. Consequently, the cell generation time (i.e., the average time between cell divisions) is simply D−1, and the population doubling time (i.e., the time it takes for the population to double in density to compensate for wash out from the growth chamber) is simply ln 2/D.
Under standard operating conditions, where μ i = D < < μi.max (the maximum growth rate of the population), μ i = D = α i G and growth rate is proportional to G, the concentration of the limiting resource (Lunzer et al. 2002). For a mixed population at quasi-steady-state in a chemostat, fitness is defined as the ratio of growth rates, wi j = μ i /μ j = α i /α j , which is independent of both G and D. Hence, changes in generation time are not expected to affect fitness during competition for an essential limiting nutrient (Lunzer et al. 2002). Arginine is not essential for growth, yet it affects fitness. Therefore, it becomes of interest to determine if changes in generation time affect fitness when fitness is a function of a resource that is neither essential nor strictly growth limiting.
(3) There are two mechanisms that allow fitness to converge on \( w_B^{K12} (\overline R ) \) that need to be distinguished. As suggested above, fitness will converge on \( w_B^{K12} (\overline R ) \) when cycle times are so short that arginine can not be displaced far from its mean, \( \overline R \). On the other hand, there may be insufficient time for cells to respond to changes in the environment which they perceive to be at a constant average \( \overline R \). Even when an environment varies dramatically (e.g., at a cycle time at 40 time units in Fig. 1B), fitness may still converge on \( w_B^{K12} (\overline R ) \). One of our goals is to determine if either or both mechanisms operate (they are not mutually exclusive).
The intensity of selection in a cyclic environment can be predicted. For example, Fig. 1A provides the relationship between fitness and arginine concentration and Fig. 1B shows how arginine concentrations change with time. Predictions are made by assuming that fitness (Fig. 1A) changes instantaneously with changes in arginine concentration (Fig. 1B). If we accurately predict fitnesses across a broad range of cycle frequencies, then we will have shown that an understanding of fitness in constant environments can be used to predict fitness in cyclic environments. If the prediction fails, then either fitness is affected by states in cyclic environments that do not occur in constant environments or fitness is affected by transient physiological states (i.e., phenotypic lag) that do not arise in constant environments.
(4) Suiter et al. (2003) showed that changes in fitness are ultimately attributable to the interaction between arginine and the ArgRK12 repressor. Precisely how selection is mediated through changes in expression of the arg regulon, or a specific gene thereof, is not known. Though we do not attempt to address explicitly the biochemical basis of selection, the data gathered shed some light on this important matter.
Materials and Methods
Strains
Construction of the strains used in these experiments was described previously (Suiter et al. 2003). Briefly, the genetic background used is derived from DD320, which, except for a small deletion spanning the lactose operon, is E. coli K12 wildtype (and which has been successfully used in many chemostat competition experiments). Alleles argRK12 (from K12 strain DD320), argRB (from wildtype B strain CGSC#5365), and argR38 (from strain CGSC#4519) were introduced into the DD320 genetic background by cotransduction using bacteriophage P1(cml clr 100), there being no convenient direct selection at argR. Allelic identities were confirmed by sequencing PCR argR amplicons. Spontaneous mutants resistant to the bacteriophage T5 (fhuA−) were isolated. All strains were stored at −80°C in 16% glycerol.
Media
Minimal medium is Davis salts (MD: 7 g K2HPO4, 2 g KH2PO4, 1 g [NH4]2SO4, 0.5 g trisodium citrate in 1 L of distilled deionized water, with 1 ml of 1 M MgSO4· 7H2O and 0.5 ml of 1% thiamine added after autoclaving) supplemented with 2 g/L glucose and 15 g/L Bacto agar for plates. Rich medium is Luria broth (LB: 5 g yeast extract, 10 g tryptone, 10 g NaCl in 1 L of distilled deionized water, with 1 g glucose and 2.5 mM CaCl2 added after autoclaving).
Competition Experiments
Chemostat competition experiments are conducted using standard methods (Lunzer et al. 2002). The medium is Davis salts, pH 7.3, supplemented with 5 μM FeSO4, 0.1 g/L glucose, and 10−3 g/L thiamine, with L-arginine added as required. Experiments are conducted in a 37°C room. Competitions in variable environments are conducted in modified chemostats having two medium inlets, with peristaltic pumps delivering media to the chemostats being turned on and off at specific intervals by a timer.
Competition experiments are conducted between pairs of strains: one carries one argR allele and is sensitive to the bacteriophage T5, while the second carries the other argR allele and is resistant to T5. The progress of competition is monitored by following the frequency of T5 resistance over time, there being no means for recombination to disrupt linkage between the alleles and the marker. Between 5 and 10 1−ml samples are stored overnight at 4°C and, the next day, stained and enumerated by flow cytometry.
Flow Cytometry
Two microliters of a 2% (w/v) solution of chloramphenicol dissolved in ethanol and 20 μl of a 5 × 1011/ml stock of bacteriophage T5 in LB medium are added to 200 μl of stored cells. Following incubation at 30°C for 1 h, 20 μl of the mixture is added to 1 ml of phosphate buffer (7 g/L K2HPO4, 2 g/L KH2PO4, 500 μM Na2EDTA, pH 8.0, passed through a 0.22-μm nitrocellulose filter to remove particulate matter) containing 10 μl of the 2% (w/v) chloramphenicol solution and 100 μM cyanine dye, YoPro-1-iodide (Molecular Probes). Samples are incubated in the dark for 30 min and then enumerated by flow cytometry.
Sensitive cells, their membranes depolarized by attachment of excess T5, accumulate the cyanine dye which intercalates into double stranded nucleic acid where it fluoresces green upon excitation by a 25-mW air-cooled 488-nm argon laser. Data acquisition in a FACSCaliber flowcytometer (Becton–Dickerson Corp.) is triggered by sideways-scattered light, with data collected for sideways (SS)- and forwards (FS)-scattered light, and fluorescence (FL) between 505 and 545 nm. Log10SS vs. Log10FS plots are gated to remove points, such as the bacteriophage T5, that are too small to be E. coli cells. Log10SS vs. Log10FL plots are bimodal, with the fluorescent T5 sensitive population well separated from the non-fluorescent T5 resistant population. Each sample is counted for approximately 40 sec (approximately 50,000 counted cells) at 30, 45, and 60 min after staining begins. Sample counts typically show little difference and are considered replicates.
Estimating Fitness
In constant environments, the selection coefficient, ŝ, is estimated as the slope of a plot the log e ratio of strains against time (measured in Dt population generations)
where N K (t) and N B (t) are the counts of argRK12 and argRB at time t hr and the y-axis intercept y 0 = log e [N K (0)/N B (0)] is the estimated loge ratio of strains at t = 0. D, the chemostat dilution rate, converts time from hours into cell generations. The mean selection coefficient among replicate experiments is estimated by unweighted pooled linear least squares regression, as described by Snedecor and Cochran (1993). The fitness of argRK12 relative to argRB is simply
The overall intensity of selection in a cyclically varying environment is determined by least-squares nonlinear regression to the empirical formula
where \( \bar s \) is the mean selection coefficient, α is the amplitude of the oscillation, π is degrees in radians, τ is the period spent in an environment, and β is the phase of the wave with respect to time. Unweighted nonlinear least squares regression is implemented using JMP (SAS Institute Inc.). Estimates of y0, \( \bar s \), α, τ, and β were obtained for each data set. The simple averages of \( \bar s \), α, and τ were statistically indistinguishable from those obtained by fitting equation (3) to the pooled data, superpositioned using estimates of y0 and β to bring each set into common register.
Arginine Assays
Isoindole products produced by derivitizing sample amines with o-pthalaldehyde (OPA) are separated by isocratic reversed-phase high pressure liquid chromatography, detected using an inline fluorimeter, and quantified with an autointegrator (Jones et al. 1981). Chemostat samples are immediately passaged through 0.45-μm filters to remove cells and stored frozen at −80°C until needed. Two hundred microliters of thawed sample is diluted with 800 μl of 1.0 mM HCl containing 75 μM aspartate as a standard. Fifty microliters of this mixture is added to 50 μl of OPA (from a 1 ml OPA, 5 μl 2-mercaptoethanol stock) and, 60 sec later, loaded onto a 100 × 4.6-mm column packed with 5 μm Absorbosphere OPA-HS (Alltech) using an autosampler. The mobile phases consist of a 1:1 ratio of buffer A (0.96 L 50 mM sodium acetate, pH 5.7, with 40 ml tetrahydrofuran passaged through a 0.45-μm nylon filter) to buffer B (absolute methanol passaged through a 0.45-μm nylon filter) at 3100 psi to produce a flow rate of 4.5 ml/min. Fluorescence detector excitation is at 325 nm, with emission monitored at 465 nm. Autointegrator peaks at 0.5, 1.25, and 3.3 min correspond to aspartate, arginine, and ammonium (in the chemostat medium). Arginine concentrations are determined from standard curves prepared using fresh chemostat medium with added arginine.
A typical chromatogram (Fig. 2) reveals three well-separated peaks corresponding to the external standard (aspartate at 0.5 min), arginine at 1.3 min, and the internal standard (ammonium at 3.35 min). Standard curves, prepared on the assumption that peak area is proportional to amount, allow arginine concentrations in media to be accurately determined.
Reverse-phase high pressure liquid chromatography with inline fluorescence detection of isoindole derivatives provides a rapid and sensitive assay of environmental arginine. Aspartate is added as an external standard, while ammonium, present in excess in the chemostat medium, provides an internal standard.
Results
Arginine Kinetics
Ambient Arginine Concentrations in Chemostats at Steady-State
Only when fresh chemostat medium is supplemented with arginine (R0) can it be detected in the growth chamber (\( \hat R \)). Hence, neither strain excretes arginine. Nor is arginine detectable in the growth chamber when fresh medium is supplemented at concentrations below 20 μM. This is because a resident population of E. coli growing on 0.01% glucose reduces the ambient arginine concentration by 19 ± 2 μM, irrespective of allele and irrespective of arginine concentrations above 20 μM in the fresh medium (Fig. 3A). With transporters saturated (K m = 20 nM [Celis 1977]) and the quantity of arginine consumed proportional to the biomass in the growth chamber (Fig. 3B), the ambient steady-state arginine concentration in the chemostat growth chamber, \( \hat R \), is best described by
Arginine assays in chemostat media. A HPLC provides an accurate estimate of the quantity of arginine in fresh medium (R0). Unconsumed arginine in spent medium (\( \hat R \)) parallels that in the fresh medium, indicating that arginine consumption is independent of concentration above 0.019 mM. B The quantity of unconsumed arginine in spent media declines in proportion to the glucose added to fresh medium, which is proportional to population density since glucose is the sole limiting nutrient. C Changing the generation time by changing the chemostat dilution rate has no effect on the level of unconsumed arginine (fresh medium is 0.01% glucose and 0.2 mM arginine). Results with strains carrying argRK12 and argRB are indistinguishable and so their means (of at least three estimates each) are shown.
where R0 is the arginine concentration in the fresh medium and \( \hat N_T /Y_R=19\,\mu M \) is the quantity of arginine consumed by a steady-state population, density \( \hat N_T \) with yield coefficient Y R .
Transient Arginine Kinetics
We need to construct a model describing the kinetics of arginine in the chemostat growth chamber after a switch in medium. We begin by noting that the quantity of arginine consumed at steady-state is proportional to population density (\(\hat N_T /Y_R \)) and independent of the steady-state growth rate of the culture (Fig. 3C). These observations imply that the rate of consumption is directly proportional to the growth rate (a seeming paradox perhaps),
where R0 is the arginine concentration in the fresh medium, R is the arginine concentration in the chemostat growth chamber, D is the chemostat dilution rate, and μ = D is the growth rate of a steady-state population, the cell density of which is not affected by environmental arginine. The seeming paradox arises because at steady-state dR/dt = 0 and μ = D so that equation (5) can be solved to yield equation (4) without contradiction. Integrating equation (5) produces
where R(t) is the ambient arginine concentration at time t and R(0) = 0. The decay from steady-state upon switching to fresh medium lacking arginine (R′0 = 0) is described by
at which point the arginine concentration has been reduced to zero, and
Equations (6) and (7), fitted using nonlinear least squares regression as implemented by JMP (SAS Institute Inc.), track the data well (Fig. 4). Thus, the steady-state arginine concentration in the growth chamber depends on R0 and \(\hat N_T \), while the fractional rate of change in R(t) is determined by D.
The kinetics of environmental arginine. A Rise in arginine concentrations as media with 0.2 mM arginine are pumped into chemosats with resident populations that had been growing on 0 mM arginine. B The decline in arginine concentrations as media with 0 mM arginine are pumped into chemostats with resident populations that had been growing on fresh media containing 0.2 mM arginine. Each point is a single estimate: argRK12 (solid symbols) and argRB (open symbols) at dilution rates 0.33 h−1 (circles) and 0.44 h−1. Neither argR allele nor dilution rates affect arginine kinetics and the pooled data in A and B were fitted to equations (6) and (7), respectively.
Alternative Kinetic Models
Arginine consumption is directly proportional to both growth rate and population density in our kinetic model. Although alternative models fit the exponential rise and fall of arginine equally well, they do not predict the final steady-state concentrations of arginine accurately. A model in which the per captita consumption rate is constant per unit time (i.e., independent of growth rate) and proportional to cell density yields the same exponent as above, but not the same steady-state concentration of arginine which now varies with dilution rate (the relationship \( \hat R=R_0 - a\hat N/D \) is rejected in Fig. 3C). A model in which the per capita consumption rate is proportional to growth rate, to cell density and to arginine concentration (unrealistic because with a K m = 20 nM arginine transporters are saturated over the entire range of concentrations measured) produces an exponent that is dependent on population density, −(1+a\( \hat N_T \))Dt, and a steady-state concentration of arginine that is directly proportional to the concentration in the fresh medium, \( \hat R=R_0 /\left( {1 + \alpha \hat N_T } \right) \)(both rejected in Fig. 3A). So while different models fit the transient kinetic data about as well as equations (6) and (7), they can be firmly rejected on the basis that they fail to predict the observed steady-state concentrations of arginine in the chemostat growth chamber.
Fitness and Generation Time
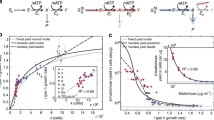
Figure 5 presents data from four chemostat competition experiments conducted with a dilution rate D = 0.44 h−1 at various concentrations of arginine. Selected against in the absence of arginine, ArgRK12 is already favored at R0 = 0.05 mM, with very little increase in fitness above R0 = 0.1 mM. These results parallel those of Suiter et al. (2003), who conducted similar experiments at a dilution rate D = 0.33 h−1. But whereas the selection against ArgRK12 in the absence of arginine here is −3.67 ± 0.05%/gen, Suiter et al. (2003) found that the selection against ArgRK12 was more intense, −10.83 ± 0.12%/gen.
The difference in selection coefficients suggests that the intensity of selection depends on generation time. In a chemostat operating at steady-state the population growth rate equals the dilution rate, the inverse of which (i.e., D−1) is the mean cell generation time (Kubitschek 1970). We therefore conducted competition experiments at various dilution rates to explore the dependency of fitness on generation time. In the absence of exogenous arginine, fitness declines linearly with generation time but rises hyperbolically with ambient arginine concentration (i.e.,\( \hat R \)). Fitting equations of the form\( \ \hat w_B^{K12}={\rm{A + }}\Delta \hat R/\left( {K + \hat R} \right) \), where A is fitness in the absence of arginine and Δ is the maximum gain in fitness when arginine is present in excess, reveals that A depends strongly on dilution rate, but that A + Δ and K are independent of it (Table 1). These observations are consistent with the following model of fitness at steady-state in the chemostat
in which it is assumed that argRB is nearly fixed in the population so that \(\hat \mu _B=D\). The derivation of equation (9) is given in the Appendix. w K12B.max is the maximum fitness of argRK12in the presence of excess arginine. Overexpression of the arg regulon by argRK12 is assumed to impose a cost to fitness: β K12 is a constant that introduces a cost that is proportional to growth rate, and χ K12 is a constant that introduces a cost that is proportional to absolute time. κ K12 is the concentration of arginine necessary to halve the cost of regulon expression in strains carrying argRK12. Analysis shows that for argRB, β B cannot be estimated independently of μB.max and that χ B = 0—i.e., argRB does not impose a cost proportional to absolute time. An r2 = 0.9972 (Table 2, Fig. 6) suggests that nonlinear regression produces an adequatef fit to the data.
The direction of selection is dependent on the availability of arginine. At 0.0 mM arginine the selection against argRK12 is 3.67 ± 0.05%/generation. Adding 0.05 mM arginine to the fresh medium causes a change in the direction of selection, which now favors argRK12 at 1.65 ± 0.03% generation, rising to 3.49 ± 0.03% generation at 0.1 mM and approaching a maximum of 4.01 ± 0.03% generation at 1.0 mM. Triplicate counts of 25,000 cells each were obtained by flow cytometry.
Regulatory Control
Experimental controls reveal that arginine affects fitness by binding to the ArgRK12 repressor, and not through any other mechanism. ArgR38 encodes an inactive ArgR repressor. Therefore, competing argR38 and argRB provides a control for the fitness effects of transcriptional regulation. As with argRK12, increasing generation time from 3 hrs to 6.7 hrs causes a noticeable decline in the fitness of argR38 relative to argR8. Unlike argRK12, adding 1 mM arginine to the chemostat feed has no impact on this decline (Fig. 7). These observations are consistent with the following model of fitness:
in which it is again assumed that argRB is nearly fixed in the population so that \( \hat \mu _B=D \). The derivation of equation (10) assumes that arginine has no effect on expression in either strain and hence no effect on fitness. Indeed, the growth rate of argRB strains in batch culture is not noticably affected by the presence of arginine (MD salts, 0.1% glucose, 0, 0.2 or 1 mM arginine). As with argRB, β38 cannot be estimated independently of μ38.max. Unlike argRB, argR38 imposes a cost in fitness proportional to absolute time (χ38 = 0.054 h−1 > 0). The fact that the addition of excess arginine to the medium has no effect on the fitness of argR38 demonstrates that the interaction between arginine and the ArgRK12 repressor ameliorates the cost to fitness of arg regulon expression.
Competition in Variable Environments
The impact of variable environments on selection was investigated by cycling between feed media supplemented with 0 and 0.2 mM arginine (Fig. 8). Oscillations in allele frequency increase with cycle length simply because there is more time for selection to drag allele frequencies up and down during each half-cycle. The overall direction of selection diminishes as the cycle length increases—Jensen’s inequality ensures that the function of the expectation (i.e., \( w_B^{K12} (\bar R) \)) exceeds the expectation of the function (i.e., \( \overline {w_B^{K12} }= \left( {w_B^{K12} (\hat R) + w_B^{K12} \left( 0 \right)} \right)/2 \)) when fitness is a concave function of ambient arginine concentrations (Fig. 1A).
The intensity of selection for argRK12 diminishes as the length of half-cycles (at 0 and 0.2 mM feed arginine) is increased from 1.3 generations (top) to 6.6 generations (bottom). Data at 1.3 and 2.6 generations are each pooled from four independently replicated experiments, those at 4 generations are pooled from two replicate experiments, and those at 5.3 and 6.6 generations are each pooled from six replicated experiments. Each circle represents 25,000 cells counted, each sample being counted three times. In total a quarter-billion cells counted by flow cytometry are represented here. The fits are for the cosine wave given by equation (3).
These results corroborate those of Suiter et al. (2003) in all essential respects. However, Suiter et al. showed not only that the overall intensity of selection diminishes as cycle lengths increase, but also that its direction depends on cycle length. This qualitative difference in the outcome of competition is largely attributable to selection against argRK12 in the absence of arginine being stronger at longer generation times. Hence, average fitness at D = 0.44 h−1, \(\overline {w_B^{K12} }=\left( {w_B^{K12} (0.181) + w_B^{K12} \left( 0 \right)} \right)/2=(1.036 \ +0.965)/2=1.001 \), is higher than at D = 0.33 h−1, \(\overline {w_B^{K12}}=\left( {w_B^{K12} (0.181) + w_B^{K12} \left( 0 \right)} \right)/2=(1.031 + 0894)/2=0.963. \)
Selection in Cyclical Environments
Selection in cyclical environments can be predicted from the kinetics of arginine in the chemostat growth chamber (Figs. 3 and 4) and the relationship between fitness and ambient arginine concentrations (Fig. 6) in constant environments. The key assumption is that fitness changes instantaneously with changes in ambient arginine concentrations. We use equations (6) and (7) to describe how arginine concentrations change with time, and equation (9) to describe the dependency of fitness on those concentrations (see Appendix for details).
The log e [NK12(t)/NB(t)] ratio of strains was simulated (equations [A23] and [A24], Appendix) for a cyclical environment with half-cycles of equal length, t1 = t2, and one feed arginine concentration set at R0 = 0.2 mM and the other at R0 = 0.0 mM. A smooth wave is produced as selection first favors one and then the other allele (Fig. 9). As expected, longer cycles reduce the overall intensity of selection and increase the amplitude of the oscillations.
Simulated selection using equations (A23) and (A24) at dilution rate D = 0.44 h−1. Dashed curves represent simulations with half-cycles of 2.25 generations (short dashes) and of 5.3 generations (long dashes). Thin lines are cosine waves (equation [3]) fitted to the simulated data. Dashed straight lines represent changes in the delivery of arginine into the chemostat growth chamber (up, 0.2 mM arginine; down, 0 mM arginine).
The simulation (Fig. 9) predicts that the delayed responses of selection following switches in media are asymmetric. The delay is short when switching to a medium with arginine (0.13 gen with half-cycles 5.3 generations long at a generation time of D−1 = 2.27 hr) but very much longer when switching to a medium without arginine (1.61 gen). The asymmetry of the delays is a consequence of the concave fitness surface (Fig. 6)—only a short time is needed to deliver the small quantity of arginine needed to produce high fitness, whereas a longer period is needed to consume most of the unused arginine.
Direct comparisons of the simulated asymmetric delays with fitted cosine waves (equation [3]) are invalid because the latter necessarily average over any asymmetries in the data (Fig. 9). One obvious approach, the piecewise fitting of three nonlinear equations (equation [A23] when R0 = 0.2 mM and equation [A23] or [A24] when R0 = 0.0 mM; see Appendix) directly to the data was found to be impractical. Nevertheless, many features seen in the simulations (Fig. 9) can be visualized in the data (Fig. 10). First, changes in the direction of selection (as predicted from the arginine kinetics) coincide with the peaks and troughs of the data, which appear a little before and a little after the peaks and troughs of the fitted cosine wave. Second, the delayed responses of selection following switches in media are asymmetric, with the change in the direction of selection being faster when switching to media with arginine than when switching to media without arginine. Third, the rise in frequency of argRK12 in the presence of arginine is both less steep and longer than its fall when the arginine runs out.
The above delays are concerned with translational shifts in time. Another type of delay is associated with the amplitude of the oscillations in the direction of selection. Simulations suggest that the amplitude of the selection data is accurately estimated by the otherwise ill-fitting cosine waves (Fig. 9). A plot of amplitude against cycle length reveals this delay as the intersection of the regression lines with the abscissa (Fig. 11). The data produce estimated delays of 1.6 and 2 generations. The simulated delay is 1.5 generations. Hence, oscillations in the direction of selection appear only when half-cycles are longer than 1.5–2 generations.
An example of the response of selection to a change in medium. The square wave (top; thin dashes) represents periods (not the arginine concentration) when 0.2 mM arginine is pumped into the chemostat growth chamber; the thick dashed curve is the predicted rise and fall in arginine concentration in the chemostat growth chamber at dilution rate D = 0.44 h−1. The thin horizontal line corresponds to an arginine concentration of 0.017 mM in the chemostat growth chamber. Where this line intersects the rise and fall in arginine concentration correspond to times where switches in the direction of selection occur (then vertical lines to bottom). The cosine curve (equation [3]), fitted to the data, averages across the asymmetry in switching times, producing an advance in the peak and a delay in the trough. Data pooled from six replicate chemostat experiments. Each circle represents 25,000 cells counted, each sample being counted three times.
Estimating the delay in the response of selection to a change in the environment. The delay is estimated as the intercept on the abscissa produced by regressing the amplitudes of the cosines wave (equation [3]) against the lengths of the half-cycles. Dots, D = 0.33 h−1; squares, D = 0.44 h−1. Solid lines are fitted to the data; dashed lines are from simulations.
Predicting Fitness in Cyclical Environments
Fitness in a cyclical environment is predicted by equations (A32) and (A33) in the Appendix. The model assumes that when argRB is at high frequencies the concentration of glucose remaining in the growth chamber, \( \hat G \), remains constant even as arginine concentrations vary. This is because the growth rate of argRBis unaffected by arginine. With \( \hat G \) constant, the growth rate of argRK12 depends only on the concentration of arginine, R, in the growth chamber. Equations (6) and (7) accurately describe the kinetics of arginine in the growth chamber. If the growth rate of argRK12 responds instantaneously to changes in R, then fitness will respond instantaneously too. Under these assumptions equations (A32) and (A33) (Appendix) predict fitness in cyclical environments.
As cycles become shorter with t → 0, fitness in (A32) converges on \({w_B^{K12} \left( {\overline R } \right)} = \left( {w_{B.{\rm{max}} }^{K12}} - \left( {w_{B.{\rm{max}} }^{K12} \beta _{K12}} + {\chi _{K12} / D} \right) / \left( {\kappa _{K12} + \overline R } \right) \right) \), which is the function of the expected ambient arginine concentration. As cycles become longer with t → ∝, fitness in (A33) converges on\( \overline {w_B^{K12} }=\left( {w_B^{K12} (0.2) + w_B^{K12} (0)} \right)/2 \), which is the expected mean fitness (the first two items of equation [A33])—selection in the transitions is now insignificant.
The predicted fit (obtained by inserting into equations [A32] and [A33] estimates for w K12B.max , βK12, χK12, κK12 obtained by fitting equation [9] and for \( \hat N_T /Y_R \) obtained by fitting equation [4], with D = 0.33 h−1 or D = 0.44 h−1), to fitnesses obtained in cyclical environments is poor (Fig. 12). Attempts to fit the entire data set (variable and constant environments) to equations (A32) and (A33) directly produce no improvement. In particular, estimates of κK12 and \( \hat N_T /Y_R \) are erratic, the latter especially so (\( \hat N_T /Y_R \) varies from 2.0 ± 0.6 μM at D = 0.33 h−1 to 42.0 ± 5.0 μM at D = 0.44 h−1, compared to a constant 19 ± 2 μM obtained by direct chemical assay). We conclude that fitness at argR in a cyclical environment can not be predicted accurately from the kinetics of arginine (equations [6], and [7]) and a knowledge of the relationship between arginine availability and fitness in constant environments (equation [9]).
Fitness in a cyclical environment cycling between 0 and 1 mM and arginine cannot be predicted (solid lines; equations [A32] and [A33]) from a knowledge of the kinetics of environmental arginine (Fig. 4) and fitnesses in constant environments (Fig. 6). Fitting equations [A32] and [A33] directly to these data improves the overall fits (dashed lines), but only if parameters are free to vary at the two dilution rates.
Discussion
The effect of generation time on selection in temporally variable environments was investigated using, as a model system, allelic variation at the argR of E. coli and cycling between high and low arginine concentrations. We determined the fitness conferred by argRK12relative to argRB in otherwise coisogenic strains in cyclic environments with different generation times, as well as monitoring fluctuations in ambient arginine concentrations in the chemostat growth chambers. Control competition experiments in which neither allele affects gene expression (argR38 and argRB) were used to assess the impact on fitness of transcriptional regulation by argRK12. Control competition experiments in constant environments were used to assess the impact of environmental variability on fitness.
Fitness in Constant Environments
The steady-state fitness of argRK12 relative to argRB (\( \hat w_B^{K12} \)) is strongly dependent on generation time in the absence of environmental arginine (Fig. 7). Addition of excess arginine eliminates the generation time effect during competition between argRK12 and argRB. Addition of arginine has no impact on fitness when argR38, which confers strong constitutive expression, is placed in competition with argRB, which confers weak constitutive expression (Fig. 7). Therefore, it is the interaction between argRK12 and arginine that ameliorates the generation time effect.
The Biochemical Mechanism of Selection Remains Unknown
Although changes in selection elicited by arginine are specifically associated with argRK12 regulated expression of the arg regulon, the biochemoical basis of selection has not been diagnosed. We agree with earlier workers (Dykhuizen 1978; Koch 1983) that overexpression does not merely impose a simple energy burden produced by excess transcription and translation. Monitoring arg regulon expression through ornithine transcarbamylase activities in chemostat grown cells at a generation time of 3 h reveals that argRK12has 50% higher expression than argRB, while argR38, which encodes a completely inactive repressor, has 6600% higher expression (Suiter et al. 2003). Yet the selection against argR38 is only 2% greater than the 10.5% seen against argRK12. And while increasing the generation time to 6.7 hrs intensifies selection against both these alleles, the selection against argR38 is now less, by 9%, than that against argRK12 (Fig. 7). An energy burden imposed by excess transcription and translation of the arg regulon does not provide a plausible explanation for the selection seen.
Nor does overproduction of arginine account for the observed fitness effects. The first step in arginine biosynthesis, catalyzed by acetylglutamate transferase, is subject to strong product inhibition which should block the massive diversion of carbon need to reduce fitness by up to 45%. Overproduction on so vast a scale is anticipated to result in excess arginine and its polyamine derivatives being excreted into the medium. Yet arginine, unless added to fresh media, has never been detected in media from the chemostat growth chambers, even from strains carrying the inactive argR38 allele. Massive overproduction of arginine does not provide a plausible explanation for the observed selection.
Arginine consumption is not a major determinant of fitness. First, arginine cannot serve as the sole source of carbon and energy for E. coli, and excess arginine is simply not consumed (Fig. 3). Therefore, the maximum benefit attainable through consumption is limited to offsetting the cost of synthesizing arginine from glucose and ammonium. Second, the kinetics of arginine in the environment indicate that the rate of consumption is dependent on growth rate and not vice versa (Fig. 4). Third, a change in ambient arginine concentration from 31 to 181 μM (50 to 200 μM in the fresh medium), well within the a range where the quantity of arginine consumed is independent of its availability (Fig. 3), produces a change in fitness from 0.988 to 1.033 (Table 2). Therefore, fitness responds to changes in ambient arginine concentrations even while the quantity of arginine consumed remains constant. Consumption is not a major determinant of fitness.
We, like others (Dykhuizen 1978; Koch 1983) investigating the energy burden hypothesis, find that data eliminate, rather than indicate, likely mechanisms of selection. Perhaps selection is generated through some metabolite pool connected, either directly or indirectly, to the intracellular pool of arginine. Such a metabolite might be toxic, affect intracellular pH, or regulate connected pathways through allostery or transcriptional regulation. So while we can demonstrate unambiguously that the ultimate cause of selection is transcriptional regulation of the argR regulon by the argRK12 allele in response arginine availability, the biochemical mechanisms underlying the selection remain mysterious.
Selection in Cyclical Environments
Competition in cyclical environments produced by alternating between fresh media with and without arginine reveals that the intensity of selection depends on the length of the cycles (Fig. 8). Intriguingly, the weak constitutive expression conferred by argRB is not only favored in the absence of arginine, but can also be favored over the regulated expression conferred by argRK12 in variable environments when cycles are sufficiently long (Suiter et al. 2003). This result demonstrates that regulation is not guaranteed adaptive in a variable environment.
Predicting Selection in Cyclical Environments
The overall intensity and direction of selection cannot be predicted from knowledge of the kinetics of arginine in the chemostat growth chamber and the relationship between fitness and ambient arginine concentrations obtained in constant environments (Figs. 4 and 6). The exception is at the extremes of environmental variability, where changes in feed arginine are either so frequent that fitness converges on \( w_B^{K12} \left( {\overline R } \right)=w_B^{K12} (0.081) \) (which is a function of the expected ambient arginine concentration) or so infrequent that fitness converges on \( \overline {w_B^{K12} }=\left( {w_B^{K12} (0.081) + w_B^{K12} \left( 0 \right)} \right)/2 \) (which is the arithmetic mean fitness). Between these extremes predictions combining fitnesses in constant environments and arginine kinetics fail to track the fitnesses in cyclical environments (Fig. 12). Although attempts to fit the model to the entire data set improved the fits somewhat, estimates of two key parameters, κK12 and\( \hat N_T /Y_R \), proved erratic with the latter never close to that estimated by direct chemical assay.
Model Failure and the Likely Role of Phenotypic Lag
There are three possible causes for failure of the model to account for the direction and intensity of selection in environments that oscillate at intermediate frequencies: (1) inadequate description of arginine kinetics and the fitness surface, (2) fitness is affected by states in cyclic environments that do not arise in constant environments, and (3) phenotypic lag, transient physiological states that do not arise in constant environments.
The first hypothesis can be eliminated because, as inspection of Figs. 3, 4, and 6 reveals, equations (4), (6), and (7), describing arginine kinetics, and equation (9), describing fitness, track the data accurately, regardless of mechanism. If fitness follows changes in arginine concentrations instantaneously then we should be predicting the outcome of competition in cyclical environments far more accurately than we do.
The second hypothesis, that states exist in cyclic environments that do not occur in constant environments, may arise by either of two means. One possibility is that some other substance in the environment, production of which must be regulated (directly or indirectly) by ArgRK12, is really the cause of selection. Extensive chemical analyses of spent medium will have to be conducted before this hypothesis can be definitively accepted or rejected. However, circumstantial evidence suggests that this hypothesis is unlikely. The asymmetric delays in the response of selection to switches in media, stronger selection over a shorter period for argRBthan weaker selection over a longer period for argRK12, the correspondence of peaks and troughs of the oscillations in selection to the concentration in ambient arginine predicted to produce selective neutrality (Fig. 10) and the simulated delays in the amplitude of the oscillations in the direction of selection (Fig. 11) match closely the estimates from the data. These observations strongly suggest that changes in the availability of arginine are, ultimately, the cause of selection. From such a perspective invoking a second substance simply multiplies hypotheses unnecessarily.
Another means to generate states unique to cyclic environments is to allow the growth rate of argRB strains to be dependent on arginine concentrations—i.e., make equation (A15) (Appendix) dependent on R. Adding arginine would then affect the growth rate of argRB, which would then affect the density of the population (argRB is assumed to be the dominant population) and this in turn would affect the concentration of glucose remaining in the growth chamber, G. Hence, glucose concentrations would oscillate in response to changes in arginine, producing combinations of R and G not present in steady-state environments, affecting the growth rates of argRB and argRK12strains in unanticipated ways, and thereby producing unexpected fitness effects. The difficulty with this hypothesis is that analysis of steady-state fitness (\( \hat w_B^{K12} \)) suggests that the growth rate of argRB depends only on the availability of glucose and is unaffected by arginine (i.e., \( \hat \mu _B=\left( {\mu _{B.{\rm{max}} } / \gamma_B } \right)\hat G\left( {1 - \beta _B } \right) \); see Appendix). This notion is buttressed by the observation that the steady-state fitness of argRB relative to the strong constitutive argR38 (\( \hat w_B^{38} \)) does not vary with arginine availability (Suiter et al. 2003). Finally, the growth rate of argRB in batch culture is not affected by arginine. For these reasons we think that the growth rate of argRB in chemostats is unaffected by arginine.
We suggest that model failure is attributable to the third cause, phenotypic lag. In an ever changing environment the current physiology of an organism matches the previous environment, not the current environment. For example, a strain carrying argRK12 and growing in the presence of arginine is at an immediate and severe disadvantage in competition with argRBif arginine is instantaneously removed because, with the argR regulon repressed, it cannot grow. Temporarily its fitness is zero, and for this period selection is far more intense than any seen in constant environments. Then again, the intensity of selection might be diminished if arginine can be stored for later use so that selection might continue on its original course for a time after all environmental arginine has disappeared. Delays in the response of an organism’s physiology may intensify or diminish the effects of selection to generate fitnesses never seen in steady-state environments.
As described in the introduction, there are two mechanisms that allow fitness to converge on \( w_B^{K12} \left( {\overline R } \right) \) that need to be distinguished: (1) short cycle times that prevent arginine concentrations being displaced far from the mean, \( \overline R \), and (2) insufficient time for cells to respond to changes in the environment which they perceive to be a constant average\( \ \overline R \). At a dilution rate of 0.33 h−1 and a half-cycle time of 2 generations the arginine concentration varies widely, between 0.03 and 0.17 mM. Yet fitness is indistinguishable from the steady-state value (a 0 generation half-cycle time). This strongly suggests that 2 generations is insufficient time for argRK12 cells to respond to changes in arginine availability in the environment. Such unresponsiveness to changes in the environment is a hallmark of phenotypic lag.
Detailed studies of transient physiology are needed to dissect the discrepancies between the predicted and observed responses of selection. Nevertheless, some general conclusions are possible because different cellular processes relax to their respective steady-states at such very different rates: an enzyme and substrate may take but milliseconds; a metabolic pathway, a few seconds to a few minutes; and transcription, translation, and RNA and protein decay may take anywhere from minutes to hours. The speeds at which enzymes and pathways relax to steady-state values suggest they should easily track changes in ambient arginine concentrations because these shift relatively slowly over many hours. Our attention is drawn to transcription, translation, and RNA and protein decay. In particular, many bacterial proteins, not subject to the rapid proteolysis characteristic of eukaryotic cells, are remarkably stable. When no longer needed bacteria simply outgrow them. Decay takes generations. We speculate that the discrepancies in fitness response to an ever changing environment are most plausibly attributable to the relatively slow rates of protein turnover.
Evolutionary Implications
We have demonstrated that fitness in a temporally variable environment cannot be predicted from fitnesses obtained in constant environments. We suggest that this is caused by phenotypic lag: growth by binary fission forces daughter cells to inherit not only the genes of the mother cell, but also her physiology. That physiology takes time to adjust to the prevailing environmental conditions. If conditions keep changing and the physiology lags, the resulting mismatch produces transient fitnesses that cannot be predicted from those seen in constant environments.
Delays in fitness responses to changing environments can affect the outcome of competition. For example, at a dilution rate D = 0.33 h−1 (Fig. 12) argRK12 is predicted to be less fit than argRB with half-cycles between 2 and 4 generations long; experimentation shows that argRK12 is fitter than argRB throughout this region (Fig. 12). Our results provide further evidence that the outcome of Darwinian selection can be influenced by physiological as well as by genetic inheritance.
Perhaps because the complex life cycles and developmental processes in higher eukaryotes are believed to wipe out physiological history with each generation, classical evolutionary theory has tended to ignore this phenomenon. Yet there are many well-documented examples where environmentally induced changes are stably inherited for many generations both in prokaryotes and in eukaryotes. Fine-grained environmental changes (i.e., those that experienced within the life-time of an organism) may also induce transient physiological states not experienced in constant environments (e.g., during acclimatization to light or altitude), producing transient fitness effects not experienced constant environments. We agree with Jablonka (1995; Lachmann and Jablonka 1996; Jablonka and Lamb 2002) and Landman (1991) that epigenetic inheritance has played important roles in evolution and with Watt (1977, 1991; Watt and Boggs 1987) that selection on transient physiological states is both common and important to adaptation.
References
Celis TFR (1977) Independent regulation of transport and biosynthesis of arginine in Escherichia coli K12. J Bactiol 130:1244–1252
Chessony PL (1985) Coexistence of competitors in spatially and temporally variable environments: a look at the combined effects of different sorts of variability. Theor Pop Biol 28:262–287
Chesson PL (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst 31:343–366
Dempster ER (1955) Maintenance of genetic heterogeneity. Cold Spring Harbor Symp Quant Biol 70:25–32
Dykhuizen D (1978) Selection for tryptophan auxotrophs of Escherichia coli in the glucose-limited chemostats as a test of the energy conservation hypothesis of evolution. Evolution 32:125–150
Dykhuizen DE, Dean AM (1994) Fitness changes across an environmental gradient. Evol Ecol 8:524–541
Ennis HL, Gorini L (1961) Control of arginine biosynthesis in strains of Escherichia coli not repressible by arginine. J Mol Biol 196:439–446
Felsenstein J (1976) The theoretical population genetics of variable selection and migration. Annu Rev Genet 10:253–280
Gillespie JH (1991) The causes of molecular evolution. Oxford University Press, Oxford
Haldane JBS, Jayakar SD (1963) Polymorphism due to selection of varying direction. J Genet 58:237–242
Hedrick PW (1986) Genetic polymorphism in heterogeneous environments: a decade later. Annu Rev Ecol Syst 17:535–566
Jablonka E, Lamb MJ (2002) The changing concept of epigenetics. Annu NY Acad Sci 981:82–96
Jablonka E, Oborny B, Molnar I, Kisdi E, Hofbauer J, Czaran T (1995) The adaptive advantage of phenotypic memory in changing environments. Phil Trans R Soc Lond B 350:133–141
Jacoby GA, Gorini L (1969) A unitary account of the repression mechanism of arginine biosynthesis in Escherichia coli. I. The genetic evidence. J Mol Biol 39:73–78
Jones BN, Pääbo S, Stein S (1981) Amino acid analysis and enzymatic sequence determination of peptides by an improved o-phtaldialdehyde precolumn labeling procedure. J Liq Chromatogr 4:565–586
Koch AL (1983) The protein burden of lac operon products. J Mol Evol 19:455–462
Kubitschek HE (1970) Introduction to research with continuous cultures. Prentice–Hall, Englewood Cliff, NJ
Lachmann M, Jablonka E (1996) The inheritance of phenotypes: an adaptation to fluctuating environments. J Theor Biol 181:1–9
Landman OE (1991) The inhertance of aquired characteristics. Annu Rev Genet 25:1–20
Lim D, Oppenheim J, Eckhardt T, Mass WK (1988) The unitary hypothesis for the repression mechanism of arginine biosynthesis in E. coli β and E. coli k12—Revisited after 18 years. In: Bissell M, Deho G, Sironi G, Torriani A (eds). Gene expression and regulation: The legacy of Luigi Gorini. Excerpta Medica, New York, pp 55–63
Lunzer M, Natarajan A, Dykhuizen DE, Dean AM (2002) Enzyme kinetics, substitutable resources and competition: from biochemistry to frequency-dependent selection in lac. Genetics 162:485–499
Meyers L. A., J. Bull 2002. Fighting change with change: Adaptive variation in an uncertain world. Trends Ecol Evol 17:551–557
Snedecor GW, Cochran WG (1993) Statistical methods. Iowa State University Press, Ames
Suiter AM, Bänziger O, Dean AM (2003) Fitness consequences of a regulatory polymorphism in a seasonal environment. Procy Natl Acad Sci USA 100:12782–12786
Sunnerhagen M, Nilge M, Otting G, Carey J (1997) Solution structure of the DNA-binding domain and model for the complex of multifunctional hexameric arginine represser with DNA. Nat Struct Biol 4:819–826
Tian G, Lim D, Oppenheim JD, Maas WK (1994) Explanation for different types of regulation of arginine biosynthesis in Escherichia coli B and Escherichia coli K12 caused by a difference between their arginine repressors. J Mol Biol 235:221–230
Van Duyne GD, Ghosh G, Mass WK, Sigler PB (1996) Structure of the oligomerization and L-arginine binding domain of the arginine represser of Escherichia coli. J Mol Biol 256:377–391
Watt WB (1977) Adaptation at specific loci. I. Natural selection on phosphoglucoisomerase of Colias butterflies: biochemical and population aspects. Genetics 87:177–194
Watt WB (1991) Biochemistry, physiological ecology, and population genetics—The mechanistic tools of evolutionary biology. Funct Ecol 5:145–154
Watt WB, Boggs CL (1987) Allelic isozymes as probes of the evolution of metabolic Organization. Isozymes Curr Top Biol Med Res 15:27–47
Acknowledgments
We thank Ben Kerr, Lauren Merlo, and two constructively critical anonymous reviewers, Stephen Abedon and another who chooses to remain anonymous for their thoughtful comments and suggestions. This work was supported by NIH grants to A.M.D.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The Basic Chemostat Model
Let the growth of the two competing populations in a chemostat have a simple birth death process:
where D is the chemostat dilution rate. N K12 and N B are the densities of the competing strains, and μ K12 and μB are their growth rates. The latter are given by
where G and R are the concentrations of glucose and arginine. The model is a modification of the classic Monod model where μi.max is the maximum growth rate of strain i (assuming expression of the arginine regulon imposes no cost to fitness) and γi is the concentration of glucose sufficient to produce μi.max/2. Two costs associated with expressing the arg regulon have been introduced: β i is proportional to growth rate and χ i proportional to absolute time. These costs are fixed in strains carrying argRB. In strains carrying argRK12, κ K12 is the concentration of arginine necessary to halve the cost of regulon expression.
Let the rates of glucose and arginine consumption be proportional to growth rates:
where G0 and R0 are the concentrations glucose and arginine in the feed medium and Y G and Y R are the yield coefficients (number of cells produced per amount of resource consumed).
Selection at Quasi-Steady-State
After inoculation, the conditions in the chemostat growth chamber rapidly approach a quasi-steady-state where competition is intense and the environment is characterized by very slowly shifting states. The growth rate of the total population. N T is zero (\( dN_T/dt=dN_{K12}/dt + dN_B/dt=0 \) ) and (A1) and (A2) sum to
where hats (^) denote quasi-steady-state values. The ambient concentrations of glucose and arginine are found by setting dG/dt = 0 and dR/dt = 0 and solving
With the environment essentially unchanging, (A1) and (A2) can be integrated to yield
Taking the log e ratio produces
Thus the slope of a plot of the loge ratio of strains against time (measured in Dt population generations) provides a direct estimate of the selection coefficient per generation, \( \hat s=(\hat \mu _{K12} - \hat \mu _B )/D \). Relative fitness is simply
In the quasi-steady-state virtually all glucose is consumed and G << γ B (Dykhuizen and Dean 1994). Let argRB be by far the most frequent competitor. Then equations (A3) and (A4) can be rewritten
Fitness is given by
where \( w_{B.{\rm{max}} }^{K12}=\left( {\mu _{K12.{\rm{max}} } / \gamma_{K12} } \right)/\left( {\left( {\mu _{B.{\rm{max}} } / \gamma_B } \right)\left( {1 - \beta _B } \right)} \right) \) is a constant that could be interpreted as the (fictional) fitness when arginine is present in excess, the dilution rate is infinite and the glucose concentration is zero. Fitting the model to the fitness data reveals that χ B is tiny and not significantly different from zero. Hence, expression of the arg regulon in strains carrying argRB does not impose a cost proportional to absolute time. The model simplifies to
Transient Arginine Kinetics
Whereas virtually all glucose is consumed (i.e.,\( \hat G \to 0 \)), arginine concentrations will range from 0 to 1 mM. The transient behavior of arginine during periods between alternating steady-states needs to be described. Substituting equation (A7) into (A6) and integrating produces
where R(t) and R(0) are the concentrations of arginine at times t and 0.
When R0 = 0 the time (t crit ) needed to consume the remaining arginine, R(0) > 0 is
Substituting R 0 = 0 into (A18) provides one solution when t < t crit , otherwise
Transient Fitness
Fitness in a variable environment can be predicted from the relationship between fitness and arginine, (A17), and the kinetics of arginine in the chemostat growth chamber, (A18)–(A20). Assume argRK12 is very rare so that μ B = D. Then the growth rate of argRK12 is simply μ k12 (t) = Dw K12 B (t), and equations (A1) and (A2) become
Following integration the log e ratio of strain densities is found to be
Fitness in a Cyclical Environment
Let the delivery of two arginine concentrations, R0.1 and R0.2, in the fresh medium alternate between two half-cycles, lengths t1 and t2. Then the ambient arginine concentration at time t1, R(t1), is the R(0) at the beginning half-cycle 2, while the ambient arginine concentration at time t 2 (R(t2), is the R(0) at the beginning of half-cycle 1. Thus,
which yield
With very long cycles most of the time is spent at one or the other quasi-steady-state, characterized by R(t 1 ) = \( \hat R_1 \) = R0.1− \( \hat N_T /Y_R \) and R(t 2 ) = \( \hat R \)2 = R0.2 − \( \hat N_T /Y_R \). With very short cycles there is hardly time to displace arginine far from its arithmetic mean, and both (A27) and (A28) converge on \( \overline R=\left( {R_{0.1} t_1 + R_{0.2} t_2 } \right)/\left( {t_1 + t_2 } \right) - \hat N_T /Y_R. \)
Expected fitness in cyclical environments is calculated as the sum of the differences in log e ratios of the strains over a full cycle divided by the total number of generations (D(t1 + t2)),
using the relationships in (A23), (A24), (A27), (A28), and (A29).
Our experiments are confined to half-cycles of equal length, t = t 1 = t 2 , with one feed arginine concentration set at zero, R0.2 = 0. The time (t crit ) needed to consume the remaining arginine is given by
and fitness by
and
For t → 0 (A32) fitness converges on \( {w_B^{K12} \left( {\overline R } \right)}=\left( {w_{B.{\rm{max}} }^{K12} -\left( {w_{B.{\rm{max}} }^{K12} \beta _{K12} + \chi _{K12} /D} \right)/\left( {\kappa _{K12} + \overline R } \right)} \right) \), which is the function of an expected ambient arginine concentration, while for t → ∞ (A33) fitness converges on the expected mean fitness \( \ \overline {w_B^{K12} }=(w_B^{K12} (R_{0.1} ) + w_B^{K12} (0))/2 \), which comprises the first row of (A33), selection in the transitions (second and third rows) being insignificant.
Assumptions
We assume that the rate of consumption of arginine is dependent on culture growth, \( D\hat N_T \), and independent of the genotypes present and the ambient arginine concentration, R(t). The approximation is justified because: (1) \( \hat N_T \) is almost constant (the maximum mass of arginine consumed is only 3.5% of the glucose consumed), (2) the quantity of arginine consumed is independent of genotype (Fig. 4), (3) the quantity of arginine consumed is constant for \( R_0 > \hat N_T /Y_R=0.02\ {\rm{ m}}M \) (Fig. 3), and (4) very low concentrations of arginine saturate the arginine transporters (K m ≈ 20 nM [Celis 1977]). Only at the last moment as the last trace of arginine disappears is this model unrealistic. The second assumption is that the growth rates of argRB strains are independent of arginine availability. The third assumption is that argRB strains are at sufficiently high frequency and that they determine the steady-state concentration of glucose in the chemostat growth chamber, \( \hat G \).
The model should not be used to describe long periods of selection at very low ambient concentrations of arginine, in the general vicinity of 0 < R0 < \( \hat N_T /Y_R \). Fluctuations between absence and presence of high ambient arginine concentrations help minimize the impact on selection of low ambient arginine concentrations in our experiments.
Our experiments are designed to test whether selection in transient states can be predicted from a knowledge of selection at quasi-steady-states. The key assumption is that fitness changes instantaneously with changes in ambient arginine concentrations—there are no delays and fitness is confined to the surface depicted in Fig. 6.
Rights and permissions
About this article
Cite this article
Suiter, A.M., Dean, A.M. Selection in a Cyclical Environment: Possible Impact of Phenotypic Lag on Darwinian Fitness. J Mol Evol 61, 153–170 (2005). https://doi.org/10.1007/s00239-004-0210-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-004-0210-9