Abstract
The Y receptors comprise a family of G-protein coupled receptors with neuropeptide Y-family peptides as endogenous ligands. The Y receptor family has five members in mammals and evolutionary data suggest that it diversified in the two genome duplications proposed to have occurred early in vertebrate evolution. If this theory holds true, it allows for additional family members to be present. We describe here the cloning, pharmacological characterization, tissue distribution, and chromosomal localization of a novel subtype of the Y-receptor family, named Y7, from the zebrafish. We also present Y7 sequences from rainbow trout and two amphibians. The new receptor is most similar to Y2, with 51–54% identity. As Y2 has also been cloned from some of these species, there clearly are two separate Y2-subfamily genes. Chromosomal mapping in zebrafish supports origin of Y7 as a duplicate of Y2 by chromosome duplication in an early vertebrate. Y7 has probably been lost in the lineage leading to mammals. The pharmacological profile of the zebrafish Y7 receptor is different from mammalian Y2, as it does not bind short fragments of NPY with a high affinity. The Y7 receptor supports the theory of early vertebrate genome duplications and suggests that the Y family of receptors is a result of these early genome duplications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The neuropeptide Y (NPY) family of peptides consists of NPY, peptide YY (PYY), pancreatic polypeptide (PP), and peptide Y (PY), where NPY and PYY are found in all vertebrates, PP only in tetrapods, and PY in acanthomorph fishes (Larhammar 1996); (Cerda-Reverter and Larhammar 2000). These peptides bind to a family of G-protein coupled receptors called the Y family. In mammals, this family has five cloned members, named Y1, Y2, Y4, Y5, and Y6 (Larhammar et al. 2001; Michel et al. 1998). Y3 was proposed from pharmacological experiments but does not seem to exist as a separate gene. In teleost fish three seemingly distinct subtypes have been found, called Ya, Yb, and Yc (Lundell et al. 1997; Starback et al. 2000). The fish receptors belong together with Y1, Y4, and Y6 to the Y1 subfamily, all of which exhibit approximately 50% sequence identity to each other, but only about 30% to Y2 and Y5. It has been suggested that the Ya receptor is the fish ortholog of the mammalian Y4 receptor, while Yb and Yc seem to be closely related copies of a fourth unique subtype not yet found outside the teleost fish (Salaneck et al. 2001). The Y2 and Y5 receptors have so far been cloned in mammals (Larhammar et al. 2001) and chicken (Holmberg et al. 2002; Salaneck et al. 2000).
The Y1, Y2, and Y5 receptor genes are located in close proximity to each other on human chromosome (Hsa) 4 (Larhammar et al. 2001) but Y2 has been separated from Y1–Y5 in both mouse and pig (Wraith et al. 2000). The gene cluster seems to be intact in chicken (Larhammar et al., unpublished data), confirming that the arrangement with Y1, Y2, and Y5 close to each other on the same chromosome is the likely ancestral configuration. Subtypes Y4 and Y6 are found on Hsa 5 and 10, respectively. As they have a higher degree of sequence identity to Y1 than to Y2 and Y5 the following scenario has been proposed for the formation of this gene family. First, an ancestral Y receptor gene underwent two duplications in an early chordate ancestor leading to a Y1-like, a Y2-like, and a Y5-like gene. In the two proposed genome duplications in early vertebrate evolution (Holland 1999; Lundin 1993), this chromosome duplicated twice. These new gene copies either acquired specialized functions and became fixed in the genome or became redundant and disappeared. It has been estimated that approximately 50% of the new gene copies are eventually lost (Wagner 1998). Today we know four Y1-like genes, three of which are present in mammals, whereas only one Y2-like and one Y5-like gene have been identified in mammals and chicken. The proposed scenario gives room for additional copies of Y2 and Y5, although they may no longer be present in all taxa.
The physiological effects of NPY and the other members of the peptide family are diverse because they work as neurotransmitters, neuromodulators, and hormones, and the receptors are present in the central nervous system as well as peripheral organs. Among the most important effects are stimulation of appetite, regulation of circadian rhythms and blood pressure, and inhibition of anxiety. Some of these effects are thought to be mediated primarily by the Y2 receptor subtype, including regulation of circadian rhythms, inhibition of presynaptic transmitter release in the central and peripheral nervous system, and modification of electrophysiological properties in the hippocampus, presumably associated with memory formation (Dumont et al. 1992; Wahlestedt and Reis 1993).
NPY and PYY are the endogenous high-affinity ligands for all Y receptors except Y4, which in mammals, but not in chicken, primarily binds PP. The mammalian and chicken Y2 receptors exhibit the same pharmacological profile, binding PYY, NPY, and also a number of truncated fragments of NPY with a high affinity (Ammar et al. 1996; Malmstrom et al. 1998; Nakamura et al. 1996; Rose et al. 1995; Sharma et al. 1998). The binding of short NPY fragments has been a hallmark of Y2 pharmacology, because the other Y receptors do not bind these shorter fragments. There is also a nonpeptidergic Y2-selective antagonist, BIIE0246 (Doods et al. 1999), that binds mammalian Y2 receptors but not the chicken ortholog (Salaneck et al. 2000).
To study further the hypothesis of the evolution of the Y receptor family, additional paralogs of Y2 and Y5 should ideally be identified. However, no such paralogs appear to be present in mammals, as homology cloning efforts and large scale sequencing projects have not revealed any Y2-like or Y5-like receptor genes. However, in our ongoing effort to unravel the evolution of the vertebrate Y receptor family, we came across distantly Y2-related sequences in fish and frog. We present here the cloning, pharmacological characterization, tissue distribution, and chromosomal localization of this novel Y2-like receptor gene, named Y7, in the zebrafish, Danio reiro. We also present partial sequences of Y7 from rainbow trout (Oncochynchus mykiss) and two amphibians, the marsh frog (Rana ridibunda) and the western clawed frog (Xenopus tropicalis), together with partial sequences of the Y2 ortholog from zebrafish and rainbow trout.
Materials and Methods
Isolation and Sequencing of the Zebrafish Y7 Receptor
Degenerate PCR with primers based on several mammalian Y2 receptors and the chicken Y2 receptor (Salaneck 2001) was performed on zebrafish genomic DNA using the following PCR conditions: 120 s at 95° for one cycle, then 30 s at 95°, touch down from 55° to 42° for 45 s, and 60 s at 72° for 20 cycles, then 30 s at 95°, 45 s at 42°, and 60 s at 72° for 20 cycles, then 5 min at 72° using stoffel Taq polymerase (Applied Biosystems, USA). One primer combination gave a product of the expected size. The forward primer had the sequence, in IUB codes, 5′-ATC AAR TTC AAR TTC AAR AGC ATG CGY ACA-3′ and the reverse primer had the sequence 5′-TAG TTG YTR TTC ATC CAN CCR TA-3′. This product was cloned into a pUC18 vector (Amersham Pharmacia Biotech, Sweden) and sequenced using the BigDye terminator sequencing kit (Applied Biosystems) and the extension products were analyzed on an ABI 310 automatic sequencer. The sequence was compared to the GenBank database using the On-Line BLASTX program and found to be similar to mammalian and chicken Y2 sequences. The cloned PCR products were labeled using the Random Primer Labeling Kit (Amersham Pharmacia Biotech) and used to screen a gridded zebrafish genomic BAC library (Genome Systems, USA) at high stringency. Two BAC clones hybridized strongly and were later confirmed to be true positives by southern hybridizations. Direct sequencing on the BAC clone was used to isolate the 3′ and 5′ ends of the zebrafish Y2 receptor gene.
Isolation of Y2 from Zebrafish and Rainbow Trout and Y7 from Rainbow Trout, Rana ridibunda, and Xenopus tropicalis
Clones were obtained by PCR as described above. Full-length clones are being sought by screening of a variety of cDNA and genomic libraries and will be reported elsewhere.
Cloning into Expression Vector
A fragment containing the entire coding region was generated from the BAC clone using Pfu-turbo DNA polymerase (Stratagene). The 5′ primer contained a HindIII restriction site and had the sequences 5′-TACGGTCTAAGCTTAGGTACATGCATGTCCTTGCTAC CGGA-3′ and the 3′ primer had a BamHI restriction site and had the sequence 5′-TACGGTCTGGATCCGATTATATACTGTG CA ACAATCAAGCT-3′. The PCR products were digested with HindIII (Amersham Pharmacia Biotech) and XhoI (Amersham Pharmacia Biotech) for 3 h, purified on a PCR purification column (Qiagen, Germany), and thereafter ligated into a modified pCEP4 vector (Marklund et al. 2002). The expression construct was sequenced and found to be identical to the genomic sequence obtained from the BAC clone.
Transfection Protocol
For transient transfections HEK 293-EBNA cells were seeded onto 90-mm dishes and grown to 50% confluence and transfected with 10 µg of the expression construct using FuGene (Roch, Germany) according to the manufacturer’s recommendations. The cells were grown for 48 h after transfection, harvested into 1 ml 25 mM HEPES buffer (pH 7.4) containing 2.5 mM CaCl2, 1 mM MgCl2, and 2 g/l bacitracin, aliquoted, and stored at −80°C until further use. For semistable transfection HEK 293-EBNA cells were transfected as above and grown for 24 h. The cells carrying the expression vector were thereafter selected for by growing them in presence of 500 µg/ml hygromycine (Gibco, Sweden) for 10 days.
Peptides and Antagonists
The zebrafish (z) NPY and PYY peptides were synthesized at Eli Lilly and Company as deduced from the cloned zebrafish genes (Soderberg et al. 1994). The porcine peptides and peptide analogs were purchased from Bachem (King of Prussia, PA). Boehringer Ingerheim, Biberach, Germany, provided the antagonists BIIE0246 and BIBP3226.
Binding Assays
For binding assays, thawed aliquots of membrane were resuspended in 25 mM HEPES buffer (pH 7.4) containing 2.5 mM CaCl2, 1 mM MgCl2, and 2 g/l bacitracin and homogenized. Binding experiments were performed in a volume of 100 µl with 2 µg of protein per assay. The reactions were incubated for 2 h at room temperature with 125I-pPYY (Amersham Pharmacia Biotech) as radioligand. Saturation experiments were carried out with twofold serial dilutions of radioligand, with nonspecific binding defined as the amount of radioactivity binding to the cell homogenate with 100 nM nonlabeled pPYY included in the reactions. Competition experiments were performed with serial dilutions of pPYY, pNPY, pNPY 2-36, pNPY 13-36, pNPY 18-36, zNPY, zPYY, p[Leu31,Pro34]NPY, BIBP3226, and BIIE0246 included in the incubation mixture. The reactions were terminated by rapid filtration through GF/C filters, which had been presoaked in 0.3% polyethyleneimine, using a TOMTEC (Orange, CT) cell harvester. The filters were washed with 5 ml of 50 mM Tris–HCl, pH 7.4, and dried at 60°. The dried filters were treated with MeltiLex A (Perkin Elmer, USA) melt-on scintillator sheets, and the radioactivity retained in the filters counted using the Wallac 1450 Betaplate counter. The results were analyzed using the Prism software package (GraphPad, San Diego, CA). Protein concentrations were measured using the Bio-R Protein Assay (BioRad) with bovine serum albumin (BSA) as standard.
cAMP Assay
Cyclic AMP was assayed on semistable cell lines expressing the zebrafish Y2 receptor treated for 20 min at 37° with 500 µg isobutylmethylxanthine. Cells were incubated with 15 µM forskolin and various concentrations of zNPY, zPYY, pNPY, and pNPY 13-36 for 30 min at 37°. Reactions were terminated by lysing the cells with 4.4 M perchloric acid. cAMP was quantified using radioassay (Amersham Pharmacia Biotech).
Reverse Transcription PCR
Total RNA was isolated from whole brain, intestine, heart, ovaries, eye, and muscle. Total RNA (5 µg) was used in cDNA synthesis using AMV reverse transcriptase (Amersham Pharmacia Biotech) and a mixture of dT18 and random octamers as primers in a total volume of 35 µl. The cDNA synthesis reaction was incubated for 60 min at 42° and terminated by heating to 60° for 10 min. The cDNA was diluted to a total of 100 µl in water. Two microliters of the diluted cDNA was used as a template in a PCR reaction under the following conditions: 60 s at 95° for one cycle, then 30 s at 95°, 30 s at 55°, and 45 s at 72° for 30 cycles, and then 72° for 5 min. The 3′ primer had the sequence 5′-TTCCATTCTCACCCTTACCATTA-3′ and the 5′ primer had the sequence 5′-CCACTGCGAAGACCACGACTACTA-3′ and the primer pair was designed to give a product of 400 bp. Three microliters of the RT-PCR reactions was analyzed on a 2% agarose gel and thereafter blotted to nylon membrane for southern hybridization. To control for genomic DNA in the RNA preparations negative controls without reverse transcriptase were performed on all samples.
Southern Hybridization
Hybridizations were performed at high stringency with a 200-bp PCR fragment labeled with 32P using the Mega prime labeling kit (Amersham Pharmacia Biotech).
Genetic Mapping
Mapping was performed on the LN54 radiation hybrid panel (Hukriede et al. 1999) using the mapping primers F-GTTTTACGCCTCATATAGCCTGTTCA, R-AAAGGCTGGTTTGTAAGTGTT GACG. The positions of the gene loci were intercalated on the HS mapping panel (Kelly et al. 2000; Postlethwait et al. 1998; Woods et al. 2000; http://zebrafish. stanford.edu/genome/Frontpage.html ). For comparative mapping, putative orthologs were defined by a BLASTX search using the zebrafish nucleotide sequence to query the human NR database at GenBank (http://www.ncbi.nlm.nih.gov/BLAST/ ), followed by using the top human hit in a TBLASTN search querying the zebrafish Unigene set (either http://www.ncbi.nlm.nih.gov/UniGene/Dr.Home.html or our own unigene set at http://zfish3.uoregon. edu/ [Alan Day and J.H.P., unpublished]). If the best human hit for a zebrafish sequence blasted back to the zebrafish Unigene that included the original zebrafish sequence, then the two sequences were considered as putative orthologs. The map locations of those sequences were obtained from LocusLink (http://www.ncbi.nlm. nih.gov/genome/guide/huma ) or GeneMap99 (http://www.ncbi. nlm.nih.gov/genemap/ ).
Phylogenetic Analyses
The alignment for the phylogenetic tree was constructed using the ClustalW 1.81 software (Thompson et al. 1994). The phylogenetic analyses were performed using the MEGA v2.1 (Kumar et al. 2001) software using the UPGMA distance method, with the human Y1 serving as an outgroup. The model for amino acid replacements were the gamma model, with a gamma parameter of 1.2. The value of the gamma parameter for this dataset were determined using Treepuzzle 5.0 (Strimmer and Haeseler 1996) on the same alignment.
Results
A 600-bp fragment from the zebrafish Y7 receptor was isolated by degenerate PCR from zebrafish genomic DNA using primers based on public sequences of Y2 from mammals (Larhammar et al. 2001) and chicken (Salaneck et al. 2000). This fragment was used as a probe for screening of a gridded zebrafish BAC library whereby two positive BAC clones were isolated, both of which were subsequently found to contain the zebrafish Y7 receptor gene. The coding part of the gene consists of one open reading frame of 372 amino acids (Fig. 1). The protein sequence displays the characteristics of a G-protein coupled receptor, that is, seven putative transmembrane regions, two well-conserved cysteines linking extra-cellular loop 1 and loop 2, and two putative glycosylation sites in the amino-terminal region. A conserved cysteine in the carboxyterminal tail could serve as a palmitoylation site to anchor the tail to the membrane.
Amino acid alignment of the Y7 receptors from zebrafish, Rana ridibunda, Xenopus tropicalis, and rainbow trout together with the Y2 receptors from zebrafish, rainbow trout, and human. The alignments were created using Lasergene DNASTAR Megalign software version 5.01 using the ClustalW algorithm. The human Y2 sequence serves as a master. Boxes mark the putativeTM regions as predicted from comparisons with the crystal structure of bovine rhodopsin. Putative glycosylation sites are boxed and shadowed boxes indicate cysteines potentially involved in disulfide bridges. Arrows indicate cysteines potentially involved palmitoylatin. Dashes show gaps introduced to optimize the alignment.
Using the same technique, partial sequences of the Y7 receptor gene were also isolated from rainbow trout, Rana ridibunda, and Xenopus tropicalis. In addition, partial sequences of the Y2 receptor gene were isolated from rainbow trout and zebrafish by PCR. The zebrafish Y7 receptor is highly divergent from the previously cloned mammalian and chicken Y2 receptors, with 51% amino acid identity to the human Y2 receptor and 54% identity to chicken Y2. The mammalian Y2 receptor sequences are approximately 90% identical to each other, while chicken and the mammalian Y2 receptors are approximately 80% identical. The zebrafish Y7 receptor is 80% identical to rainbow trout Y7 and about 66% identical to Y7 from the frogs Rana ridibunda and Xenopus tropicalis when comparing the partial sequences available. The degree of identity between Y7 and Y2 is approximately the same as the identity between members of the Y1 subfamily, suggesting that Y2 and Y7 arose from a common ancestor at approximately the same time as the Y1 subfamily. When Y7 is compared to other Y-receptor subtypes the identity is considerably lower, approximately 25% to Y1, Y4, Y5, and Y6 from mammals. The alignment in Fig. 1 shows that the N- and C-terminals are, as for other G-protein coupled receptors, the most divergent regions.
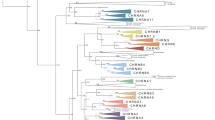
The phylogenetic tree in Fig. 2 shows that the Y2 and Y7 sequences form separate branches that diverged before the split of teleost fish from the lineage leading to mammals and chicken, i.e., before the divergence of ray-finned and lobe-finned fish. The stability of the tree topology was determined using the bootstrap method with 1000 replicates and all branches with less then 50% support were collapsed. The tree was calculated using the UPGMA distance method with gamma correction. The same tree topologies were obtained using the neighbor-joining and the parsimony methods.
Phylogenetic tree of the Y2 subfamily of receptors based on the data shown in Fig. 1 and previously published sequences retrieved from the public databases. Alignments were made using ClustalW 1.81 software. The phylogenetic tree was constructed using the MEGA software using the UPGMA method, with the human Y1 serving as an outgroup. Stability of the branches was assessed using bootstrapping with 1000 replicas. All branches with less then 50% bootstrap support were collapsed.
The coding part of the zebrafish Y7 receptor was transferred to a modified pCEP-4 expression vector (Marklund et al. 2002) using PCR with a proofreading Taq polymerase and transfected into the HEK-293 EBNA cell line. Cells were harvested 48 h after transfection and membranes prepared for binding assays. The membranes showed specific binding of 125I-pPYY in a concentration-dependent manner with a K d value of 9.4 pM (SE = 2.1; N = 3) and a B max of 202 fmol/mg protein (SE = 2; N = 3). The endogenous zebrafish peptides NPY and PYY were potent inhibitors of radioligand binding and also the porcine peptides had a high affinity for the receptor. On the other hand, the truncated forms of NPY had a considerably lower affinity for the zebrafish Y7 receptor than what is normal for the Y2 receptor subtype. The fragment NPY 3-36 showed a 10-fold lower affinity, while NPY 13-36 had an almost 100-fold lower affinity than intact pPYY. The fragment pNPY 18-36 showed no binding to the receptor, while it binds to mammalian and chicken Y2 receptors with an affinity of approximately 10 nM (Salaneck et al. 2000; Sharma et al. 1998). Also the Y2-specific antagonist BIIE0246 showed no binding to the zebrafish Y7 receptor, as is also the case for the chicken Y2 receptor (Salaneck et al. 2000). Thus, BIIE0246 seems to function as an antagonist only at the mammalian Y2 receptors and not on nonmammalian Y2 and Y7 receptors. All binding constants for the tested ligands are shown in Table 1.
In cAMP assays, functional coupling to G- proteins was shown by inhibition of cAMP production. The most potent ligand tested was pPYY, with a 10-fold higher EC50 value then zPYY, zNPY, and pNPY. The ligand with the lowest potency among the tested was, as expected from the binding data, pNPY 13-36 (Table 1).
A rough tissue distribution profile was obtained with reverse transcription PCR (RT-PCR) (Fig. 3) on total RNA isolated from different tissues. The Y7 mRNA was primarily expressed in the gastrointestinal tract and eye and there seems to be some expression also in the brain, whereas muscle was negative. To exclude the possibility of contamination of genomic DNA in the RNA samples, negative controls were performed by excluding reverse transcriptase from the reactions. All samples were found to be negative (data not shown).
RT-PCR analyses of Y7 gene expression in zebrafish tissues. RT-PCR using total RNA extractions from brain, eye, muscle, and gastrointestinal tract (GI). For all tissues a negative control excluding reverse transcriptase was run to exclude the possibility of contamination of genomic DNA (data not shown).
The zebrafish Y7 gene was mapped to LG14 (Fig. 4) in a region showing extensive conserved synteny with the distal third of the long arm of Hsa5, the location of the human locus NPY6R at 5q31 (Gregor et al. 1996). Some loci on LG14 are related to loci on Hsa4 but these similarities are more likely to reflect the more ancient paralogous relationship thought to result from chromosome duplications in an ancient vertebrate or prevertebrate. This is supported by the mapping of zebrafish Y2 to LG1, a region with even greater similarity to Hsa4.
Chromosomal localization of the Y2 and Y7 genes in the zebrafish. The Y7 gene is located in LG14 (LG for linkage group) in a region orthologous to the human chromosome 5, which harbors the Y6 gene, The region containing the zebrafish Y2 gene is orthologous to human chromosome 4, which has the Y1, Y2, and Y5 gene cluster.
Discussion
In this paper we present the cloning and characterization of a novel subtype of the Y-receptor family, a Y2-related receptor that we have named Y7. The status of Y7 as a distinct subtype is demonstrated by its presence in two different vertebrate lineages, bony fish (zebrafish and rainbow trout) and amphibians (western clawed frog and marsh frog) and by our cloning of Y2 from the two species of bony fish (Fig. 1 and Fig. 2).
Previously cloned Y2 receptors from several different orders of mammals and chicken show a nearly constant rate of evolution over time (Salaneck et al. 2000). Assumption of a similar rate of evolution for the Y7 receptor suggests that the duplication that generated Y2 and Y7 from a common ancestor took place early in vertebrate evolution. An early time point for the duplication leading to Y2 and Y7, i.e., before the split of teleost fish and tetrapods, is also shown by our cloning of Y7 from two amphibians, Rana ridibunda and Xenopus tropicalis. The sequence segments available for comparison display 66% identity to the zebrafish and rainbow trout Y7 sequences. The latter two are 80% identical. The presence of Y7 in amphibians excludes the possibility that the fish Y7 gene could be a result of the genome duplication basal in the teleost lineage (Taylor et al. 2001). The paralogous nature of Y7 and Y2 is further supported by the chromosomal localization of these genes in the zebrafish genome. The Y7 gene was found to be located in LG 14, which is paralogous to Hsa5, on which the Y6 receptor gene is found (Fig. 4). The zebrafish Y2 receptor maps to a region on LG1 in the zebrafish genome which is paralogous to Hsa4, where the human Y2 receptor gene is located together with Y1 and Y5.
The zebrafish Y7 receptor was functionally expressed in HEK-293 EBNA cells and characterized by ligand binding studies. The peptides and peptide fragments used display the following rank order of potency: pPYY > zNPY > pNPY > pNPY 2-36 > zPYY > pNPY 13-36, with no binding detected for p[Leu31, Pro34]NPY and pNPY 18-36. The sensitivity to N-terminal truncation of peptide ligands makes Y7 distinct from the mammalian and chicken Y2 receptors, as high-affinity binding of truncated NPY is a characteristic of Y2 pharmacology, with pNPY 18-36 being one of the most selective agonists available for mammalian Y2 receptors. This suggests that the zebrafish Y7 receptor has a different way of binding NPY, with more interaction points near the N-terminal part of the peptide like the Y1 subfamily (except Ya) and Y5. This is evolutionarily interesting since the typical Y2 profile seems to have been conserved over the 300 million years separating chicken from mammals (Salaneck et al. 2000). The peptide p[Leu31, Pro34]NPY, a variant of pNPY modified to be more PP-like (Gehlert et al. 1997), lacked binding to Y7, which was less surprising, as it does not bind to Y2 in mammals. This peptide has, on the other hand, been found to bind the chicken Y2 receptor with a high affinity (Salaneck et al. 2000). This observation will give ideas for future mutagenesis studies to elucidate the amino acids that are important for binding, both in the receptors and in the peptide ligands. The antagonist BIIE0246 developed toward mammalian Y2 did not bind to the zebrafish Y7 receptor. This was expected, as it does not even bind to the chicken Y2 receptor (Berglund et al. 2002; Salaneck et al. 2000). Thus, the pharmacological profile of zebrafish Y7 differs considerably from the previously characterized Y2 receptors adding further support to its status as a separate subtype.
The cAMP data presented here show that despite the high degree of diversity in the C-terminal tail, the Y7 receptor couples to Gi proteins as seems to be the case for all other Y-receptors tested. The rank order of potency for inhibition of cAMP production was the same as in the competition experiments (Table 1).
The tissue distribution profile for Y7 mRNA as detected by RT-PCR is very broad and seems to differ from the Y2 expression profile in rat and mouse, where expression is mainly seen in the CNS (Goumain et al. 1998; Gustafson et al. 1997; Naveilhan et al. 1998). It should be noted that the data presented are not quantitative, thus more detailed studies are required, preferably by in situ hybridization or real-time PCR.
These new results corroborate the previously proposed scenario (Wraith et al. 2000) shown in Fig. 5 regarding how this gene family has expanded during evolution. A single Y-receptor gene in a chordate ancestor underwent two local duplications resulting in three genes, the ancestors of the Y1 subfamily, the Y2 subfamily, and the Y5 subfamily. This was followed by the two genome duplications early in the vertebrate lineage (Holland 1999; Lundin 1993). No single vertebrate lineage has so far been found to have all of these but our discovery of Y7 and its chromosomal localization in the zebrafish add important data that support the hypothesis. It is quite possible that some lineages may have yet additional subtypes, but with the presently available information, the most parsimonious scheme seems to be the one shown in Fig. 5. This assumes only three gene losses, two after the first chromosome duplication (tetraplodization) and one after the second. Subsequently, Yb may have been lost in the lineage leading to mammals. It remains to be seen if other losses have occurred in the teleost fish lineage. Obviously, more complicated schemes are possible involving additional gene losses and such schemes remain possible as gene losses have been found to occur frequently after gene duplications (Wagner 1998). The apparent differences in NPY receptor repertoire between fish and mammals make physiological comparisons complicated. For instance, NPY receptor subtypes recently proposed to be involved in feeding in goldfish (Narnaware and Peter 2001) may have to be reevaluated to include the novel receptors Y7 and Yb. Other complicating factors are the possible absence of some subtypes (Y1), as well as differences in pharmacological profile for orthologous receptors.
Hypothetical scheme for the evolution of the Y-receptor family. The single ancestral Y-receptor was first duplicated twice locally to create the first Y-receptor gene cluster in a prevertebrate. The family then expanded in the chromosome duplication events in early vertebrate evolution. The crossed-out genes indicate that these genes were probably lost after the chromosomal duplicationevent. Data from other vertebrates are required to confirm this scheme.
Delineation of the gene duplication events that generated the multiple receptor subtypes is a prerequisite for comparative studies. This is also an absolute requirement to increase our understanding of the evolution of complex regulatory networks such as feeding. Our discovery of the Y7 receptor in fish and frogs adds strong support to the concept of chromosome duplications or tetraplodization in early vertebrate evolution, major events that probably had far-reaching consequences for the expansion and diversification of vertebrate classes.
References
DA Ammar DM Eadie DJ Wong YY Ma LF, Jr Kolakowski TL Yang-Feng DA Thompson (1996) ArticleTitleCharacterization of the human type 2 neuropeptide Y receptor gene (NPY2R) and localization to the chromosome 4q region containing the type 1 neuropeptide Y receptor gene. Genomics 38 392–398 Occurrence Handle10.1006/geno.1996.0642 Occurrence Handle1:CAS:528:DyaK2sXksFeltQ%3D%3D Occurrence Handle8975716
MM Berglund R Fredriksson E Salaneck D Larhammar (2002) ArticleTitleReciprocal mutations of neuropeptide Y receptor Y2 in human and chicken identify amino acids important for antagonist binding. FEBS Lett 518 5–9 Occurrence Handle10.1016/S0014-5793(02)02534-6 Occurrence Handle1:CAS:528:DC%2BD38XjsVSku7o%3D Occurrence Handle11997008
JM Cerda-Reverter D Larhammar (2000) ArticleTitleNeuropeptide Y family of peptides: structure, anatomical expression, function, and molecular evolution. Biochem Cell Biol 78 371–392 Occurrence Handle10.1139/bcb-78-3-371 Occurrence Handle1:CAS:528:DC%2BD3cXltlKntbw%3D Occurrence Handle10949087
H Doods W Gaida HA Wieland H Dollinger G Schnorrenberg F Esser W Engel W Eberlein K Rudolf (1999) ArticleTitleBIIE0246: A selective and high affinity neuropeptide Y Y(2) receptor antagonist. Eur J Pharmacol 384 R3–R5 Occurrence Handle10.1016/S0014-2999(99)00650-0 Occurrence Handle1:CAS:528:DyaK1MXotVOiu7k%3D Occurrence Handle10611450
Y Dumont JC Martel A Fournier S St-Pierre R Quirion (1992) ArticleTitleNeuropeptide Y and neuropeptide Y receptor subtypes in brain and peripheral tissues. Prog Neurobiol 38 125–167 Occurrence Handle10.1016/0301-0082(92)90038-G Occurrence Handle1:CAS:528:DyaK38XhsVeqsLs%3D Occurrence Handle1312243
DR Gehlert DA Schober SL Gackenheimer L Beavers R Gadski I Lundell D Larhammar (1997) ArticleTitle[125I]Leu31, Pro34-PYY is a high affinity radioligand for rat PP1/Y4 and Y1 receptors: evidence for heterogeneity in pancreatic polypeptide receptors. Peptides 18 397–401 Occurrence Handle10.1016/S0196-9781(96)00346-4 Occurrence Handle1:CAS:528:DyaK2sXivFClt7o%3D Occurrence Handle9145427
M Goumain T Voisin AM Lorinet M Laburthe (1998) ArticleTitleIdentification and distribution of mRNA encoding the Y1, Y2, Y4, and Y5 receptors for peptides of the PP-fold family in the rat intestine and colon. Biochem Biophys Res Commun 241 52–56 Occurrence Handle10.1006/bbrc.1998.8647
P Gregor Y Feng LB DeCarr LJ Cornfield ML McCaleb (1996) ArticleTitleMolecular characterization of a second mouse pancreatic polypeptide receptor and its inactivated human homologue. J Biol Chem 271 27776–27781 Occurrence Handle10.1074/jbc.271.44.27776 Occurrence Handle1:CAS:528:DyaK28XmvVWkt70%3D Occurrence Handle8910373
EL Gustafson KE Smith MM Durkin MW Walker C Gerald R Weinshank TA Branchek (1997) ArticleTitleDistribution of the neuropeptide Y Y2 receptor mRNA in rat central nervous system. Brain Res Mol Brain Res 46 223–235 Occurrence Handle10.1016/S0169-328X(97)00017-X Occurrence Handle1:CAS:528:DyaK2sXislemsL0%3D Occurrence Handle9191097
PW Holland (1999) ArticleTitleGene duplication: past, present and future. Semin Cell Dev Biol 10 541–547 Occurrence Handle10.1006/scdb.1999.0335 Occurrence Handle1:CAS:528:DyaK1MXnvFOmsb0%3D Occurrence Handle10597638
SK Holmberg S Mikko T Boswell R Zoorob D Larhammar (2002) ArticleTitlePharmacological characterization of cloned chicken neuropeptide Y receptors Y1 and Y5. J Neurochem 81 462–471 Occurrence Handle10.1046/j.1471-4159.2002.00817.x Occurrence Handle1:CAS:528:DC%2BD38XjsFGktrk%3D Occurrence Handle12065655
NA Hukriede L Joly M Tsang J Miles P Tellis JA Epstein WB Barbazuk FN Li B Paw JH Postlethwait TJ Hudson LI Zon JD McPherson M Chevrette IB Dawid SL Johnson M Ekker (1999) ArticleTitleRadiation hybrid mapping of the zebrafish genome. Proc Natl Acad Sci USA 96 9745–9750 Occurrence Handle10.1073/pnas.96.17.9745 Occurrence Handle1:CAS:528:DyaK1MXlsVymt7w%3D Occurrence Handle10449765
PD Kelly F Chu IG Woods P Ngo-Hazelett T Cardozo H Huang F Kimm L Liao YL Yan Y Zhou SL Johnson R Abagyan AF Schier JH Postlethwait WS Talbot (2000) ArticleTitleGenetic linkage mapping of zebrafish genes and ESTs. Genome Res 10 558–567
S Kumar K Tamura IB Jakobsen M Nei (2001) ArticleTitleMEGA2: Molecular evolutionary genetics analysis software. Bioinformatics 17 1244–1245 Occurrence Handle1:CAS:528:DC%2BD38XmtVCktQ%3D%3D Occurrence Handle11751241
D Larhammar (1996) ArticleTitleEvolution of neuropeptide Y, peptide YY and pancreatic polypeptide. Regul Pept 62 1–11 Occurrence Handle10.1016/0167-0115(95)00169-7 Occurrence Handle1:CAS:528:DyaK28XjtFWit7g%3D Occurrence Handle8738876
D Larhammar A Wraith MM Berglund SK Holmberg I Lundell (2001) ArticleTitleOrigins of the many NPY-family receptors in mammals. Peptides 22 295–307 Occurrence Handle10.1016/S0196-9781(01)00331-X Occurrence Handle1:CAS:528:DC%2BD3MXisVWntLs%3D Occurrence Handle11287083
I Lundell MM Berglund P Starback E Salaneck DR Gehlert D Larhammar (1997) ArticleTitleCloning and characterization of a novel neuropeptide Y receptor subtype in the zebrafish. DNA Cell Biol 16 1357–1363 Occurrence Handle1:CAS:528:DyaK2sXotFSnu7c%3D Occurrence Handle9407007
LG Lundin (1993) ArticleTitleEvolution of the vertebrate genome as reflected in paralogous chromosomal regions in man and the house mouse. Genomics 16 1–19 Occurrence Handle10.1006/geno.1993.1133 Occurrence Handle1:CAS:528:DyaK3sXkt1Wnsr4%3D Occurrence Handle8486346
RE Malmstrom T Hokfelt JA Bjorkman C Nihlen M Bystrom AJ Ekstrand JM Lundberg (1998) ArticleTitleCharacterization and molecular cloning of vascular neuropeptide Y receptor subtypes in pig and dog. Regul Pept 75–76 55–70 Occurrence Handle10.1016/S0167-0115(98)00053-6
U Marklund M Bystrom K Gedda A Larefalk K Juneblad S Nystrom AJ Ekstrand (2002) ArticleTitleIntron-mediated expression of the human neuropeptide Y Y(1) receptor. Mol Cell Endocrinol 188 85–97 Occurrence Handle10.1016/S0303-7207(01)00738-9 Occurrence Handle1:CAS:528:DC%2BD38XitFWntro%3D Occurrence Handle11911949
MC Michel A Beck-Sickinger H Cox HN Doods H Herzog D Larhammar R Quirion T Schwartz T Westfall (1998) ArticleTitleXVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev 50 143–150 Occurrence Handle1:CAS:528:DyaK1cXitlylsrc%3D Occurrence Handle9549761
M Nakamura Y Aoki D Hirano (1996) ArticleTitleCloning and functional expression of a cDNA encoding a mouse type 2 neuropeptide Y receptor. Biochim Biophys Acta 1284 134–137 Occurrence Handle10.1016/S0005-2736(96)00166-6 Occurrence Handle1:CAS:528:DyaK28Xlsl2hsr8%3D Occurrence Handle8914576
YK Narnaware RE Peter (2001) ArticleTitleNeuropeptide Y stimulates food consumption through multiple receptors in goldfish. Physiol Behav 74 185–190 Occurrence Handle10.1016/S0031-9384(01)00556-X Occurrence Handle1:CAS:528:DC%2BD3MXmvVyktr4%3D Occurrence Handle11564467
P Naveilhan I Neveu E Arenas P Ernfors (1998) ArticleTitleComplementary and overlapping expression of Y1, Y2 and Y5 receptors in the developing and adult mouse nervous system. Neuroscience 87 289–302 Occurrence Handle10.1016/S0306-4522(98)00141-9 Occurrence Handle1:CAS:528:DyaK1cXlt1eitbc%3D Occurrence Handle9722158
JH Postlethwait YL Yan MA Gates et al. (1998) ArticleTitleVertebrate genome evolution and the zebrafish gene map. Nature Genet 18 345–349 Occurrence Handle1:CAS:528:DyaK1cXit1egsrs%3D Occurrence Handle9537416
PM Rose P Fernandes JS Lynch ST Frazier SM Fisher K Kodukula B Kienzle R Seethala (1995) ArticleTitleCloning and functional expression of a cDNA encoding a human type 2 neuropeptide Y receptor. J Biol Chem 270 29–38 Occurrence Handle10.1074/jbc.270.1.29 Occurrence Handle7814389
E Salaneck SK Holmberg MM Berglund T Boswell D Larhammar (2000) ArticleTitleChicken neuropeptide Y receptor Y2: structural and pharmacological differences to mammalian Y2(1). FEBS Lett 484 229–234 Occurrence Handle10.1016/S0014-5793(00)02164-5 Occurrence Handle1:CAS:528:DC%2BD3cXotVCkurs%3D Occurrence Handle11078884
E Salaneck R Fredriksson ET Larson JM Conlon D Larhammar (2001) ArticleTitleA neuropeptide Y receptor Y1-subfamily gene from an agnathan, the European river lamprey. A potential ancestral gene. Eur J Biochem 268 6146–6154 Occurrence Handle10.1046/j.0014-2956.2001.02561.x Occurrence Handle1:CAS:528:DC%2BD3MXptVGqu78%3D Occurrence Handle11733009
P Sharma SK Holmberg H Eriksson AG Beck-Sickinger L Grundemar D Larhammar (1998) ArticleTitleCloning and functional expression of the guinea pig neuropeptide Y Y2 receptor. Regul Pept 75–76 23–28 Occurrence Handle10.1016/S0167-0115(98)00049-4
C Soderberg VA Pieribone J Dahlstrand L Brodin D Larhammar (1994) ArticleTitleNeuropeptide role of both peptide YY and neuropeptide Y in vertebrates suggested by abundant expression of their mRNAs in a cyclostome brain. J Neurosci Res 37 633–640 Occurrence Handle1:STN:280:ByuA3c%2FmsVI%3D Occurrence Handle8028041
P Starback A Wraith H Eriksson D Larhammar (2000) ArticleTitleNeuropeptide Y receptor gene y6: multiple deaths or resurrections? Biochem Biophys Res Commun 277 264–269 Occurrence Handle10.1006/bbrc.2000.3656 Occurrence Handle1:CAS:528:DC%2BD3cXntFOqu70%3D Occurrence Handle11027673
K Strimmer A Haeseler (1996) ArticleTitleQuartet puzzling: A quartet maximum likelihood method for reconstructing tree topologies. Mol Biol Evol 13 964–969 Occurrence Handle1:CAS:528:DyaK28XltlSmsLk%3D
JS Taylor de Van Y Peer I Braasch A Meyer (2001) ArticleTitleComparative genomics provides evidence for an ancient genome duplication event in fish. Philos Trans R Soc Lond B Biol Sci 356 1661–1679 Occurrence Handle1:CAS:528:DC%2BD38XitFCnsw%3D%3D Occurrence Handle11604130
JD Thompson DG Higgins TJ Gibson (1994) ArticleTitleCLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22 4673–4680 Occurrence Handle7984417
A Wagner (1998) ArticleTitleThe fate of duplicated genes: Loss or new function? Bioessays 20 785–788 Occurrence Handle10.1002/(SICI)1521-1878(199810)20:10<785::AID-BIES2>3.0.CO;2-M Occurrence Handle1:STN:280:DyaK1M3gs1emug%3D%3D Occurrence Handle10200118
C Wahlestedt DJ Reis (1993) ArticleTitleNeuropeptide Y-related peptides and their receptors—Are the receptors potential therapeutic drug targets? Annu Rev Pharmacol Toxicol 33 309–352 Occurrence Handle10.1146/annurev.pa.33.040193.001521 Occurrence Handle1:CAS:528:DyaK3sXkvVKisrY%3D Occurrence Handle8494343
IG Woods PD Kelly F Chu P Ngo-Hazelett YL Yan H Huang JH Postlethwait WS Talbot (2000) ArticleTitleA comparative map of the zebrafish genome. Genome Res 10 1903–1914 Occurrence Handle1:CAS:528:DC%2BD3MXht1Y%3D Occurrence Handle11116086
A Wraith A Tornsten P Chardon I Harbitz BP Chowdhary L Andersson LG Lundin D Larhammar (2000) ArticleTitleEvolution of the neuropeptide Y receptor family: Gene and chromosome duplications deduced from the cloning and mapping of the five receptor subtype genes in pig. Genome Res 10 302–310 Occurrence Handle10.1101/gr.10.3.302 Occurrence Handle1:CAS:528:DC%2BD3cXitVWktrg%3D Occurrence Handle10720571
Acknowledgements
We thank Christina Bergqvist for excellent technical assistance, Nina Mohell for expert advice on the pharmacological studies, and Björn Carlsson for help with the cAMP assays. Finally, we thank Isabelle Lihrmann and Hubert Vaudry for providing Rana ridibunda genomic DNA and Michael Conlon for providing Xenopus tropicalis genomic DNA. This work was supported by a grant from the Swedish Research Council for Natural and Engineering Sciences (NFR). R.F. was supported by a grant to D.L. from the National Network for the Neurosciences (NNN) of the Swedish Strategic Funds (SSF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fredriksson, R., Larson, E., Yan, YL. et al. Novel Neuropeptide Y Y2-Like Receptor Subtype in Zebrafish and Frogs Supports Early Vertebrate Chromosome Duplications . J Mol Evol 58, 106–114 (2004). https://doi.org/10.1007/s00239-003-2529-z
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00239-003-2529-z