Abstract
Purpose
Cerebral hyperperfusion syndrome (CHS) is a critical complication after carotid artery stenting (CAS). However, few CAS studies have evaluated immediate and temporary changes in ipsilateral cerebral blood flow (CBF) quantitatively. The study was performed to evaluate immediate changes in CBF after CAS and subsequent CBF changes in patients with cerebral hyperperfusion (HP) using 123I-IMP SPECT.
Methods
The subjects were 223 patients with chronic extracranial carotid artery stenosis who underwent CAS in our department between March 2010 and March 2020. Quantitative CBF and cerebrovascular reactivity to acetazolamide in the middle cerebral artery were assessed before CAS by 123I-IMP SPECT. CBF was also measured immediately after CAS by 123I-IMP SPECT. When HP was detected, CBF was measured again 3 and 7 days after CAS.
Results
The median (interquartile range) ipsilateral quantitative CBF change after CAS was − 0.1% (− 9.5–8.2%), and the upper value of the 95% CI of the quantitative CBF change was 48.2%. Thus, we defined HP after CAS as an increase in quantitative CBF of > 48.2% compared with the preoperative value. Of 223 patients, 5 (2.2%) had HP, and 4 of these patients (80%) developed CHS. In the CHS patients, HP was maintained for about 3 days and improved after about 7 days.
Conclusion
An immediate CBF increase of > 48.2% after CAS may lead to development of CHS. In CHS after CAS, HP persisted for about 1 week and postoperative management may be required for at least 1 week.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cerebral hyperperfusion syndrome (CHS) is a significant complication after carotid artery stenting (CAS) and can lead to severe morbidity and mortality [1]. CHS may be caused by focal cerebral damage as a result of cerebral hyperperfusion (HP), which is defined as a major increase in ipsilateral cerebral blood flow (CBF) after revascularization [2]. CHS occurs earlier after CAS than carotid endarterectomy (CEA); that is, CHS has a peak prevalence in the first few postoperative days, compared with postoperative day (POD) 6 for CEA [1, 3]. This difference in onset time suggests that postoperative CBF changes may differ between CEA and CAS.
In assessment of CBF after CEA, ipsilateral increases of > 100% in CBF compared with baseline are related to CHS [2, 4, 5]. For this reason, HP after CEA is defined as an ipsilateral increase in CBF of at least 100% from preoperative CBF. Moreover, patients with an immediate increase of > 100% in CBF after CEA and a further progressive increase of > 200% from baseline after (POD) 3 tend to progress to CHS after CEA [6]. In CAS, most reports have diagnosed CHS based on symptoms, presence of postoperative intracerebral hemorrhage or subarachnoid hemorrhage, and absence of additional ischemic lesions [1], and CBF changes have mainly been evaluated qualitatively. Regarding quantitative ipsilateral CBF changes, positron emission tomography (PET) has shown a rapid increase in mean CBF from 37.6 ml/100 ml/min before CAS to 49.0 ml/100 ml/min in 1–7 days after CAS [7]. However, the changes in ipsilateral CBF immediately after CAS and the time course of these changes in the acute phase of HP are unclear.
Several imaging modalities and techniques are used to investigate HP and CHS, including transcranial Doppler, computed tomography (CT), magnetic resonance imaging (MRI), PET, and single photon emission CT (SPECT). SPECT is reliable for assessment of regional brain perfusion. Current tracers used to measure CBF with SPECT include N-isopropyl-p-[I-123] iodoamphetamine (123I-IMP), 99mTc-hexamethyl-propyleneamine oxime (99mTc-HMPAO), and 99mTc-bicisate ethylcysteinate dimer (99mTc-ECD). Of these tracers, 123I-IMP is reliable for quantification of regional CBF and regional cerebral hemodynamic reserve by measuring the cerebrovascular reactivity (CVR) to acetazolamide challenge [6, 8] and can also be used to detect postoperative HP quantitatively, especially if HP persists for several days [5].
Assessment of CBF after CAS is important in evaluation of HP and prediction of CHS. However, compared with CEA, the quantitative ipsilateral change and time course of CBF after CAS are unclear. In this study, we investigated the association between immediate CBF changes after CAS and the incidence of CHS, and subsequent CBF changes in HP using quantitative 123I-IMP SPECT.
Methods
Patient population
A retrospective chart review was performed for patients with chronic extracranial carotid artery stenosis treated with CAS at our hospital between March 2010 and March 2020. The protocol was approved by the Institutional Review Board (IRB). The requirement for patient consent was waived by the IRB because of the registry-based study design at our institution only and the minimal risk to participants. Patients with extracranial carotid artery stenosis and residual function (mRS 0, 1, or 2) who underwent CAS were enrolled in the study. CAS was indicated if extracranial carotid artery stenosis was > 50% in symptomatic patients or > 70% in asymptomatic patients. The degree of stenosis was calculated from digital subtraction angiography images using the NASCET criteria [9]. Overall, a total of 223 patients were enrolled in the study.
CAS procedure and protocol after CAS
For at least 7 days before CAS, each patient received oral dual antiplatelet treatment of aspirin (100 mg/day), clopidogrel (75 mg/day), or cilostazol (200 mg). Under local anesthesia, all procedures were performed using a femoral approach with an 8-ft-long sheath. At the beginning of the procedure, heparin was given intravenously to achieve an activated clotting time > 250 s. The 8-ft-guiding catheter was introduced into the ipsilateral common carotid artery. Depending on the nature of the lesion, we selected from five types of embolic protection devices: distal balloon protection using PercuSurge GuardWire (Medtronic AVE; n = 112); distal filter protection using FilterWire Ez (Boston Scientific; n = 90); proximal balloon protection using Optimo (Tokai Medical Products) and distal balloon protection using PercuSurge Guardwire (n = 12); proximal balloon protection using Optimo and distal filter protection using FilterWire Ez (n = 5); or proximal balloon protection using Mo.Ma Ultra (Medtronic) and distal filter protection using FilterWire Ez (n = 4). In most cases, a 3.0- or 3.5-mm balloon catheter was used to predilate the stenosis. Three types of stents were placed in the stenotic lesion: Precise (Cordis; n = 74); Protage (Medtronic; n = 20); or Wallstent (Boston Scientific; n = 129). Post-dilation was performed with a percutaneous transluminal angioplasty balloon for residual stenosis. When a distal balloon protection device was used, an aspiration catheter was used to remove debris from the treated vessel before the balloon was deflated and antegrade flow in the vessel was restored. The median total occlusion time was 9 min (8–11 min). The final residual stenosis rate (%) was evaluated using NASCET measurement criteria by complete angiography.
All patients received maintenance oral antiplatelet treatment starting on the day after the procedure. Blood pressure (BP) and heart rate (HR) were monitored continuously in the intraoperative period and appropriately in the neurocritical care unit. Hypotension was defined as systolic blood pressure (SBP) < 90 mmHg, hypertension as SBP > 160 mmHg, and bradycardia as HR < 50 bpm. In the intraoperative period, if hypotension occurred and persisted, a vasopressor was administered intravenously. SBP management was continued in the neurointensive care unit. SBP was maintained at < 140 mmHg after CAS because of concerns of HP. If HP or CHS was diagnosed or suspected, SBP was maintained at < 120 mmHg [10].
Postoperative MRI was performed within 3 days after CAS. A diffusion-weighted imaging (DWI)-positive lesion was defined as a lesion with a high intensity in the hemisphere ipsilateral to the treated carotid artery stenosis territory. Of the 223 patients, 3 with pacemakers were excluded from this analysis because MRI was not performed. Therefore, postoperative DWI-positive lesions were evaluated in 220 patients.
CBF measurements by SPECT
In all cases, preoperative resting CBF (rCBF) and CVR were assessed before CAS within 2 weeks, and rCBF was assessed immediately after CAS; that is, 30 min to 1 h after CAS. Preoperative rCBF and CVR were measured by 123I-IMP SPECT using the dual-table autoradiographic (DTARG) method, and postoperative rCBF was measured by 123I-IMP SPECT using the autoradiographic method, as described in detail elsewhere [11]. For SPECT with drug challenge, acetazolamide (17 mg/kg) was administered intravenously, and CVR is then calculated as follows: CVR = 100 × [(Diamox rCBF—rCBF)/rCBF] [12]. Tomographic images were reconstructed and CBF was quantified using the QSPECT image reconstruction package [13]. Quantitative image data obtained via the QSPECT/DTARG method at rest and after acetazolamide challenge were represented with a three-dimensional stereotactic surface projection [14]. CBF was then measured by two methods using automated region-of-interest (ROI) settings and analytical software: SEE-JET software [15] and NEURO FLEXER [16].
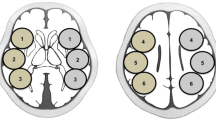
Using the SEE-JET program, CBF was automatically measured at rest and after acetazolamide challenge, and ROIs were automatically set for the territories of the anterior cerebral artery (ACA), middle cerebral artery (MCA), and posterior cerebral artery (PCA) in the brain surface of each hemisphere (Fig. 1A, B) [17]. We measured preoperative rCBF and CVR and postoperative rCBF in the ipsilateral MCA territory. The quantitative CBF change was then calculated as follows: quantitative CBF change (%) = 100 × [(postoperative rCBF − preoperative rCBF)/preoperative rCBF]. In this study, HP was defined as the upper value of the 95% CI of the immediate quantitative CBF change. If HP was detected, rCBF was also measured on PODs 3 and 7.
ROI placement for measuring CBF using the SEE-JET program (A, B) and NEURO FLEXER (C, D). SEE-JET automatically measures CBF in territories of the anterior cerebral artery (light blue), middle cerebral artery (orange), and posterior cerebral artery (yellow) in the brain surface of each hemisphere. NEURO FLEXER automatically sets ROIs in the territories of the middle cerebral artery (C) and cerebellum (D) in the brain parenchyma of each hemisphere
Using the NEURO FLEXER program, CBF was automatically measured and ROIs were set automatically for the territories of the ACA, MCA, PCA, basal ganglia, thalamus, pons, vermis, and cerebellum (Fig. 1C, D). Using rCBF measured automatically in the ROI of the ipsilateral MCA and cerebellum regions in the brain parenchyma, the qualitative CBF ratio (MCA/cerebellum ratio) was calculated as follows: qualitative CBF ratio (%) = 100 × (rCBF in ipsilateral MCA territory/rCBF in ipsilateral cerebellum). The qualitative CBF change was then calculated as follows: postoperative qualitative CBF ratio − postoperative qualitative CBF ratio [18].
CHS was diagnosed by the following criteria: (1) unilateral headache, face pain, eye pain, seizure, deterioration of consciousness, and/or development of focal neurological signs; and (2) immediate increase in CBF after CAS measured using 123I-IMP SPECT without findings of an additional ischemic lesion on postoperative CT or MRI [19].
Statistical analysis
Descriptive statistics are shown as medians (interquartile range (IQR)) and 95% confidence interval (CI). Statistical analysis was performed with JMP Pro 14 for Windows. Continuous and categorical variables were evaluated by Mann–Whitney U test and χ2 test, respectively. A difference with P < 0.05 was defined as significant. Scatter plots and linear regression lines were calculated using Spearman correlation analysis to examine correlations between quantitative and qualitative CBF changes.
Results
Immediate quantitative CBF changes and relationship with CHS
Immediate quantitative CBF changes in all patients are shown in Fig. 2. The median (IQR) of these changes was − 0.1% (− 9.5–8.2%). An increase in quantitative CBF occurred in 111 (49.8%) patients and a decrease occurred in 112 (50.2%). The upper value of the 95% CI of the quantitative CBF changes was 48.2%. Thus, we defined HP after CAS as a quantitative increase in CBF of > 48.2% compared with the preoperative value. Of the 223 patients, 5 (2.2%) had HP. The median (IQR) of the quantitative CBF changes in patients with HP was 73.0% (59.2 − 79.7%). Of the 5 patients with HP, 4 (80%) developed CHS, whereas none of the 218 patients without HP had development of CHS (P < 0.001).
Quantitative CBF changes after CAS in all patients. The median (IQR) increase was − 0.1% (− 9.5–8.2%). The upper value of the 95% CI for the quantitative increase in CBF was 48.2%. HP was defined as an increase of quantitative CBF of > 48.2% (dotted line). There were 5 patients (2.2%) with HP. Four patients with CHS are indicated by arrows
Cases of CHS
Of 4 patients with CHS, case 1 experienced headache 4 h after CAS, with a 59.2% quantitative increase in CBF, case 2 had eye pain and deterioration of consciousness 2 h after CAS, with a 53.7% increase in CBF, case 3 had deterioration of consciousness with restlessness 1 day after CAS, with a 79.7% increase in CBF, and case 4 experienced headache and CT showed intracerebral hemorrhage 2 days after CAS, with a 73.0% increase in CBF, as shown in Fig. 3.
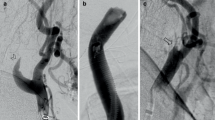
Angiogram in a 61-year-old man with severe left extracranial carotid artery stenosis with asymptomatic stenosis (83%) (A). A post-CAS angiogram showed that stenosis improved (B). Preoperative 123I-IMP SPECT at rest (C) and post-acetazolamide challenge (D) indicated a resting CBF of 23.77 mL/100 g/min and CVR of 6.6% in the middle cerebral artery territory, as measured by the SEE-JET program. 123I-IMP SPECT immediately (E), 3 days (F), and 7 days (G) after CAS showed ipsilateral increases in quantitative CBF of 41.12, 38.99, and 34.40 mL/min/100 g, respectively, again measured with the SEE-JET program. CT on postoperative day 2 showed intracranial hemorrhage on the ipsilateral side (H)
Time course of quantitative CBF changes of HP
Time courses of quantitative CBF changes, which are immediately, 3 days, and 7 days after CAS in the 5 patients with HP, are shown in Fig. 4. In the 4 patients with CHS, HP persisted and CBF slowly decreased to < 48.2% from days 3 to 7. In the non-CHS case with HP, CBF rapidly decreased to < 48.2% within 3 days.
Time courses of postoperative quantitative CBF increases in 5 patients with HP. Four patients in whom a CBF increase of > 48.2% (dotted line) was maintained for over 3 days developed CHS (arrows), and in these cases HP improved after 3 to 7 days. In contrast, in the non-CHS case, HP rapidly improved within 3 days
Qualitative CBF changes and comparison with quantitative CBF changes
Immediate qualitative CBF changes in all patients are shown in Fig. 5. The median (IQR) of these changes was − 0.3% (− 7.0–8.1%) and the upper value of the 95% CI was 28.3%. In the 4 CHS cases, the increases in qualitative CBF were 38.0% in case 1, 28.5% in case 2, 22.2% in case 3, and 15.2% in case 4; that is, there was variation in qualitative CBF changes in the 4 patients with CHS. There was a correlation between quantitative and qualitative CBF changes (R2 = 0.442), as shown by the scatter plot and linear regression line in Fig. 6.
Qualitative CBF changes after CAS in all patients. The median (IQR) increase was − 0.3% (− 7.0–8.1%). The upper value of the 95% CI for the increase in qualitative CBF was 28.3% (dotted line). Of 4 patients with CHS (arrows), 2 (50.0%) had a qualitative increase in CBF > 95% CI and 2 (50.0%) had an increase < 95% CI
Correlation of quantitative and qualitative CBF changes in a scatter plot with a linear regression line. The 95% CIs are shown for quantitative CBF changes (horizontal dotted line) and qualitative CBF changes (vertical dotted line). Correlation coefficient: R2 = 0.442. Four patients with CHS are indicated by arrows
Patient demographics and comorbidities
Of the 223 patients in the study (Table 1), most were male (n = 192, 86.1%) and the median age was 74 years. The underlying conditions were hypertension (n = 190, 85.2%), diabetes mellitus (n = 83, 37.2%), and dyslipidemia (n = 125, 56.1%); and 148 patients (66.4%) had symptomatic events. NASCET was 81.5% (72.5–90.0%), preoperative rCBF was 29.4 (25.5–33.5) mL/100 g/min, and CVR was 22.0% (7.7–36.5%). Intraoperative hypotension and bradycardia occurred in 102 (45.7%) and 70 (31.4%) cases, and hypotension and hypertension at the end of CAS were present in 27 (12.1%) and 21 (9.4%) cases, respectively. The final residual stenosis rate was 6.0% (0.0–19.5%). Of 220 patients who underwent MRI, 20 (9.1%) had a postoperative DWI-positive lesion, and 3 patients with pacemakers did not develop HP.
The 5 patients with HP were significantly more frequently female (60.0% vs. 12.8%, P = 0.020), had a significantly lower CVR [− 3.2% (− 27.0–27.6%) vs. 22.3% (8.3–36.6%), P < 0.016], and had a significantly higher SBP at the end of CAS [150 (132–152) vs. 122 (103–142) mmHg, P < 0.032].
Discussion
This study quantified ipsilateral CBF changes after CAS using 123I-IMP SPECT. CHS after CAS is most common in patients with an immediate quantitative increase of CBF of > 48.2% from baseline. In CHS patients, an increase in CBF > 48.2% lasted for 3 to 7 days. This is the first report of measurement of ipsilateral changes and the time course of CBF after CAS using quantitative 123I-IMP SPECT.
In studies of CBF in CEA, HP is usually defined as an increase of ipsilateral CBF of > 100% using 123I-IMP SPECT, and CHS is most common in patients with HP [4, 5]. Unlike the CBF change after CEA, our study showed that cases of CHS after CAS do not have an increase in CBF of > 100%. An ipsilateral increase of > 48.2% in CBF using 123I-IMP SPECT was sufficient to cause CHS and a change of CBF of < 48.2% did not result in development of CHS. This suggests that a lower increase in CBF may lead to CHS after CAS compared to that after CEA. The exact mechanism of CHS is unclear, but two interlinked and synergistic mechanisms may lead to an increase in CBF: impairment of autoregulation and postoperative hypertension [1, 5, 20]. The difference in increased CBF between CEA and CAS may be caused by the difference between these two mechanisms.
A preoperative decrease in CVR is a significant risk factor in both CEA and CAS [1, 5]. Moreover, both preoperative reduced CVR and intraoperative cerebral ischemia during clamping of the ICA in CEA are involved in deterioration of autoregulatory mechanisms and occurrence of HP [21, 22]. Thus, in addition to preoperative chronic ischemia, intraoperative ischemia affects CBF changes. In this study, a preoperative decrease in CVR was significantly associated with HP, as seen in CEA. However, regarding intraoperative ischemia, the occlusion time in CAS (about 9 min), even if using a distal balloon, was similar to those in previous studies [23, 24], and shorter than the clamping time in CEA (about 30 min) [21, 22, 25]. Thus, intraoperative ischemia is likely to be milder in CAS and the shorter intraoperative period of ischemia may partly explain the lower elevation of CBF after CAS compared to that after CEA.
Postoperative hypertension has also been associated with CHS after CEA [20]. In this study, postoperative elevation of SBP was significantly associated with HP. There are two factors that affect BP after CEA and CAS: baroreflex and catecholamines, and these differ between CEA and CAS. CEA causes a baroreceptor reflex failure [26] and much higher surges of catecholamines [27]. However, CAS causes baroreceptor stimulation by the balloon and stent [28] and a less marked catecholamine response [27]. These different responses of baroreceptors and catecholamines may influence postoperative hemodynamics and the extent of the increases in CBF in CAS and CEA.
A major difference in CHS between CEA and CAS is the peak onset time. In CEA, CHS peaks on POD 6, whereas in CAS, the peak is in the first few days [1, 3]. Regarding CBF change after CEA, patients with an immediate increase of > 100% in CBF after CEA using 123I-IMP SPECT and a progressive increase of > 200% after POD 3 tend to develop CHS [5]. In this study, patients with an immediate increase of > 48.2% in CBF after CAS and a sustained increase of > 48.2% after POD 3 tended to develop CHS. The time course after CEA and CAS differs in patterns of postoperative BP responses influenced by baroreceptors or catecholamines [26,27,28,29]. Thus, there is a possibility that the time course of CBF changes after CEA and CAS also differs, and these different time courses may be related to the different peak onset times of CHS after CEA and CAS. In CEA, a subsequent progressive increase in CBF after several days may cause the peak onset time on POD 6 in CHS. Moreover, in CAS, the absence of a progressive increase in CBF may reflect CHS not increasing beyond the first few days. This clinical feature seems to be related to the different time courses in CBF changes found for CEA in a previous study [5] and for CAS in the current study.
In post-CAS management, strict control of BP is recommended during the postoperative period to prevent HP and CHS [10]. However, the timing and duration of intensive treatment for hypertension are uncertain. In this study, HP was associated with postoperative SBP elevation. Regarding the timing of intensive BP lowering therapy, the current study suggests that this treatment should be started as soon as possible after CAS, especially in cases at high risk for HP. For the treatment duration, strict control of BP is recommended until cerebral autoregulation is restored. The current study showed that HP persisted for about 1 week in CHS patients. Thus, for HP in CHS, antihypertensive therapy is suggested to be required for at least 1 week.
About half of the patients in the study had immediate quantitative decreases in CBF despite revascularization. The exact reason for the CBF decrease after CAS is uncertain; however, the following two mechanisms are conceivable to explain hypoperfusion after CAS. First, hypotension due to a baroreceptor reflex failure is a concern and systemic hypotension could lead to decreased CBF when it exceeds the cerebral autoregulation capacity [30, 31]. Second, there may be ischemic complications such as microembolism during the procedure or stent thrombosis, which may lead to occurrence of no territory of reperfusion [32]. In this study, cases without HP with a decrease in CBF had a significantly higher decrease in SBP at the end of CAS and more postoperative DWI-positive lesions. Thus, hypotension or thromboembolic complications may affect the decrease in CBF.
There have been many reports on qualitative CBF changes in the contralateral MCA or ipsilateral cerebellum, and the MCA/cerebellum ratio change has been shown to be useful for prediction of CHS [18]. In this study, patients with CHS had increased qualitative CBF changes and there was a correlation between quantitative and qualitative CBF changes; however, there was also more variability in the qualitative CBF changes in patients with CHS compared with quantitative CBF changes. Therefore, our results suggest that quantitative CBF changes may be more useful for prediction of CHS after CAS.
There are several limitations of this study. First, the results should be carefully interpreted because of the small study population at a single center. More cases are required to validate the findings. Second, we examined CBF only using 123I-IMP SPECT, rather than transcranial Doppler, CT, MRI, or PET. Changes in CBF after CAS require validation by methods other than 123I-IMP SPECT. Third, the definition of HP was given by the upper value of the 95%CI. The small number of cases causes may result in overfitting, and it is necessary to accumulate more cases. Fourth, 123I-IMP SPECT measurement of CBF after CAS on PODs 3 and 7 was limited to patients with HP or CHS. Thus, the time course in patients without HP was not determined. Fifth, we examined hospital outcomes and did not observe delayed CHS after CAS [1], but 8% of cases of CHS after CAS are reported to occur after hospital discharge. Thus, the time course of CBF changes in this study does not represent all cases of CAS. Sixth, the HP patients were mainly female. Gender differences have been shown to not be associated with CHS [1], but evaluation in more cases may be important to validate the results.
Conclusion
An immediate quantitative increase of > 48.2% in ipsilateral CBF measured by 123I-IMP SPECT may cause development of CHS. In CHS after CAS, HP persisted for about 1 week and postoperative management may be required for at least 1 week.
Availability of data and material
Data will be made available on request.
Code availability
Not applicable.
References
Huibers AE, Westerink J, de Vries EE et al (2018) Cerebral hyperperfusion syndrome after carotid artery stenting: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg 56:322–333
Piepgras DG, Morgan MK, Sundt TM Jr et al (1988) Intracerebral hemorrhage after carotid endarterectomy. J Neurosurg 68:532–536
Ogasawara K, Sakai N, Kuroiwa T et al (2007) Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting: retrospective review of 4494 patients. J Neurosurg 107:1130–1136
van Mook WN, Rennenberg RJ, Schurink GW et al (2005) Cerebral hyperperfusion syndrome. Lancet Neurol 4:877–888
Ogasawara K, Yukawa H, Kobayashi M et al (2003) Prediction and monitoring of cerebral hyperperfusion after carotid endarterectomy by using single-photon emission computerized tomography scanning. J Neurosurg 99:504–510
Kuhl DE, Barrio JR, Huang SC et al (1982) Quantifying local cerebral blood flow by N-isopropyl-p-[123I]iodoamphetamine (IMP) tomography. Nucl Med 23:196–203
Matsubara S, Moroi J, Suzuki A et al (2009) Analysis of cerebral perfusion and metabolism assessed with positron emission tomography before and after carotid artery stenting. J Neurosurg 11:28–36
Nishizawa S, Tanada S, Yonekura Y et al (1989) Regional dynamics of N-isopropyl-(123I)p-iodoamphetamine in human brain. Nucl Med 30:150–156
North American Symptomatic Carotid Endarterectomy Trial (1991) Methods, patient characteristics, and progress. Stroke 22:711–720
Abou-Chebl A, Reginelli J, Bajzer CT et al (2007) Intensive treatment of hypertension decreases the risk of hyperperfusion and intracerebral hemorrhage following carotid artery stenting. Catheter Cardiovasc Interv 69:690–696
Oka F, Ishihara H, Kato S et al (2013) Cerebral hemodynamic benefits after contralateral carotid artery stenting in patients with internal carotid artery occlusion. AJNR Am J Neuroradiol 34:616–621
Yamada S, Kobayashi M, Watanabe Y et al (2014) Quantitative measurement of blood flow volume in the major intracranial arteries by using 123i-iodoamphetamine SPECT. Clin Nucl Med 39:868–873
Kim KM, Watabe H, Hayashi T et al (2006) Quantitative mapping of basal and vasareactive cerebral blood flow using split-dose 123I-iodoamphetamine and single photon emission computed tomography. Neuroimage 33:1126–1135
Minoshima S, Frey KA, Koeppe RA et al (1995) A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med 36:1238–1248
Mizumura S, Nakagawara J, Takahashi M et al (2004) Three-dimensional display in staging hemodynamic brain ischemia for JET study: objective evaluation using SEE analysis and 3D-SSP display. Ann Nucl Med 18:13–21
Ogura T, Hida K, Masuzuka T et al (2009) An automated ROI setting method using Neurostat on cerebral blood flow SPECT images. Ann Nucl Med 23:33–41
Minoshima S, Koeppe RA, Frey KA et al (1994) Anatomic standardization: linear scaling and nonlinear warping of functional brain images. J Nucl Med 35:1528–1537
Iwata T, Mori T, Tajiri H et al (2011) Predictors of hyperperfusion syndrome before and immediately after carotid artery stenting in single-photon emission computed tomography and transcranial color-coded real-time sonography studies. Neurosurgery 68:649–655
Wang S, Han J, Cheng L et al (2017) Risk factors and preventive measures of cerebral hyperperfusion syndrome after carotid artery interventional therapy. Exp Ther Med 14:2517–2520
Bouri S, Thapar A, Shalhoub J et al (2011) Hypertension and the post-carotid endarterectomy cerebral hyperperfusion syndrome. Eur J Vasc Endovasc Surg 41:229–237
Komoribayashi N, Ogasawara K, Kobayashi M et al (2006) Cerebral hyperperfusion after carotid endarterectomy is associated with preoperative hemodynamic impairment and intraoperative cerebral ischemia. J Cereb Blood Flow Metab 26:878–884
Suga Y, Ogasawara K, Saito H et al (2007) Preoperative cerebral hemodynamic impairment and reactive oxygen species produced during carotid endarterectomy correlate with development of postoperative cerebral hyperperfusion. Stroke 38:2712–2717
Horie N, Kitagawa N, Morikawa M et al (2005) Monitoring of regional cerebral oxygenation by near-infrared spectroscopy in carotid arterial stenting: preliminary study. Neuroradiology 47:375–379
Schluter M, Tubler T, Mathey DG et al (2002) Feasibility and efficacy of balloon-based neuroprotection during carotid artery stenting in a single-center setting. J Am Coll Cardiol 40:890–895
Kuroda H, Ogasawara K, Hirooka R et al (2009) Prediction of cerebral hyperperfusion after carotid endarterectomy using middle cerebral artery signal intensity in preoperative single-slab 3-dimensional time-of-flight magnetic resonance angiography. Neurosurgery 64:1065–1071
Timmers HJLM, Wieling W, Karemaker JM et al (2004) Baroreflex failure: a neglected type of secondary hypertension. Neth J Med 62:151–155
Barry MC, Hendriks JM, Alberts G et al (2004) Comparison of catecholamine hormone release in patients undergoing carotid artery stenting or carotid endarterectomy. Endovasc Ther 11:240–250
Dangas G, Laird JR, Satler LF et al (2000) Postprocedural hypotension after carotid artery stent placement: predictors and short- and long-term clinical outcomes. Radiology 215:677–683
Lim S, Javorski MJ, Nassoiy SP et al (2019) Long-term hemodynamic effects after carotid artery revascularization. Vasc Endovascular Surg 53:297–302
McKevitt FM, Sivaguru A, Venables GS et al (2003) Effect of treatment of carotid artery stenosis on blood pressure: a comparison of hemodynamic disturbances after carotid endarterectomy and endovascular treatment. Stroke 34:2576–2581
Bladin CF, Chambers BR (1994) Frequency and pathogenesis of hemodynamic stroke. Stroke 25(2):2179–2182
Orlandi G, Fanucchi S, Fioretti C et al (2001) Characteristics of cerebral microembolism during carotid stenting and angioplasty alone. Arch Neurol 58:1410–1413
Author information
Authors and Affiliations
Contributions
T.N. studied the concept and design and helped in the analysis and interpretation and preparation of the article. F.O. helped in the interpretation of data and critical revision of the article for important intellectual content. K.O. carried out data analysis and interpretation and helped in the preparation of the article. H.I. helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or non-financial interests to disclose.
Ethical approval
This study was conducted retrospectively from data obtained for clinical purposes. The study was approved by the IRB of Yamaguchi University School of Medicine.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nishimoto, T., Oka, F., Okazaki, K. et al. Relationship between cerebral hyperperfusion syndrome and the immediate change of cerebral blood flow after carotid artery stenting evaluated by single-photon emission computed tomography. Neuroradiology 64, 1157–1164 (2022). https://doi.org/10.1007/s00234-021-02822-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02822-8