Abstract
Purpose
The lifetime risk of developing amyotrophic lateral sclerosis (ALS) increases in the elderly, and greater age at symptom onset has been identified as a negative prognostic factor in the disease. However, the underlying neurobiological mechanisms are still poorly investigated. We hypothesized that older age at symptom onset would have been associated with greater extra-motor cortical damage contributing to worse prognosis, so we explored the relationship between age at symptom onset, cortical thinning (CT) distribution, and clinical markers of disease progression.
Methods
We included 26 ALS patients and 29 healthy controls with T1-weighted magnetic resonance imaging (MRI). FreeSurfer 6.0 was used to identify regions of cortical atrophy (CA) in ALS, and to relate age at symptom onset to CT distribution. Linear regression analyses were then used to investigate whether MRI metrics of age-related damage were predictive of clinical progression. MRI results were corrected using the Monte Carlo simulation method, and regression analyses were further corrected for disease duration.
Results
ALS patients exhibited significant CA mainly encompassing motor regions, but also involving the cuneus bilaterally and the right superior parietal cortex (p < 0.05). Older age at symptom onset was selectively associated with greater extra-motor (frontotemporal) CT, including pars opercularis bilaterally, left middle temporal, and parahippocampal cortices (p < 0.05), and CT of these regions was predictive of shorter survival (p = 0.004, p = 0.03).
Conclusion
More severe frontotemporal CT contributes to shorter survival in older ALS patients. These findings have the potential to unravel the neurobiological mechanisms linking older age at symptom onset to worse prognosis in ALS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by the progressive loss of upper and lower motor neurons in the cerebral cortex, brainstem, and spinal cord.
The mean or median age at symptom onset is highly variable, typically ranging from 51 to 66 years [1, 2]. This variability is also influenced by genetic factors, with younger age of onset reported in familial forms of ALS, and older age of onset observed in patients with sporadic forms of the disease [1], who represent the vast majority of cases [3].
ALS is an age-related neurodegenerative disease. As such, the lifetime risk of developing the disease increases in the elderly, even if it has been suggested that above the age of 75 years there may be a decline in incidence [2].
Notably, recent studies further suggest that age at symptom onset might influence a wide range of clinical features. Older ALS patients more frequently develop frontotemporal dementia (FTD) [4,5,6], exhibit a generalized onset, and manifest faster disease progression and shorter survival compared to younger cases [7, 8].
Despite these considerations, the neurobiological mechanisms contributing to worse prognosis in ALS patients with older age at symptom onset are still largely unknown.
Cross-sectional structural magnetic resonance imaging (MRI) studies have shown that ALS is a multisystem disorder, with cortical atrophy encompassing motor and extra-motor regions [9, 10]. However, recent longitudinal investigations suggest that, over the course of the disease, motor cortical atrophy does not further advance [11], while extra-motor brain regions further degenerate [12, 13].
It is therefore plausible to hypothesize that what contributes to the faster disease course observed in older ALS patients might be a more severe age-related involvement of extra-motor brain regions from the onset of the disease.
In this work, we therefore aimed at using structural MRI to test the hypothesis that older age at symptom onset would have been associated with greater extra-motor cortical degeneration leading to worse prognosis in ALS.
Methods
Subjects
As part of a larger study established in 2009 and still in operation, all patients diagnosed with ALS at our Center, who agree to participate, undergo a thorough baseline clinical evaluation (all the details are provided below) and are followed longitudinally with clinical evaluations at regular intervals (2–4 months). A standard form is used for collecting clinical information at each follow-up visit, including therapeutic interventions, and all the evaluations are performed by a team of experienced neurologists (C.C., C.C., and G.M.) operating at the Center.
From this large dataset, the present retrospective study selected all the ALS cases who were meeting the following inclusion criteria: (1) a diagnosis of possible, probable, or definite ALS according to revised El Escorial criteria [14]; (2) complete clinical characterization including time of symptom onset, age at symptom onset, and site of symptom onset (bulbar or spinal); (3) MRI examination with 3D T1-weighted sequences; (4) full baseline clinical evaluation performed either on the same date of the MRI examination, or within a maximum of 30 days from the MRI scan, including the ALS functional rating scale revised (ALSFRS-r) [15], the Medical Research Council (MRC) scale, and the clinical progression rate estimation (calculated with the formula (48 − ALFRS − r score at the time of the evaluation)/disease duration); and (5) absence of overt FTD or clinical evidence of cognitive/behavioral impairment (including negative informant report of behavioral and/or social deficits).
An additional sample of demographically matched healthy controls (HC) was selected from our HC dataset which includes all subjects recruited among spouses of patients and by word of mouth fulfilling the following inclusion criteria: (1) absence of any psychiatric and/or neurological disorder (as ascertained from clinical interview, neurological assessment, and absence of any impairment in daily life activities), (2) absence of any history of cerebrovascular events, (3) MRI examination with 3D T1-weighted sequences, and (4) no territorial infarcts nor significant small vessel disease at routine MRI (Fazekas score > 1) [16].
These selection procedures resulted in the final sample of the present study: 26 ALS patients (11 females, 15 males; mean age 61.26 years) and 29 healthy controls (HC) (10 females, 19 males; mean age 59.55 years).
Written informed consent was obtained from each participant according to the institution’s procedures and the Declaration of Helsinki. Retrospective analysis of the data was approved by the Hospital Review Board.
MRI acquisition
All subjects underwent a routine MRI protocol, including a standardized T1-weighted brain volumetric scan acquired with a 1.5-T system (SIGNA; General Electric Healthcare, Milwaukee, WI, USA) using an eight-channel high-resolution brain array coil. All the clinical scans were reviewed by an experienced neuroradiologist to exclude the presence of pathological findings independent from ALS.
The T1 volumetric sequence obtained was a sagittal three-dimensional spoiled gradient recalled (3D SPGR) sequence with the following parameters: TR 28 ms, TE 6 ms, flip angle 30°, FOV 256 × 256 mm, in-plane matrix 256 × 256, slice thickness 1.3 mm.
MRI analysis
Cortical reconstruction and estimation of cortical thickness were performed on the 3D SPGR images using the FreeSurfer image analysis suite, version 6.0 (http://surfer.nmr.mgh.harvard.edu/) [17]. After registration to Talairach space and intensity normalization, the process involved automatic skull stripping, which removes extra-cerebral structures, cerebellum, and brainstem, by using a hybrid method combining watershed algorithms and deformable surface models. Images were then carefully checked for skull stripping errors. After this step, images were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF); cerebral hemispheres were separated; and subcortical structures divided from cortical components. The WM/GM boundary was tessellated, and the surface was deformed following intensity gradients to optimally place WM/GM and GM/CSF borders, thus obtaining the WM and pial surfaces [18].
The results of this segmentation procedure were inspected visually, and, afterward, surface inflation and registration to a spherical atlas were performed [18]. Finally, cortical thickness was estimated as the average shortest distance between the WM boundary and the pial surface.
Statistical analysis
All data were analyzed using CRAN R Version 3.4.1. Normal distribution assumption was checked by means of Shapiro-Wilk test. T tests and Chi-square tests were applied, as appropriate, to compare demographic features between ALS patients and HC.
Using general linear models in FreeSurfer, a vertex-by-vertex analysis was used to assess differences of cortical thickness between ALS patients and HC, and to perform univariate regression analyses relating age at symptom onset to cortical thinning in the ALS group.
Mean cortical thickness values from regions showing cortical thinning related to older age at onset were then entered in linear regression models to test their role as significant predictors of clinical markers of poor prognosis (clinical progression rate and survival (calculated as the time in months from symptom onset to death)).
All the MRI results were corrected for multiple comparisons using the Monte Carlo simulation method implemented in FreeSurfer (p < 0.05) and regression analyses were further corrected for disease duration at the time of the MRI exam.
Results
Demographic and clinical features
ALS patients and HC were matched in terms of age and gender (Table 1). The majority of patients (N = 21, 80.76%) had a spinal onset form of ALS, the mean age at symptom onset was 60.07 years (SD = 9.56), mean disease duration was 14.69 months (SD = 12.22), mean ALSFRS-r score was 39.95 (SD = 5.35), mean MRC score was 131.42 (SD = 17.47), and the mean clinical progression rate was 0.72 (SD = 0.64) (Table 1). Two patients were found to carry ALS-associated genetic mutations (C9orf72 expansion in one case, and TARDBP mutation in the other case).
MRI findings
Cortical thickness comparison between ALS patients and healthy controls
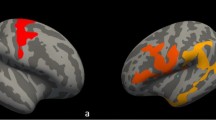
Compared to HC, ALS patients showed cortical atrophy mainly encompassing motor regions (precentral and paracentral cortices bilaterally) (p < 0.05) (Fig. 1).
Peaks of cortical atrophy were also observed in the cuneus bilaterally as well as in the right superior parietal cortex (p < 0.05) (Fig. 1). Results remained significant when we applied a more stringent threshold (p < 0.01). When we repeated the analysis excluding the genetic cases (N = 2), the results remained largely unchanged (Supplementary Figure 1).
Cortical thinning related to older age at symptom onset in ALS patients
Older age at symptom onset was selectively associated with a predominant frontotemporal pattern of cortical thinning, including pars opercularis bilaterally as well as left middle temporal and parahippocampal cortices (p < 0.05) (Fig. 2). When we repeated the analysis excluding the genetic cases (N = 2), the results remained largely unchanged (Supplementary Figure 2).
Prognostic role of age-related cortical thinning
Mean cortical thickness values of the left parahippocampal and middle temporal cortices were significant predictors of shorter survival (respectively, coeff = 0.03, t value = 4.15, p < 0.001, and coeff = 0.03, t value= 3.43, p = 0.002) (Fig. 3). No significant associations were observed between age-related cortical thickness measures and the rate of clinical progression.
Discussion
To our knowledge, this is the first study investigating the cortical correlates of older age at symptom onset in ALS and exploring their prognostic role in the disease.
Our results confirmed the initial hypothesis of greater extra-motor cortical degeneration related to older age at symptom onset, and further revealed a focal frontotemporal distribution of disease burden. Moreover, in line with our expectation, the degree of extra-motor damage was predictive of worse prognosis.
Relative to HC, cortical atrophy in the ALS group mainly encompassed motor regions, with a widespread bilateral pattern, in accordance with classical MRI findings in the disease [19,20,21].
However, in line with the emerging literature of extra-motor cortical involvement in ALS [9, 10], we further found peaks of cortical atrophy in the parietal and occipital areas, confirming that cortical pathology in ALS also extends beyond the motor cortex.
Intriguingly, while widespread cortical involvement was observed compared to HC, the regression analysis with age at symptom onset revealed a focal, frontotemporal pattern of atrophy.
ALS onset has been postulated to be driven by a progressive damage of specific central nervous system (CNS) regions. While this damage might remain subclinical for several decades, it makes those affected especially prone to the consequences of age-related neuronal attrition [2]. Neural degeneration is indeed a gradual process, and it is plausible that it must rely on a vulnerable substrate in order to propagate. In line with this observation, our finding of greater frontotemporal damage associated with older age at symptom onset suggests that previously reported age-related frontotemporal atrophy [22] might provide a vulnerable substrate for faster and more severe disease propagation in older ALS patients. Accordingly, we further found that age-related frontotemporal thinning exerted a significant negative impact on survival, providing a neurobiological explanation for the worse prognosis frequently observed in older cases.
Furthermore, the anatomical distribution of cortical damage in our study well matches with other negative prognostic features previously reported in older ALS patients.
Firstly, patients with older age at symptom onset more frequently develop cognitive impairment or overt FTD over the course of the disease [4,5,6]. Accordingly, in older patients, we observed greater cortical thinning encompassing frontal regions bilaterally and left temporal cortices, in line with the previously reported patterns of cortical atrophy in overt FTD [23] and ALS-FTD cases [24]. Moreover, the same pattern of atrophy has been recently found to be associated with the development of cognitive and behavioral deficits in ALS [25].
Secondly, older age at symptom onset has been consistently identified as a predictor of worse outcome in ALS [7, 8].
Our observation of greater age-related pars opercularis thinning is therefore in agreement with previous evidences of a significant association between more extensive atrophy in extra-motor gray matter regions, pars opercularis in particular [26], and a faster rate of disease progression in ALS.
The greater extra-motor cortical thinning we observed and its relation to shorter survival might also suggest a more advanced disease state in patients with older age at onset, in agreement with recent longitudinal studies reporting a progressive involvement of frontotemporal regions related to disease progression in ALS [12, 13].
Notably, by embedding disease duration as a covariate in the statistical model, we were able to rule out a possible effect of longer symptom duration on the observed extra-motor pattern of atrophy. Our results, indeed, point toward the hypothesis of a greater burden of subclinical cortical damage in patients with older age at disease onset.
Intriguingly, the triggering factors of this subclinical damage are still unknown, even if an interindividual heterogeneity in susceptibility to ageing might contribute to the explanation, with recent evidences suggesting that certain individuals age better and others worse than expected, termed “delta aging” [27, 28].
This exploratory study is not without limitations. The first shortcoming deals with the relatively small ALS sample size. Patients were indeed selected based on the availability of homogeneous T1-weighted sequences and complete clinical characterization, as well as full clinical evaluation within a maximum of 30 days from the date of the MRI exam. As a result, the intrinsic variability of the ALS sample in terms of age at MRI could not be mitigated by further selecting more homogeneous cases. The second limitation is the absence of a standardized cognitive evaluation to better characterize the cognitive status of the included patients. While all patients had no clinical evidence of overt FTD or cognitive impairment, it is not possible to rule out the presence of subtle neuropsychological deficits in some of the cases. Yet, the mild degree of eventual cognitive alterations in a subset of patients would not be expected to significantly influence the obtained MRI findings. Despite these limitations, our work provides a preliminary contribution to unravel the neurobiological mechanisms linking older age at symptom onset to worse prognosis in ALS.
Conclusions
In conclusion, our study demonstrated the presence of significant age-related brain structural changes in ALS, and further proved that these alterations are predictive of reduced survival in this devastating disease. Future studies in larger, ideally more homogeneous, and deeply phenotyped samples are warranted to further prove our findings, and to investigate genetic and environmental factors triggering the onset of the clinical manifestations of the disease.
Code availability
Not applicable.
Data Availability
Raw data are available upon appropriate request.
References
Longinetti E, Fang F (2019) Epidemiology of amyotrophic lateral sclerosis: an update of recent literature. Curr Opin Neurol 32(5):771–776. https://doi.org/10.1097/WCO.0000000000000730
Turner MR, Barnwell J, Al-Chalabi A, Eisen A (2012) Young-onset amyotrophic lateral sclerosis: historical and other observations. Brain 135(Pt 9):2883–2891. https://doi.org/10.1093/brain/aws144
Renton AE, Chiò A, Traynor BJ (2014) State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 17(1):17–23. https://doi.org/10.1038/nn.3584
Lomen-Hoerth C, Murphy J, Langmore S, Kramer JH, Olney RK, Miller B (2003) Are amyotrophic lateral sclerosis patients cognitively normal? Neurology 60(7):1094–1097. https://doi.org/10.1212/01.wnl.0000055861.95202.8d
Irwin D, Lippa CF, Swearer JM (2007) Cognition and amyotrophic lateral sclerosis (ALS). Am J Alzheimers Dis Other Dement 22(4):300–312. https://doi.org/10.1177/1533317507301613
Montuschi A, Iazzolino B, Calvo A, Moglia C, Lopiano L, Restagno G, Brunetti M, Ossola I, Lo Presti A, Cammarosano S, Canosa A, Chio A (2015) Cognitive correlates in amyotrophic lateral sclerosis: a population-based study in Italy. J Neurol Neurosurg Psychiatry 86(2):168–173. https://doi.org/10.1136/jnnp-2013-307223
Chio A, Logroscino G, Hardiman O, Swingler R, Mitchell D, Beghi E et al (2009) Prognostic factors in ALS: a critical review. Amyotroph Lateral Scler 10(5-6):310–323. https://doi.org/10.3109/17482960802566824
Calvo A, Moglia C, Lunetta C, Marinou K, Ticozzi N, Ferrante DG et al (2017) Factors predicting survival in ALS: a multicenter Italian study. J Neurol 264(1):54–63. https://doi.org/10.1007/s00415-016-8313-y
Grosskreutz J, Kaufmann J, Frädrich J, Dengler R, Heinze HJ, Peschel T (2006) Widespread sensorimotor and frontal cortical atrophy in amyotrophic lateral sclerosis. BMC Neurol 6:17. https://doi.org/10.1186/1471-2377-6-17
Agosta F, Ferraro PM, Riva N, Spinelli EG, Chio’ A, Canu E et al (2016) Structural brain correlates of cognitive and behavioral impairment in MND. Hum Brain Mapp 37(4):1614–1626. https://doi.org/10.1002/hbm.23124
Schuster C, Kasper E, Machts J, Bittner D, Kaufmann J, Benecke R, Teipel S, Vielhaber S, Prudlo J (2014) Longitudinal course of cortical thickness decline in amyotrophic lateral sclerosis. J Neurol 261(10):1871–1880. https://doi.org/10.1007/s00415-014-7426-4
Walhout R, Westeneng HJ, Verstraete E, Hendrikse J, Veldink JH, van den Heuvel MP, van den Berg LH (2015) Cortical thickness in ALS: towards a marker for upper motor neuron involvement. J Neurol Neurosurg Psychiatry 86(3):288–294. https://doi.org/10.1136/jnnp-2013-306839
Senda J, Atsuta N, Watanabe H, Bagarinao E, Imai K, Yokoi D, Riku Y, Masuda M, Nakamura R, Watanabe H, Ito M, Katsuno M, Naganawa S, Sobue G (2017) Structural MRI correlates of amyotrophic lateral sclerosis progression. J Neurol Neurosurg Psychiatry 88(11):901–907. https://doi.org/10.1136/jnnp-2016-314337
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1(5):293–299. https://doi.org/10.1080/146608200300079536
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 169(1-2):13–21. https://doi.org/10.1016/s0022-510x(99)00210-5
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149(2):351–356. https://doi.org/10.2214/ajr.149.2.351
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97(20):11050–11055. https://doi.org/10.1073/pnas.200033797
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9(2):179–194. https://doi.org/10.1006/nimg.1998.0395
Mezzapesa DM, D’Errico E, Tortelli R, Distaso E, Cortese R, Tursi M, Federico F, Zoccolella S, Logroscino G, Dicuonzo F, Simone IL (2013) Cortical thinning and clinical heterogeneity in amyotrophic lateral sclerosis. PLoS One 8(11):e80748. https://doi.org/10.1371/journal.pone.0080748
Bede P, Bokde A, Elamin M, Byrne S, McLaughlin RL, Jordan N et al (2013) Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): a neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. J Neurol Neurosurg Psychiatry 84(7):766–773. https://doi.org/10.1136/jnnp-2012-302674
Qin Y, Zhang S, Jiang R, Gao F, Tang X, Zhu W (2018) Region-specific atrophy of precentral gyrus in patients with amyotrophic lateral sclerosis. J Magn Reson Imaging 47(1):115–122. https://doi.org/10.1002/jmri.25765
Chow TW, Binns MA, Freedman M, Stuss DT, Ramirez J, Scott CJ et al (2008) Overlap in frontotemporal atrophy between normal aging and patients with frontotemporal dementias. Alzheimer Dis Assoc Disord 22(4):327–335. https://doi.org/10.1097/WAD.0b013e31818026c4
Omer T, Finegan E, Hutchinson S, Doherty M, Vajda A, McLaughlin RL et al (2017) Neuroimaging patterns along the ALS-FTD spectrum: a multiparametric imaging study. Amyotroph Lateral Scler Frontotemporal Degener 18(7-8):611–623. https://doi.org/10.1080/21678421.2017.1332077
Lillo P, Mioshi E, Burrell JR, Kiernan MC, Hodges JR, Hornberger M (2012) Grey and white matter changes across the amyotrophic lateral sclerosis-frontotemporal dementia continuum. PLoS One 7(8):e43993. https://doi.org/10.1371/journal.pone.0043993
Consonni M, Contarino VE, Catricala’ E, Dalla Bella E, Pensato V, Gellera C et al (2018) Cortical markers of cognitive syndromes in amyotrophic lateral sclerosis. Neuroimage Clin 19:675–682. https://doi.org/10.1016/j.nicl.2018.05.020
Shellikeri S, Myers M, Black SE, Abrahao A, Zinman L, Yusunova Y (2019) Speech network regional involvement in bulbar ALS: a multimodal structural MRI study. Amyotroph Lateral Scler Frontotemporal Degener 20(5-6):385–395. https://doi.org/10.1080/21678421.2019.1612920
Rhinn H, Abeliovich A (2017) Differential aging analysis in human cerebral cortex identifies variants in TMEM106B and GRN that regulate aging phenotypes. Cell Syst 4(4):404–15.e5. https://doi.org/10.1016/j.cels.2017.02.009
Pandya VA, Patani R (2020) Decoding the relationship between ageing and amyotrophic lateral sclerosis: a cellular perspective. Brain 143(4):1057–1072. https://doi.org/10.1093/brain/awz360
Acknowledgements
The authors are grateful to patients and healthy volunteers for participating in the study.
Funding
This work was supported by grants from Italian Ministry of Health (5x1000 2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethic approval
Retrospective analysis of the data was approved by the IRCCS Ospedale Policlinico San Martino Review Board.
Consent to participate
Written informed consent was obtained from each participant according to the institution’s procedures and the Declaration of Helsinki.
Consent for publication
Not applicable.
Conflict of interest
We declare that we have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Figure 1.
Three-dimensional reconstructed MR imaging maps show regions of significant cortical atrophy on the inflated surface in the left (L) and right (R) hemispheres in ALS patients (excluding the two genetic cases) compared to healthy controls (Montecarlo, p< 0.05). (JPG 273 kb)
Supplementary Figure 2.
Three-dimensional reconstructed MR imaging maps show regions of significant cortical thinning related to older age at symptoms onset on the inflated surface in the left (L) and right (R) hemispheres in ALS patients (excluding the two genetic cases) (Montecarlo, p< 0.05). (JPG 322 kb)
Rights and permissions
About this article
Cite this article
Ferraro, P.M., Cabona, C., Meo, G. et al. Age at symptom onset influences cortical thinning distribution and survival in amyotrophic lateral sclerosis. Neuroradiology 63, 1481–1487 (2021). https://doi.org/10.1007/s00234-021-02681-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02681-3