Abstract
Purpose
Non-aneurysmal subarachnoid hemorrhage (NA-SAH) is a clinical-radiological entity with a different prognosis than aneurysmal SAH (A-SAH). The purpose of this study is to assess the predictive value of the modified Fisher Scale (mFS) for neurological complications in patients with this diagnosis.
Methods
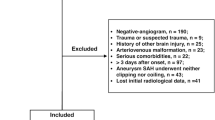
We recruited patients admitted at our hospital services between 2009 and 2017 who were diagnosed with spontaneous SAH, with either perimesencephalic (PM-SAH) or diffuse pattern (D-SAH), an initial negative angio-CT, and at least one digital subtraction angiography of brain vessels discarding underlying brain aneurysms or other vascular malformations.
Results
The retrospective observational study included 116 patients. The mean age was 54.4, and the sample included predominantly male subjects (62.9%). Hunt and Hess (HH) scores on admission ranged from 3 to 5 in 18.1% of patients. The prevalence of hydrocephalus requiring ventricular drainage was 18.1%. The prevalence of symptomatic vasospasm was 4.3%. A modified Rankin Scale (mRS) 0–2 at discharge was found in 95.6%. In a multivariate logistic regression for the presence of neurological complications including age, sex, admission HH 3-5 compared with < 3, mFS 4 compared with mFS < 4, D-SAH compared with PM-SAH, and mRS score at discharge of 0–2 compared with > 2, the only significant predictors were mFS 4 compared with mFS < 4 (OR 4.47 (95% CI 1.21, 16.66) p value = 0.03) and D-SAH compared with PM-SAH (OR 7.10 (95% CI 1.24, 40.8) p value = 0.03).
Conclusion
In patients with NA-SAH, a mFS score of 4 and/or a D-SAH bleeding pattern in non-contrast cranial CT on admission predicted the development of relevant neurological complications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-aneurysmal subarachnoid hemorrhage (NA-SAH) is a clinical-radiological entity which comprises almost 15% of cases of subarachnoid hemorrhage [1,2,3]. NA-SAH appears to follow a more benign clinical outcome compared with aneurysm-associated SAH (A-SAH) [4,5,6]. However, some cases may develop complications such as hydrocephalus and/or vasospasm, which require emergency treatment.
The modified Fisher Scale (mFS) is a radiological scale applied on non-contrast CT widely used to predict the probability of vasospasm after SAH secondary to intracranial aneurysm rupture (A-SAH) [7]. It was developed from the original Fisher Scale, which was modified to account for patients with thick cisternal blood and concomitant intraventricular or intraparenchymal hemorrhage (see “Methods” for grading description and reference). Despite its wide use as a predictor of vasospasm in A-SAH, the application of mFS in NA-SAH has never been tested and remains unknown.
NA-SAH has also been classified according to the radiologic distribution patterns of the blood into perimesencephalic subarachnoid hemorrhage (PM-SAH) and diffuse subarachnoid hemorrhage (D-SAH). Recent studies suggest that these two subtypes of NA-SAH may also show different clinical courses [8,9,10,11], yet the value of this sub-classification in predicting specific neurological complications has not been determined.
In this study, we assess the population of patients with NA-SAH at our center during an 8-year time period and analyze the value of the mFS and bleeding pattern as tools for the prognosis of neurologic complications (hydrocephalus requiring ventricular drainage and/or symptomatic vasospasm).
Methods
We retrospectively reviewed the radiology and medical record database of all patients admitted at our hospital between January 1, 2009 and December 31, 2017. For the purpose of this study, we included those patients with (1) spontaneous SAH (either PM-SAH or D-SAH) and (2) an initial angio-CT with at least one digital subtraction angiography (DSA) of brain vessels discarding underlying brain aneurysms or other vascular malformations. Cases of isolated cortical or intraventricular subarachnoid hemorrhage were excluded. Patients with no available imaging studies in the radiology database were also excluded.
Imaging protocol
At our institution, all patients with spontaneous SAH detected in the initial non-contrast head CT (NCCT) are taken into an emergency angio-CT. NCCT and angio-CT are then performed following standard protocols, using a 16 or 64 section helix CT scanner. Axial images are obtained using a 120–140 kV and a 0.625–2.5-mm section thickness reconstruction process. MPR, MIP, and volume rendering reconstructions are used to assess the images. The iodine contrast is administered with a power injector at a dose and rate of 50 ml and 4 ml/s, respectively. Patients without evidence of brain aneurysms in the initial emergency angio-CT must undergo a DSA within the following 24–48 h. The procedure is performed using a femoral or brachial arterial access. The internal and external carotids and the vertebral arteries are selectively catheterized and injected. The standard projections are anterior-posterior and lateral. Oblique and/or rotational 3D are obtained at the discretion of the neurologic intervention specialist in charge.
Image analysis
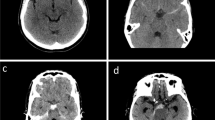
For the purpose of the study, an experienced interventional neuroradiology specialist and a senior resident physician analyzed the images. The cases were classified according to the bleeding pattern (PM-SAH or D-SAH) and the mFS grade (1 to 4), following the criteria below:
-
Bleeding pattern. PM-SAH: (1) The epicenter of the hemorrhage is immediately anterior to the midbrain, (2) possible extension of the hemorrhage to the posterior aspect of the interhemispheric fissure or to the base of the Sylvian ridge, (3) absence of a significant amount of intraventricular blood (sedimented blood within the occipital horns included), and (4) absence of brain hematoma [8, 9, 12]. D-SAH: Any SAH beyond the previous limits.
-
mFS score: Criteria published by Frontera et al. [7] were followed. Grade 1, thin focal or diffuse SAH, no IVH; 2, thin focal or diffuse SAH, IVH present; 3, thick focal or diffuse SAH, no IVH; 4, thick focal or diffuse SAH, IVH present.
Data collection
Medical records were reviewed with the assistance of an experienced stroke neurologist. The patients’ age, sex, Hunt and Hess score on admission, relevant neurological complications (symptomatic vasospasm and/or hydrocephalus requiring ventricular drainage), and mRS score at discharge [13, 14] were recorded. Hunt and Hess score values ranging 3 to 5 were considered as severe for symptoms at the time of onset and mRS > 2 as a poor clinical outcome.
Statistical analysis
Normally distributed data are presented as mean ± SD and non-normally distributed data as median and interquartile range (IQR). Categorical variables are expressed as percentages. A categorized analysis between groups was performed comparing the two types of NA-SAH (PM-SAH and D-SAH) and neurological complications. Student’s t test was used for continuous variables, and χ2 test for categorical variables. Odds ratios (OR) and 95% confidence intervals (CI) were calculated at first with univariate analysis to identify association between each variable with type of NO-SAH and a second time with uni- and multivariate logistic regression analysis to identify the association between each variable with neurological complications. Statistical significance was set at p < 0.05, and all analyses were done with Statistical Package for the Social Sciences Software Version 22.0 (SPSS, Chicago, IL, USA).
Results
A total of 116 patients fulfilled the aforementioned criteria and were included in the study.
Baseline population demographics
Table 1 (1st row) shows the baseline demographics of the global population. The mean age was 54.4 years, with a predominant male majority (62.9%). Hunt and Hess scores on admission ranging from 3 to 5 were found in 18.1% of patients. A mFS score of 4 was found in 33.6% of subjects. The proportion of patients who presented at least one neurological complication (symptomatic vasospasm or hydrocephalus requiring ventricular drainage) was 19.8%, being hydrocephalus more frequent than vasospasm (18.1 and 4.3%, respectively). A mRS 0–2 score at the time of discharge was considered as a good clinical outcome, found in 97.4% of the patients.
Blood distribution pattern
To test specifically the value of the blood pattern distribution to predict the development of neurological complications in NA-SAH, we considered the differences between patients with PM-SAH and those with D-SAH (Table 1, 2nd and 3rd rows). Sixty patients (51.7%) showed a PM-SAH, and 56 patients (48.3%) showed a D-SAH. No differences were found between both subgroups in regard to age and gender. The number of patients with an admission Hunt and Hess score between 3 and 5 and a mFS score of 4 was higher in those patients with D-SAH (p < 0.001). When considered as a group, relevant neurological complications (symptomatic vasospasm and hydrocephalus requiring ventricular drainage) were more frequent in patients with D-SAH (p < 0.001). However, this difference is due solely to the higher number of patients with hydrocephalus in the D-SAH group (p < 0.001), with an insignificant number of patients with vasospasm (p = 0.15). Good clinical outcome (assessed by a mRS 0–2 at discharge) was superior in patients with PM-SAH compared with those with D-SAH (p = 0.02).
Predictors of neurological complications
We performed univariate and multivariate analysis to detect which of the variables involved may be related to the onset of relevant neurological complications. Results of the analyses are summarized in Table 2. In a multivariate logistic regression for the presence of neurological complication, which included as predictors age, sex, admission Hunt and Hess 3–5 compared with < 3, mFisher 4 compared with mFisher < 4, D-SAH compared with PM-SAH, and mRS at discharge of 0–1 compared with > 1, the only significant predictors were mFisher (OR 4.47 (95% CI 1.21, 16.66) p value = 0.03) and D-SAH compared with PM-SAH (OR 7.10 (95% CI 1.24, 40.8) p value = 0.03).
Modified Fisher Scale
Table 3 shows the relation between the mFS grade and the number of patients that develop neurological complications (either vasospasm, hydrocephalus, or both). As it can be seen, the proportion of patients with neurological complications increased with the mFS grade (2.4%, 9.5%, 14.3%, and 46.2% for grades 1, 2, 3, and 4, respectively).
Discussion
In this study, we analyze the use of the mFS and radiological bleeding patterns (PM-SAH or D-SAH) for clinical assessment in a series of 116 patients with NA-SAH. Our results suggest that both of these tools are useful for prediction of neurological complications (mainly hydrocephalus requiring ventricular drainage and symptomatic vasospasm in second instance) in this setting, albeit with different meanings and implications than for aneurysmal SAH.
The main objective of our study was to focus on the applicability of mFS. This scale has been widely used in aneurysmal SAH settings, accurately predicting symptomatic vasospasm [7]. However, its meaning in patients with NA-SAH has not been tested, leading to confusion on the usefulness of mFS grading patients when there is no underlying aneurysm as cause of the bleeding.
Our patient series show that there is a correlation between the mFS score and the prevalence of relevant neurological complications in NA-SAH. However, this relation is statistically significant only for patients with a score of 4 (p < 0.001, OR 35.14), whereas scores of 2 and 3 do not show statistical significance (p 0.21 and 0.09, respectively). These results differ from those observed for aneurysmal SAH, which show a linear correlation between the mFS value and vasospasm that was also significant for scores of 2 and 3 [7].
Interestingly, the main symptomatic neurological complication in our series was hydrocephalus requiring ventricular drainage, rather than symptomatic vasospasm. Hydrocephalus was observed in 21 of the 23 patients who presented at least one neurological complication (91.3%), and it was significantly more common in patients with D-SAH than in PM-SAH (p < 0.001, Table 1), especially in the subset of patients with a mFS score of 4. The dilatation of the ventricular system is well established as the main complication in cases of NA-SAH [12]. Lin and Sprenker reported 12 out of 33 (36.3%) and 17 out of 26 (65.3%) patients with D-SAH that presented hydrocephalus requiring ventricular shunting in their respective series [15, 16]. Our study confirms not only that hydrocephalus is the most frequent complication of NA-SAH but also that this correlation may be predicted through the mFS and radiological blood patterns. Patients with a mFS score of 4 and/or a D-SAH are more likely to develop acute dilation of the ventricular system requiring ventricular drainage, while patients with a mFS score between 1 and 3 and/or a PM-SAH show a significantly lower prevalence of this complication. This finding is probably related to larger amounts of blood found in mFS score 4 patients [17, 18].
It is also noteworthy that the correlation between symptomatic vasospasm and mFS scores in our patient series with NA-SAH was poor. Only one patient with a mFS score of 1 and four with a mFS score of 4 developed this complication. This data supports conclusions from previous publications reporting a low prevalence of this complication in patients with NA-SAH [19]. As suggested in previous studies, the low prevalence of vasospasm found in patients with NA-SAH could be explained by the theoretical origin of NA-SAH in the venous system [20, 21] and the low concentration of oxyhemoglobin (key substance generating vasospasm), rather than blood quantity. This hypothesis is also supported by the similarly low prevalence of vasospasm found in both subtypes of patients with NA-SAH. Regardless, larger amounts of blood in patients with D-SAH showed no difference in vasospasm rates when compared with PM-SAH patients (p = 0.15, Table 1).
Finally, we analyzed differences within the remaining clinical and radiological variables between PM-SAH and D-SAH populations (Table 1, 2nd and 3rd rows). Our results support the idea that, despite being a non-aneurysmal disease, D-SAH has a more aggressive entity than PM-SAH. Patients with D-SAH show a worsened Hunt and Hess score on admission (p < 0.001) and worse mRS scores at discharge (p = 0.02), besides the abovementioned higher rate of hydrocephalus (p < 0.001) and a mFS score of 4.
Limitations of this research include selection bias due to the study taking place at a large, level 3 hospital and the retrospective nature of the study. Additional prospective studies are needed to confirm the conclusions of our findings.
Conclusion
In patients with NA-SAH, a mFS score of 4 and/or a D-SAH bleeding pattern in non-contrast cranial CT on admission predicted the development of relevant neurological complications. In this setting, hydrocephalus requiring ventricular drainage was much more frequent than symptomatic vasospasm. Patients with D-SAH had a more aggressive clinical course than those with PM-SAH.
References
Marder CP, Narla V, Fink JR, Tozer Fink KR (2014) Subarachnoid hemorrhage: beyond aneurysms. AJR Am J Roentgenol 202(1):25–37
Kawamura Y, Narumi O, Chin M, Yamagata S (2011) Variant deep cerebral venous drainage in idiopathic subarachnoid hemorrhage. Neurol Med Chir (Tokyo) 51(2):97–100
Daenekindt T, Wilms G, Thijs V, Demaerel P, Van Calenbergh F (2008) Variants of the basal vein of Rosenthal and perimesencephalic nonaneurysmal hemorrhage. Surg Neurol 69(5):526–529
Konczalla J, Platz J, Schuss P, Vatter H, Seifert V, Güresir E (2014) Non-aneurysmal non-traumatic subarachnoid hemorrhage: patient characteristics, clinical outcome and prognostic factors based on a single-center experience in 125 patients. BMC Neurol 14:140
Zhong W, Zhao P, Wang D, Li G, Sun H, Chen H et al (2014) Different clinical characteristics between perimesencephalic subarachnoid hemorrhage and diffuse subarachnoid hemorrhage with negative initial angiography. Turk Neurosurg 24(3):327–332
Pyysalo LM, Niskakangas TT, Keski-Nisula LH, Kähärä VJ, Öhman JE (2011) Long term outcome after subarachnoid haemorrhage of unknown aetiology. J Neurol Neurosurg Psychiatry 82(11):1264–1266
Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES, Macdonald RL, Mayer SA (2006) Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher Scale. Neurosurgery 59(1):21–27 discussion 21-7
van Gijn J, van Dongen KJ, Vermeulen M, Hijdra A (1985) Perimesencephalic hemorrhage: a nonaneurysmal and benign form of subarachnoid hemorrhage. Neurology 35:493–497
Rinkel GJ, Wijdicks EF, Vermeulen M, Ramos LM, Tanghe HL, Hasan D, Meiners LC, van Gijn J (1991) Nonaneurysmal perimesencephalic subarachnoid hemorrhage: CT and MR patterns that differ from aneurysmal rupture. AJNR Am J Neuroradiol 12:829–834
Coelho LG, Costa JM, Silva EI (2016) Non-aneurysmal spontaneous subarachnoid hemorrhage: perimesencephalic versus non-perimesencephalic. Rev Bras Ter Intensiva 28(2):141–146
Konczalla J, Schmitz J, Kashefiolasl S, Senft C, Seifert V, Platz J (2015) Non-aneurysmal subarachnoid hemorrhage in 173 patients: a prospective study of long-term outcome. Eur J Neurol 22(10):1329–1336
Mensing LA, Vergouwen MDI, Laban KG, Ruigrok YM, Velthuis BK, Algra A, Rinkel GJE (2018) Perimesencephalic hemorrhage: a review of epidemiology, risk factors, presumed cause, clinical course, and outcome. Stroke 49(6):1363–1370
Hunt WE, Hess RM (1968) Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 28(1):14–20
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J (1988) Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19:604–607
Sprenker C, Patel J, Camporesi E, Vasan R, Loveren HV, Chen H et al (2015) Medical and neurologic complications of the current management strategy of angiographically negative nontraumatic subarachnoid hemorrhage patients. J Crit Care 30(1):216.e7–216.11
Lin N, Zenonos G, Kim AH, Nalbach SV, Du R, Frerichs KU et al (2012) Angiogram-negative subarachnoid hemorrhage: relationship between bleeding pattern and clinical outcome. Neurocrit Care 16(3):389–398
Schuss P, Hadjiathanasiou A, Brandecker S, Wispel C, Borger V, Güresir Á, Vatter H, Güresir E (2019) Risk factors for shunt dependency in patients suffering from spontaneous, non-aneurysmal subarachnoid hemorrhage. Neurosurg Rev 42(1):139–145
Mohan M, Islim AI, Rasul FT, Rominiyi O, deSouza RM, Poon M et al (2019) Subarachnoid haemorrhage with negative initial neurovascular imaging: a systematic review and meta-analysis. Acta Neurochir (Wien) 161(10):2013–2026
Whiting J, Reavey-Cantwell J, Velat G, Fautheree G, Firment C, Lewis S, Hoh B (2009) Clinical course of nontraumatic, nonaneurysmal subarachnoid hemorrhage: a single-institution experience. Neurosurg Focus 26(5):E21
Raya A, Zipfel GJ, Diringer MN, Dacey R, Derdeyn CP, Rich KM et al (2014) Pattern not volume of bleeding predicts angiographic vasospasm in nonaneurysmal subarachnoid hemorrhage. Stroke 45(1):265–267
Kolias AG, Sen J, Belli A (2009) Pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage: putative mechanisms and novel approaches. J Neurosci Res 87(1):1–11
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
The Clinical Investigation Ethics Committee (CEIC) of Hospital Clínico Universitario Virgen de la Arrixaca approved the research protocol. All the procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Páez-Granda, D., Parrilla, G., Díaz-Pérez, J. et al. Are modified Fisher Scale and bleeding pattern helpful predictors of neurological complications in non-aneurysmal subarachnoid hemorrhage?. Neuroradiology 63, 253–257 (2021). https://doi.org/10.1007/s00234-020-02524-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02524-7