Abstract
Purpose
Differential diagnosis between central neurocytoma and ependymoma is very important for making preoperative scheme. We explored the application of diffusion-weighted imaging (DWI) combined with apparent diffusion coefficient (ADC) in differential diagnosis between both.
Methods
The data of preoperative MR plain and contrast-enhanced scan, DWI and ADC values of neoplastic solid parts from 18 cases with central neurocytoma and 19 cases with lateral ventricular ependymoma, were retrospectively analyzed. Mann-Whitney test was used for the comparison of ADC values between central neurocytoma and ependymoma. The application of ADC values in the differential diagnosis between central neurocytoma and ependymoma was evaluated by ROC curve.
Results
The lesions showed hyperintensity-dominant mixed signal intensity on DWI and mean ADC was (0.65 ± 0.13) × 10−3 mm2/s in the 18 cases with central neurocytoma. In the 19 cases with ependymoma, 13 had hyperintensity-dominant mixed signal intensity on DWI and 6 had hypointensity-dominant mixed signal intensity on DWI, and mean ADC was (1.20 ± 0.23) × 10−3 mm2/s. The mean ADC value was significantly higher in the 19 cases with ependymoma than in the 18 cases with central neurocytoma (P < 0.001). The ADC of 0.87 × 10−3 mm2/s might be used as a threshold for differential diagnosis between central neurocytoma and ependymoma with an area under ROC curve of 0.98 ± 0.02 and a 95% confidence interval of 0.95–1.00. Its sensitivity, specificity, and accuracy were 90%, 100%, and 90%, respectively.
Conclusion
There is a certain overlap in MRI imaging features between central neurocytoma and ependymoma. DWI combined with ADC value can improve peoperative diagnostic accuracy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Central neurocytoma originating from the Monro foramen accounts for 0.1% of the primary brain tumors. Its clinical symptoms usually occur within less than 6 months and mainly include headache and intracranial hypertension [1]. Since its clinical symptoms and imaging features are similar to those of ependymoma, it is difficult to distinguish them before operation. Moreover, central neurocytoma requires preoperative embolism to prevent excessive intraoperative bleeding due to its abundant blood supply, which is different from ependymoma. Therefore, accurate diagnosis is important before operation. At present, magnetic resonance diffusion-weighted imaging (DWI) has been widely used in the diagnosis and differential diagnosis of central nervous system neoplasms, but the application of DWI combined with apparent diffusion coefficient (ADC) in differential diagnosis between central neurocytoma and ependymoma is rarely reported. The purpose of this study was to explore the application of DWI combined with ADC in the differential diagnosis between central neurocytoma and ependymoma.
Subjects and methods
All study methods were approved by Institutional Review Board and Ethics Committee of our Hospital. The informed consent was obtained from all subjects.
Subjects
Clinical and imaging data of 18 cases with central neurocytoma and 19 cases with ependymoma, which were confirmed by operation, pathology, and immunohistochemistry in our hospital between January 2012 and October 2018, were retrospectively analyzed. In the 18 cases with central neurocytoma, 10 were male and 8 female with a mean age of 29.7 years (range 16–45). In the 19 cases with ependymoma, 12 were male and 7 female with a mean age of 36.2 years (range 7–58). All patients underwent pre-operative 3.0 T MRI plain scan, MRI enhanced scan, DWI, and ADC. Clinical manifestations were intracranial hypertension such as headache, dizziness, nausea and vomiting, and average disease course was 0.8–9 months in all patients.
MRI
Head plain scan and enhanced scan were performed using Siemens 3.0 T Verio MR scanner (MAGNETOM Verio, Siemens AG Healthcare, Erlangen, Germany) in all cases. Gradient recalled echo (GRE) T1WI (TR = 1800 ms, TE = 20 ms) parameters were as follows: slice thickness 5.0 mm, slice interval 1.0 mm, field of view 220 mm × 220 mm, and matrix 256 × 256. Turbo spin echo (TSE) T2WI (TR = 4500 ms, TE = 96 ms) parameters were as follows: echo time 10 ms, echo train length 8, and average number of excitation 2. DWI parameters were as follows: TR 4500 ms/TE 98 ms, slice thickness 5.0 mm, slice interval 1.0 mm, matrix 256 × 256, and b-values (0 and 1000 s/mm2). After injection of 0.1 mmol/kg of Gd-DTPA (Bayer Schering Pharma, Germany), enhanced MRI was performed.
Image analysis and measure
Every image was analyzed by two senior doctors together who specialized in imaging diagnosis of the central nervous system and were blind to this study. When their diagnoses were inconsistent, they finally reached agreement by discussion. MR DWI images were loaded on a computer for processing to obtain corresponding ADC images, and then the ADC images were imported into Siemens workstations to measure the ADC values of neoplastic solid parts. When measuring ADC, a region of interest (ROI) was first selected according to MR T1WI, T2WI, and enhanced images. The ROI should avoid cystoid (round shadow with long T1 and T2 signal, as well as clear boundary), necrotic (irregular shadow with long T1 and T2 signal, as well as unclear boundary), and hemorrhagic (equal T1 and short T2 signal shadow, or short T1 and long T2 signal shadow) areas. The ROI area was 15–20 mm2 with b = 1000 s/mm2. Three adjacent slices were selected from the lesion center to measure ADC values, and the ROI areas were the same as much as possible. Each slice was measured three times in different areas, and the mean ADC value of this slice was calculated, and then the average ADC value of the three slices was calculated as the ADC value of the lesion.
Statistical analysis
Statistical treatment was performed using SPSS19.0 software. Mann-Whitney test was used to compare the ADC values of neoplastic solid parts between central neurocytoma and ependymoma. Statistical significance was established at P < 0.01. The ROC curve was used to determine ADC optimal threshold for differential diagnosis between central neurocytoma and ependymoma. The area under ROC curve, sensitivity, specificity, and accuracy were calculated.
Results
Imaging features of central neurocytoma and ependymoma
In the 18 cases with central neurocytoma, all lesions were located in the body of lateral cerebral ventricle. Of the 18 cases, the lesions were bilateral in 5 and unilateral in 13, were lobulated (Fig. 1) or irregular in 14 and round-like in 4, had cystoid variation and/or necrosis in 13, hemorrhage in 9, and vascular flow void effect in 12 (Fig. 1b). The neoplastic solid parts showed slightly low signal intensity on T1WI and slightly high signal intensity on T2WI, while neoplastic necrosis or cystoid variation showed long T1 and long T2 signals. The lesions showed heterogeneous moderate to obvious enhancement on MR enhanced scan in 14 cases (Fig. 1c). In the 19 cases with ependymoma, 9 had lesions in the body of lateral cerebral ventricle, 5 in the anterior horn of lateral ventricle (Fig. 2), and 5 in the posterior horn of lateral ventricle (Fig. 3). The positions of these lesions were similar to that reported by Lejeune et al. [2]. Of the 19 cases with ependymoma, the lesions were lobulated in 13 and round or round-like in 6, were cystic-solid mixed mass in 12, and solid mass in 7. The neoplastic solid parts showed equal or low signal intensity on T1WI (Figs. 2a and 3a) as well as equal, low, or slightly high signal intensity on T2WI (Fig. 2b and 3b), while neoplastic necrosis or cystoid variation showed long T1 and long T2 signals. In the 19 cases, 5 had intratumoral hemorrhage, 2 mild cerebral edema around the brain ventricle, 5 vascular flow void effect in the lesion, and 12 hydrocephalus. The lesions showed slight enhancement on MR-enhanced scan in 13 (Fig. 2c) and moderate to obvious enhancement in 6 cases (Fig. 3c) (Table 1).
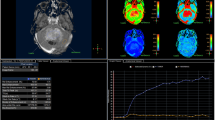
A 27-year-old man with lobulated central neurocytoma located in the Monro foramen of lateral ventricle. a Heterogeneous equal and low signal intensity on T1WI. b Equal and high signal intensity with vascular flow void effect on T2WI. c Heterogeneous moderate enhancement with lamellar nonenhancement areas and vascular flow void effect on enhanced T1WI. d Hyperintensity-dominant mixed signal intensity on DWI. e ADC showing hypointensity-dominant mixed signal intensity. f Round and uniform tumor cells with eosinophilic cytoplasm, and lots of blood capillary among these cells (HE × 200). DWI diffusion-weighted imaging, ADC apparent diffusion coefficient
A 26-year-old man with round-like ependymoma located in the body and anterior horn of the left lateral ventricle. a Cystic-solid mixed mass with clear boundary, the neoplastic solid part showing equal signal intensity on T1WI. b The neoplastic solid part showing equal and low mixed signal intensity on T2WI. c No enhancement on enhanced T1WI. d The neoplastic solid part showing hypointensity-dominant mixed signal intensity and the neoplastic cystoid part showing equal signal intensity on DWI. e The neoplastic solid part showing equal and low mixed signal intensity and the neoplastic cystoid part showing high signal intensity on ADC. f Lots of blood capillary and perivascular chrysanthemum-like appearance (HE × 200). DWI diffusion-weighted imaging, ADC apparent diffusion coefficient
A 56-year-old man with lobulated ependymoma located in the posterior horn of the right lateral ventricle. a Heterogeneous and slight low signal intensity with mottling high signal intensity areas on T1WI. b Heterogeneous and slight high signal intensity with surrounding brain edema on T2WI. c Heterogeneous moderate enhancement on sagittal image. d Slightly limited diffusion with surrounding liquid signals on DWI. e Equal and low signal intensity on ADC. f MRS showing increased Cho with loose-bodied lip peak and without NAA peak. DWI diffusion-weighted imaging, ADC apparent diffusion coefficient, MRS magnetic resonance spectroscopy, Cho cholin, Lip lipid, NAA N-acetylaspartate
Comparisons of DWI and ADC between central neurocytoma and ependymoma
In the 18 cases with central neurocytoma, the neoplastic solid parts showed hyperintensity-dominant mixed signal intensity on DWI, but its corresponding ADC showed hypointensity-dominant mixed signal intensity, namely that DWI hyperintensity would have ADC hypointensity (Fig. 1 d and e). Of the 19 cases with ependymoma, the neoplastic solid parts showed hyperintensity-dominant mixed signal intensity on DWI and hypointensity-dominant mixed signal intensity on ADC in 13 (Fig. 3 d and e), and the neoplastic solid parts showed hypointensity-dominant mixed signal intensity on DWI and equal and low mixed signal intensity on ADC in 6 (Fig. 2 d and e). The mean ADC value was 0.65 ± 0.13 × 10−3 mm2/s (range 0.46–1.09 × 10−3 mm2/s) in central neurocytoma, and 1.20 ± 0.23 × 10−3 mm2/s (range 0.92–1.73 × 10−3 mm2/s) in ependymoma. The ADC value was significantly higher in ependymoma than in central neurocytoma (P < 0.001). The ADC of 0.87 × 10−3 mm2/s might be used as a threshold for differential diagnosis between central neurocytoma and ependymoma with an area under ROC curve of 0.98 ± 0.02 (Fig. 4) and a 95% confidence interval of 0.95–1.00. Its sensitivity, specificity, and accuracy were 90%, 100%, and 90%, respectively. The lesions with more than 0.87 × 10−3 mm2/s of ADC may be diagnosed as ependymoma, and the lesions with less than 0.87 × 10−3 mm2/s of ADC may be diagnosed as central neurocytoma.
Pathology
Central neurocytoma
The gross appearance of tumor section was grayish white or grayish red with moderate texture. Under microscope, the tumor is composed of small cells with round nuclei, less cytoplasm, spotted chromatin, and perinuclear halo (Fig. 1f), and calcification could be seen in some tumors. In most central neurocytoma, synaptophysin was strongly positive, but glial fibrillar acidic protein (GFAP) was negative [3]. In some cases, neuron-specific enolase (NSE) staining was positive. In a few of cases with negative synaptophysin, the diagnosis depended on cellular ultrastructure. Under electron microscope, the cytoplasm contained a lot of Golgi complex, mitochondria, parallel microtubules, dense core particles and transparent vesicles, and synapses could be seen.
Ependymoma
The gross appearance of tumor section was red and lobulated with brittle texture. Under light microscope, ependymoma cells have moderate density, large round or oval nuclei with rare mitosis, and calcification or necrosis could be seen. Under low power microscope, the tumor tissue sections showed leopard skin-like change, which was one of the diagnostic bases for ependymoma. Under high power microscope, the tumor cells were arranged into a “palisade-like” structure around tumor vessel wall. The central vessel was surrounded by long cell processes containing glial fiber without cell nuclei, followed by its periphery consisting of tumor cell nuclei, showing chrysanthemum-like or perivascular pseudochrysanthemum-like appearance (Fig. 2f). Immunohistochemistry showed positive GFAP, vimentin, and fibronectin [4].
Discussion
Significance of preoperative differential diagnosis between central neurocytoma and ependymoma
Central neurocytoma, a rare tumor, was first raised by Hassoun in 1982. Central neurocytoma is usually located in the interventricular foramen (Monro foramen) of the lateral ventricles and only accounts for 0.1% of the primary tumors originating from central nervous system. In 1993, WHO classified central neurocytoma as an independent tumor belonging to WHO grade II glioma [5], because it may originate from neurons or neuroblasts with bidirectional differentiation potential in transparent septum or ependyma and is different from ganglioneuroma and neuroblastomas under light microscope. Ependymoma originates from meningeal remnants of ependyma or other parts. It belongs to central nervous system glioma and WHO grade II [5]. Ependymoma accounts for about 3/4 of ependymal cell tumors and 1.2–7.8% of intracranial tumors [6]. It mostly occurs in children, specifically in 5- to 15-year-old children with an annual incidence rate of about 0.2–0.8/100,000. Ependymoma is usually located in the ventricle with expansive growth. After anaplasia, the malignant degree of ependymoma markedly increases to grade III or IV. In 2016, WHO added a subtype of ependymoma, RELA fusion gene positive ependymoma with specific L1CAM expression [7]. This subtype is usually supratentorial neoplasms and has worse prognosis than typical ependymoma.
At present, the best treatment for central neurocytoma and ependymoma is surgery for trying to completely remove lesions and relieve obstructive hydrocephalus. However, for central neurocytoma, due to its abundant blood supply, it is not completely removed usually and requires preoperative embolism to prevent excessive intraoperative bleeding. Therefore, the preoperative accurate diagnosis is very important for the choice of surgical scheme. This study aimed to improve the differential diagnosis between central neurocytoma and ependymoma by retrospective analyses of MRI features, DWI and ADC values from 18 cases with central neurocytoma and 19 cases with ependymoma whose diagnoses had been confirmed by surgery, pathology, and immunohistochemistry.
Analyses of imaging features for central neurocytoma and ependymoma
In this study, the central neurocytoma was located in lateral ventricular septum pellucidum or Monro foramen and was connected with the septum pellucidum in all the 18 cases. Of the 18 cases, the lesions were lobulated or irregular in 14 and round or round-like in 4 with clear boundary. The lesions were mainly cystic-solid mixed, accompanied by calcification and vascular flow void effect. The neoplastic solid parts showed equal or slightly low signal intensity on T1WI and equal or slightly high signal intensity on T2WI. The most lesions showed heterogeneous moderate to obvious enhancement on MR enhanced scan [8,9,10]. The central neurocytoma was limited to the body and anterior horn of lateral ventricle without invasion into the adjacent brain parenchyma. In this study, ependymoma was also lobulated, round or round-like with clear boundary. Stippled calcification or cystoid variation were seen in these lesions commonly without edema in the surrounding brain parenchyma. The neoplastic solid parts showed equal or low signal intensity on T1WI as well as equal or slightly high signal intensity on T2WI. The lesions showed various degrees of enhancement on MR enhanced scan. Ependymoma often grows along the brain ventricle [11]. In a word, cystoid variation, vascular flow void effect, and enhancement were more marked in central neurocytoma than in ependymoma, but morphology and other imaging features were similar between both, so it is difficult to distinguish central neurocytoma from ependymoma.
Analyses of DWI and ADC for central neurocytoma and ependymoma
DWI is closely related to diffusion velocity of water molecules. The main factors affecting the diffusion velocity of water molecules include the distributions of cell membrane and basilar membrane, ratio of nucleus to cytoplasm, and the content of macromolecular substances in cytoplasm [12, 13]. In pathological state, the change in the distributions of intra- and extracellular macromolecular substances such as proteins, and the destruction of cell membrane and basilar membrane affect diffusion velocity of water molecules, resulting in abnormal DWI signal. DWI signal attenuation mainly depended on water molecular ADC (mm2/S) and dispersion sensitivity coefficient b value (s/mm2) [14]. ADC value is negatively correlated with DWI signal, namely that ADC value increases, the dispersion of water molecules accelerates and DWI signal decreases, and vice versa. According to DWI signal and ADC value, a qualitative diagnosis can be made and biological behavior can be evaluated for most lesions. For example, due to rapid growth of malignant tumor, tumor cells are large and dense, and the extracellular space is relatively narrow, limiting water molecular dispersion with a marked reduction of ADC value [15]. ADC value is closely correlated with tumor cell density as well as tumorous benignancy and malignancy. The lower the ADC value is, the higher the tumor cell density is, namely that the ADC value is negatively correlated with tumor cell density [16]. In addition, the ratio of nucleus to cytoplasm is also one of the important factors to determine ADC value. The high ratio of nucleus to cytoplasm caused by dense arrangement of tumor cells limits water molecular dispersion, leading to high DWI signal and low ADC value. In this study, central neurocytoma showed hyperintensity-dominant mixed signal intensity on DWI with a mean ADC value of 0.65 ± 0.13 × 10−3 mm2/s in the 18 cases, and ependymoma showed hyperintensity or slight hyperintensity on DWI in 13 cases and hypointensity on DWI in 6 cases with a mean ADC value of 1.20 ± 0.23 × 10−3 mm2/s. This may be that the cell density was relatively low and the blood supply was relatively small in ependymoma, and 12 cases had necrotic and cystoid ependymoma, leading to mean ADC elevation in the 19 cases with ependymoma.
In lesions, cystoid, necrotic, and hemorrhagic areas as well as tumor cell density are all associated with water molecular diffusion, finally affecting the ADC value. In addition, images on different slices will influence the ADC value due to the heterogeneity of tumor, different MR systems with specific scanning sequences and parameters also will influence the ADC value, and scanners with different field strengths also have various ADC values [14], so it is necessary to explore ADC thresholds of different MR systems in clinical practice. Therefore, there is no unified ADC threshold for differential diagnosis between benign and malignant tumors. In this study, the ADC value was significantly higher in ependymoma than in central neurocytoma. The ADC of 0.87 × 10−3 mm2/s might be used as a threshold for differential diagnosis between central neurocytoma and ependymoma with an area under ROC curve of 0.98 ± 0.02, a 95% confidence interval of 0.95–1.00, a sensitivity of 90%, a specificity of 100%, and a accuracy of 90%, which is consistent with that reported by Wang et al. [17].
Recently, Chen et al. [18] have used ADC histogram to differentiate ventricular ependymomas, choroid plexus papillomas (CPPs), and central neurocytomas, and their results suggest that the ADC histogram analysis may help to discriminate ependymomas, CPPs, and central neurocytomas. Chen et al. [18] estimated a threshold value of 86.50 for perc.50% to predict ependymomas from central neurocytomas (AUC = 0.97, sensitivity = 97.20%, specificity = 85.70%). ADC histograms contain more information of tumor tissue than mean ADC value. ADC histogram parameters include mean value, variance, skewness, kurtosis, and percentile values. Positive skewness indicates that there is high cellularity or possibility of malignancy, while negative skewness suggests a large amount of cystic or edematous tissue. In this study, we explored the application of mean ADC in differential diagnosis between central neurocytoma and ependymoma. Our methods and results were similar with that of above study, but ADC histogram analysis requires special software, so it is not suitable to wide application in clinical practice.
There are some limitations in this study. Firstly, the sample size was relatively small. Secondly, histogram parameters were relatively less. We will accumulate cases and carry out an analysis of multiparameters in large sample.
In summary, although there are some similar imaging features between central neurocytoma and lateral ventricular ependymoma, the ADC value of ependymoma is significantly higher than that of central neurocytoma. Therefore, the imaging features combined with ADC value is very important for differential diagnosis between central neurocytoma and ependymoma and also provides a basis for making preoperative scheme.
References
Yang I, Ung N, Chung LK, Nagasawa DT, Thill K, Park J, Tenn S (2015) Clinical manifestations of central neurocytoma. Neurosurg Clin N Am 26:5–10
Lejeune JP, Reyns N, Baroncini M, Peltier J, Le Gars D (2011) Ependymomas of the lateral ventricle. A series of 27 cases with review of the literature. Neurochirurgie 57:206–209
Jaiswal S, Vij M, Rajput D, Mehrotra A, Jaiswal AK, Srivastava AK, Behari S, Krishnani N (2011) A clinicopathological, immunohistochemical and neuroradiological study of eight patients with central neurocytoma. J Clin Neurosci 18:334–339
Sun S, Wang J, Zhu M, Beejadhursing R, Gao P, Zhang X, Jiao L, Jiang W, Ke C, Shu K (2018) Clinical, radiological, and histological features and treatment outcomes of supratentorial extraventricular ependymoma: 14 cases from a single center. J Neurosurg 128:1396–1402
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Thompson YY, Ramaswamy V, Diamandis P, Daniels C, Taylor MD (2015) Posterior fossa ependymoma: current insights. Childs Nerv Syst 31:1699–1706
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Muly S, Liu S, Lee R, Nicolaou S, Rojas R, Khosa F (2018) MRI of intracranial intraventricular lesions. Clin Imaging 52:226–239
Chen H, Zhou R, Liu J, Tang J (2012) Central neurocytoma. J Clin Neurosci 19:849–853
Tlili-Graiess K, Mama N, Arifa N, Kadri K, Hasni I, Krifa H, Mokni M (2014) Diffusion weighted MR imaging and proton MR spectroscopy findings of central neurocytoma with pathological correlation. J Neuroradiol 41:243–250
Delmaire C, Boulanger T, Leroy HA, Tempremant F, Pruvo JP (2011) Imaging of lateral ventricle tumors. Neurochirurgie 57:180–192
Al-Sharydah AM, Al-Arfaj HK, Saleh Al-Muhaish H, Al-Suhaibani SS, Al-Aftan MS, Almedallah DK, Al-Abdulwahhab AH, Al-Hedaithy AA, Al-Jubran SA (2019) Can apparent diffusion coefficient values help distinguish between different types of pediatric brain tumors? Eur J Radiol Open 6:49–55
Soni N, Srindharan K, Kumar S, Bhaisora KS, Kalita J, Mehrotra A, Mishra P (2018) Application of diffusion tensor imaging in brain lesions: a comparative study of neoplastic and non-neoplastic brain lesions. Neurol India 66:1667–1671
Bulakbasi N, Guvenc I, Onguru O, Erdogan E, Tayfun C, Ucoz T (2004) The added value ofthe apparent diffusion coefficient calculation to magneticresonance imaging in the differentiation and grading of malignant braintumors. J Comput Assist Tomogr 28:735–746
Stadnik TW, Chaskis C, Michotte A, Shabana WM, van Rompaey K, Luypaert R, Budinsky L, Jellus V, Osteaux M (2001) Diffusion-weighted MR imaging of intracerebral masses: comparison with conventional MR imaging and histologic findings. AJNR Am J Neuroradiol 22:969–976
Lee EJ, terBrugge K, Mikulis D, Choi DS, Bae JM, Lee SK, Moon SY (2011) Diagnostic value of peritumoral minimum apparent diffusion coefficient for differentiation of glioblastoma multiforme from solitary metastatic lesions. AJR Am J Roentgenol 196:71–76
Wang CY, Cheng JL, Nie YF, Pang BB, Yan J, Song YM (2015) The diagnostic value of ADC combined with DWI in the differentiation of the central neurocytoma and ependymoma. Radiol Practice 30:1011–1015
Chen C , Ren CP , Zhao RC , Ding JW , Cheng JL. Histogram Analysis Parameters ADC for Distinguishing Ventricular Neoplasms of Ependymoma, Choroid Plexus Papilloma, and Central Neurocytoma.Med Sci Monit. 2019 Aug 7;25:5886-5891. https://doi.org/10.12659/MSM.915398
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study has been approved by the ethics committee of Second Hospital Affiliated to Lanzhou University.
Informed consent
The patients reported in this study gave written informed consent to participate.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, Pf., Ma, L., Ye, Bq. et al. Application of diffusion-weighted imaging combined with apparent diffusion coefficient in differential diagnosis between central neurocytoma and ependymoma. Neuroradiology 62, 439–445 (2020). https://doi.org/10.1007/s00234-019-02342-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-019-02342-6