Abstract
Purpose
Carotid near-occlusion is a tight atherosclerotic stenosis of the internal carotid artery (ICA) resulting in decrease in diameter of the vessel lumen distal to the stenosis. Near-occlusions can be classified as with or without full collapse, and may have high peak systolic velocity (PSV) across the stenosis, mimicking conventional > 50% carotid artery stenosis. We aimed to determine how frequently near-occlusions have high PSV in the stenosis and determine how accurately carotid Doppler ultrasound can distinguish high-velocity near-occlusion from conventional stenosis.
Methods
Included patients had near-occlusion or conventional stenosis with carotid ultrasound and CT angiogram (CTA) performed within 30 days of each other. CTA examinations were analyzed by two blinded expert readers. Velocities in the internal and common carotid arteries were recorded. Mean velocity, pulsatility index, and ratios were calculated, giving 12 Doppler parameters for analysis.
Results
Of 136 patients, 82 had conventional stenosis and 54 had near-occlusion on CTA. Of near-occlusions, 40 (74%) had high PSV (≥ 125 cm/s) across the stenosis. Ten Doppler parameters significantly differed between conventional stenosis and high-velocity near-occlusion groups. However, no parameter was highly sensitive and specific to separate the groups.
Conclusion
Near-occlusions frequently have high PSV across the stenosis, particularly those without full collapse. Carotid Doppler ultrasound does not seem able to distinguish conventional stenosis from high-velocity near-occlusion. These findings question the use of ultrasound alone for preoperative imaging evaluation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carotid near-occlusion is a tight atherosclerotic stenosis of the internal carotid artery (ICA) resulting in decrease in diameter (or “collapse”) of the vessel lumen distal to the stenosis. Carotid near-occlusion may be classified as with or without full collapse [1, 2]. Near-occlusion with full collapse results in a string like ICA lumen distal to the stenosis and has many pseudonyms including string sign, pseudo-occlusion, atheromatous pseudo-occlusion, and slim sign [1]. Near-occlusion without full collapse has more subtle reduction in ICA caliber distal to the stenosis and is easily overlooked on vascular imaging studies, which may lead to spurious quantification of stenosis (Fig. 1) [3]. The prevalence of near-occlusion is not well established; however, it is common in patients with symptomatic carotid stenosis, accounting for up to 11–23% of cases in studied populations [2, 4,5,6]. Distinguishing near-occlusion from “conventional” ≥ 50% stenosis (defined as 50–99% ICA stenosis excluding near-occlusions) is important as the risk of stroke, and therefore management may differ [2, 6,7,8]. Post hoc analysis of combined North American Symptomatic Carotid Endarterectomy Trial (NASCET) and European Carotid Surgery Trial (ECST) data revealed a lower stroke risk with carotid near-occlusion compared to conventional stenosis; however, near-occlusions with and without full collapse were combined into one group for analysis with only 16 of 262 near occlusion cases having full collapse [2]. A more recent study suggests that patients with symptomatic near-occlusion with full collapse may have a significantly higher short-term risk of ipsilateral ischemic stroke when compared with conventional stenosis and that patients with near-occlusion without full collapse have a very low short-term risk of ipsilateral stroke [6].
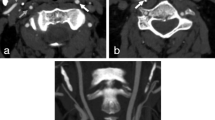
Right-sided near-occlusion without full collapse. a CTA coronal maximum intensity projection (MIP). Distal to a severe ICA stenosis (white arrowhead), the ICA (black arrowhead) is small in caliber but not threadlike. The distal ICA is smaller than the contralateral side (white arrow) and similar in caliber to the ipsilateral ECA (black arrow). b & c Ultrasound findings mimic conventional stenosis
Carotid near-occlusions may have high, low, or unrecordable velocity at or distal to the stenosis on carotid Doppler ultrasound examination [9,10,11]. The presence of a very narrow stenosis lumen associated with low ICA peak systolic velocity (PSV) is highly specific for diagnosing near-occlusion with full collapse [10, 11], but may not have a high sensitivity as PSV for this group may also be “normal” or high. Using only this ultrasound criterion, cases of high-velocity near-occlusion with full collapse may be misdiagnosed as conventional stenosis. Similarly, near-occlusions without full collapse are reported to almost always have high PSV in the stenosis [6], and may thus be misdiagnosed as conventional stenosis. The sensitivity of the low flow finding for all near-occlusions is not well established and there are currently no ultrasound criteria to distinguish near-occlusion with high PSV in the stenosis (high-velocity near-occlusion) from conventional stenosis. The aim of this study was to determine how frequently near-occlusions have high PSV across the stenosis and determine whether systolic and diastolic velocities in the common carotid artery (CCA) and ICA, as well as other less commonly utilized Doppler ultrasound parameters can distinguish high-velocity near-occlusions from conventional stenosis.
Methods
Patient selection
This multisite study included patients from Sunnybrook Health Sciences Centre (Toronto, Canada) and Norrlands University Hospital (Umeå, Sweden) with retrospective collection of cases and blinded hypothesis-driven analysis. The study was approved by local research ethics committees.
At Sunnybrook, patients were selected from two pre-existing carotid stenosis databases, obtained from two independent ultrasound laboratories (Vascular laboratory and Radiology Department). Patients were examined between August 2007 and June 2015. Those with an ultrasound and CTA performed within 30 days of each other were further evaluated. All CTA examinations included a delayed phase allowing assessment of delayed vascular filling. Clinical information was obtained from medical records. Patients were excluded if they had co-morbidities that could affect carotid artery hemodynamics: ipsilateral acute malignant infarct or chronic infarction of > 1/3 of the ipsilateral cerebral hemisphere, acute brain hemorrhage, carotid artery dissection, aortic stenosis or insufficiency, intraluminal thrombus, atrial fibrillation or flutter, ipsilateral carotid endarterectomy or stenting prior to or between imaging examinations, CTA performed for trauma, carotid web, ICA fibromuscular dysplasia or Takayasu arteritis, occlusion of either carotid artery on CTA, severe ipsilateral distal tandem stenosis, or poor quality studies. CTA examinations of the remaining 240 patients were reviewed by a neuroradiologist (MM—5 years experience). Additional exclusions were 154 patients with < 50% stenosis on CTA, one patient with a CCA stenosis and one patient whose CTA could not be retrieved. The final combined Sunnybrook cohort comprised 84 patients.
The data from Norrlands are previously published in the Additional Neurological Symptoms before Surgery of the Carotid Arteries (ANSYSCAP): a Prospective study [6]. The ANSYCAP study prospectively recruited 230 consecutive patients with symptomatic conventional stenosis or near-occlusion that underwent a preoperative evaluation at Umeå Stroke Centre, Norrlands University Hospital, between August 2007 and December 2009 [12]. For this study, all cases with both good quality CTA and ultrasound examinations performed within 30 days of each other were selected (n = 68). Carotid Doppler ultrasound was the standard imaging technique during the ANSYSCAP study period, whereas CTA was used in only some cases. Use of CTA was either due to poor quality ultrasound (cases not included in this analysis) or where the attending physician generally preferred CTA over ultrasound. Only single phase CTA was performed. Patients were excluded according to the same criteria as for the Sunnybrook cases. After exclusions, 52 Umeå cases were included, giving a combined total study population of 136 patients.
CTA analysis
All CTA examinations of the selected patients were blindly double reviewed by readers with expertise in carotid stenosis and near-occlusion imaging. AF (42 years experience) read all examinations, with RA (15 years experience) or EJ (2 years intensive training in near-occlusion diagnosis) as second readers. Findings on CTA were classified as near-occlusion with full collapse, near-occlusion without full collapse, or conventional stenosis. Distinguishing conventional stenosis and near-occlusion without full collapse was based on the CTA criteria proposed by Bartlett et al. [4] and required evaluation of the entire arterial circulation of the neck and circle of Willis. Particular attention was drawn to differentiating true near-occlusion without full collapse from a circle of Willis anatomical variant that results in asymmetric ICA calibers, such as an ipsilateral fetal posterior cerebral artery or an ipsilateral anterior cerebral artery trunk supplying bilateral pericallosal arteries [1]. These cases were classified and analyzed as conventional ≥ 50% stenosis. Near-occlusion cases typically had an ICA stenosis diameter of < 1.3 mm (often < 1.0 mm) with similar caliber of the ipsilateral distal ICA and ECA [4]. Anatomical variants typically had a more modest ICA stenosis diameter of > 1.3 mm and a distal ipsilateral ICA caliber larger than the ECA. Where there was initial disagreement between the 2 CTA readers, final classification was reached by consensus. Only the side of greater stenosis was included for analysis of any patient with bilateral ICA stenosis.
Carotid ultrasound analysis
PSV and end diastolic velocity (EDV) in the CCA and point of maximum ICA stenosis (defined as the point of maximum PSV in the proximal ICA, and hereafter referred to as ICA stenosis) were recorded. Values were obtained by reviewing images, detailed reports, or from the prospectively collected Umeå database. Examinations had been performed by experienced sonographers using a mix of color Doppler, pulse waved Doppler, and power Doppler reflecting usual clinical practice. A PSV threshold value of 125 cm/s was used to designate high (≥ 125 cm/s) and low (< 125 cm/s) velocity in the ICA stenosis [13]. Mean velocity in the CCA and ICA stenosis were each calculated using the formula mean velocity = (PSV-EDV)/3 + EDV [14]. Pulsatility index (PI) in the CCA and ICA stenosis were each calculated using the formula (PSV-EDV)/mean velocity. A PSV ratio was calculated by dividing the PSV in the stenosis by the PSV in the CCA. An EDV ratio and PI ratio were each calculated in the same fashion. This resulted in 12 Doppler ultrasound parameters for analysis. Two patients had an EDV in the CCA of 0 cm/s, resulting in an incalculably high EDV ratio and were therefore arbitrarily assigned a very high EDV ratio of 25.
Statistical analysis
Statistical analyses were performed using MedCalc for Windows, version 15.8 (MedCalc Software, Ostend, Belgium). Doppler ultrasound values and quantitative patient demographic variables were compared between the conventional stenosis and near-occlusion groups using an independent sample two-sided t test (normally distributed parameters) or a two-tailed Mann-Whitney test (non-normally distributed parameters). Correction for multiple testing was applied via a Bonferroni adjustment. Qualitative demographic and co-morbidity variables were compared with Fischer’s exact test. With CTA, as the gold standard, receiver operating characteristic (ROC) curves were calculated for each of the 12 ultrasound velocity parameters; thresholds were set where the sum of sensitivity and specificity was highest (maximum value of the Youden index) [15]. A p value < 0.05 was regarded as statistically significant.
Results
Of the 136 study population patients, 82 had conventional stenosis and 54 had near-occlusion (Table 1). Of the near-occlusion cases, 40 (74–95% CI 62–86%) had high velocity (> 125 cm/s) in the ICA stenosis, 7 (13%) had low velocity and 7 (13%) were mistaken for occlusion. The proportion of near-occlusions that had high velocity was similar in all three ultrasound laboratories (73–75%); however, the prevalence of near-occlusion ranged between 29 and 60% (p = 0.007, χ 2 test) between the ultrasound laboratories. There were no statistically significant differences in patient demographic and co-morbidity data between the high-velocity near-occlusion and conventional stenosis groups (Table 2). All near-occlusions that were misdiagnosed as occlusion on ultrasound had full collapse. Five (5/7; 71%) cases of low-velocity near-occlusion had full collapse; the two (2/7; 29%) without full collapse had either a very long stenosis or a borderline CTA classification between with and without full collapse. Of the 40 near-occlusions with high velocity in the ICA stenosis, three had a PSV in the stenosis of 125–230 cm/s (50–69% stenosis according to Grant et al.) [13]. Of these, two were without full collapse and were classified as < 50% stenosis in the clinical ultrasound report (due to a higher lab specific threshold for 50% stenosis), and one had full collapse and was classified as near-occlusion in the clinical ultrasound report due to a longitudinally extensive stenosis visible on B-mode imaging.
There was a significant difference in 10 of 12 ultrasound parameters between the high-velocity near-occlusion and conventional stenosis groups (Table 3). High-velocity near-occlusions had significantly higher values for PSV ICA stenosis, PSV ratio, EDV ICA stenosis, EDV ratio, mean velocity ICA stenosis, mean velocity ratio, PI CCA, and PI ratio. They had significantly lower values for EDV CCA and mean velocity CCA and no significant difference in PSV CCA and PI stenosis. After adjustment for multiple comparisons, nine of these ten findings remained statistically significant (p < 0.05), the exception was mean velocity in the CCA.
ROC curve data is presented in Table 4. Area under the curve (AUC) values for the 12 ultrasound parameters range between 0.54 and 0.73. No single ultrasound parameter had both high sensitivity and specificity to distinguish near-occlusion from conventional stenosis when utilizing optimum threshold criteria as calculated via ROC analysis. If threshold values were set to maintain ≥ 90% sensitivity, specificities ranged from 3% (PI ratio) to 39% (PSV Ratio, mean velocity ICA). A PSV ratio of ≥ 3.9 and a mean velocity in ICA of ≥ 145 cm/s had respectively 92% (36/39) and 90% (36/40) sensitivity, 39% (28/72) and 39% (30/77) specificity, 45% (36/80) and 43% (36/83) positive predictive value, and 90% (28/31) and 88% (30/34) negative predictive value.
Combinations of any two variables were evaluated by manually comparing all 66 possible combinations in scatter-plots and adjusting threshold values to produce maximum specificity while maintaining ≥ 90% sensitivity. Thirteen combinations had ≥ 50% specificity. These had similar performance: 90–98% sensitivity, 50–56% specificity. The best result was CCA EDV ≤ 12 cm/s and/or ICA PSV ≥ 410 cm/s, which had 98% (39/40) sensitivity, 53% specificity (41/78), 51% positive predictive value (39/76), and 98% negative predictive value (41/42).
Distinguishing high-velocity near-occlusion with full collapse from all other cases with PSV ≥ 125 cm/s was explored. The 95% CI of AUC intersected 0.5 in all parameters except PI CCA (0.77, 95% CI 0.62–0.92) and CCA EDV (0.72, 95% CI 0.53–0.91). CCA PI of ≥ 1.75 was 89% sensitive and 61% specific, and CCA EDV ≤ 12 cm/s was 78% sensitive and 64% specific to distinguish high-velocity near-occlusion with full collapse from remaining cases. A CCA EDV threshold of ≤ 12 cm/s has been a previously suggested threshold for near-occlusion with full collapse [16]; we further evaluated its performance of all high-velocity near-occlusions, rendering 67% sensitivity and 75% specificity.
The CCA findings was compared between the 40 high-velocity near-occlusions and the seven near-occlusions with low flow velocity in the stenosis (< 125 cm/s). In the low flow velocity near-occlusion, the CCA EDV tended to be lower (mean 11.7 vs 8.3 cm/s; p = 0.18) and CCA PI tended to be higher (mean 1.86 vs 2.15; p = 0.14); whereas CCA PSV (mean 68 vs 65 cm/s; p = 0.68) and CCA mean velocity (mean 31 vs 27 cm/s; p = 0.35) were similar.
The effect of excluding the 27 cases with a contralateral ≥ 50% stenosis was explored. The share of near-occlusions that had high (> 125 cm/s) velocity in the stenosis remained high (79%, 33/42). The only Doppler value to significantly alter as a result was PI ratio, becoming more similar between the groups (0.81 vs 0.70; p = 0.08; AUC 0.61) compared to without the exclusions (0.83 vs 0.67; p = 0.002; AUC 0.68).
The mean interval between CTA and ultrasound examinations was 6.8 days, and the interval was ≤ 7 days in 67% of patients. Exploring the effect of shortening the inclusion criteria interval to ≤ 7 days between examinations revealed no relevant change in the main findings: 71% (24/34) of near-occlusion cases had high velocity on ultrasound (compared with 74% with an interval of ≤ 30 days) and the AUCs were essentially unchanged (all new calculated values were within 0.06 of the original AUCs).
Discussion
The main study finding was that in three separate laboratories, near-occlusions frequently had high PSV in the ICA stenosis, particularly those without full collapse. Other important findings were differences in ultrasound parameters between high-velocity near-occlusions and conventional stenoses on a group level; however, due to considerable overlap, these were insufficient for diagnostic or screening purposes. These findings question the use of ultrasound alone for preoperative imaging evaluation as ultrasound may misdiagnose a significant proportion of near occlusions (those with high velocity) as conventional high-grade stenosis.
The most important design feature of this study is the interpretation of near-occlusion on the reference test (in this case, CTA) to which the ultrasound findings were compared. A detailed review of the angiographic and ultrasound literature pertaining to near-occlusion has been recently published [1, 17], but a few salient points are highlighted: near-occlusion without full collapse is often overlooked in clinical and scientific studies including those examining Doppler ultrasound [1]. Most near-occlusions analyzed in NASCET and ECST were near-occlusions without full collapse [1, 17]. Separating near-occlusions from conventional stenosis is important as it may direct management. In addition, recent findings suggest a differing stroke risk between near occlusions with and without full collapse [6]. Larger studies evaluating stroke risk in near-occlusion subgroups is required in order to develop appropriate management strategies. Thus, identifying all near-occlusions (not just those with full collapse) is required. Studies analyzing the ultrasound findings of a tight stenosis with low flow velocities have included only near-occlusion with full collapse in the definition of near-occlusion [10, 11]. This “low flow” criterion was highly specific (98.8%) and moderately sensitive (71%) for diagnosing near-occlusion with full collapse [1]. However, our study found that only 13% of all near-occlusion cases had low velocity on ultrasound, (15% if excluding mistaken occlusions), the remainder were high-velocity near-occlusions with an ICA stenosis PSV ≥ 125 cm/s. Most cases of near occlusion will be missed if only the “low flow” ultrasound criterion is used. A moderate delay between ultrasound and CTA examinations (in this study up to 30 days) is unlikely to account for the large difference in sensitivity between this and prior studies. Previous studies have had longer delays (3 months [9], unspecified [10] and 52 days [11]). In addition, we found no clear difference between short (≤ 7 days) and moderate (≤ 30 days) delay between examinations. Identifying near-occlusions without full collapse by careful analysis of CTA, and including them as near occlusions for analysis likely explains the difference in sensitivity between this and previous studies.
Doppler ultrasound is currently widely used clinically, however the knowledge of prognosis and treatment of near-occlusion is based on angiographic techniques [2, 8]. Conventional angiography was used in the randomized trials of more than 25 year ago, not CTA, and is thus often used or desired as the reference standard [2, 18]. Undertaking conventional angiographic studies today is ethically problematic due to the procedural risk. In addition, the principle of assessing reduced artery size—the hallmark of near-occlusions—is very similar in both CTA and conventional angiography, but not with ultrasound. Indeed, CTA accurately detects near-occlusion compared with conventional angiography [19], whereas ultrasound often overlooks near-occlusion without full collapse [9]. As a result, CTA is a reasonable reference standard for near-occlusion to compare with ultrasound.
Near-occlusion without full collapse rarely (6%) showed low flow velocity, whereas 24% of near-occlusions with full collapse had low flow velocity (36% if excluding mistaken occlusions). Ultrasound descriptions of near-occlusion include reducing and variable velocity, as well as high velocities [20, 21]. The 2003 ultrasound velocity criteria consensus states that near-occlusion may have high, low, or undetectable PSV in the ICA [13], but references only one supporting study in which the angiographic definition of near-occlusion without full collapse is not well described [9]. The first study suggesting near-occlusions (both with and without full collapse) frequently have high flow velocity was published in 2015 [6]. Our study independently validates this finding with data from two additional laboratories, reiterating that near occlusions (both with and without full collapse) frequently have high PSV in the stenosis.
We evaluated if ultrasound could separate high-velocity near-occlusion and conventional stenosis. No parameter produced a high sensitivity and specificity. We failed to validate a previously proposed criterion for near-occlusion (CCA EDV ≤ 12 cm/s) [16], likely because that original study examined CCA regardless of findings in the ICA. With sensitivity set high (≥ 90%), specificities were too poor (3–39%) to be diagnostic of high-velocity near-occlusion. Specificities of ultrasound were too low even as a screening test to select for further evaluation with CTA. The most specific parameters at ≥ 90% sensitivity (PSV ratio and mean velocity ICA) had a 43–45% positive predictive value and 71–72% (80/111, 83/117) of all cases were positive. If 70% of cases with a suggested diagnosis of stenosis are in question, they should all be evaluated further with CTA where ICA stenosis velocity is high to allow definitive diagnosis of near occlusion. We compared all possible pairs of parameters and adjusted thresholds for ultrasound to best fit the data. Even with this approach, the results did not improve, possibly because ultrasound parameters are often not independent of each other.
We conclude that ultrasound cannot distinguish high-velocity near-occlusion from conventional stenosis well enough to be diagnostic or used as a screening tool for selection to CTA. We therefore question the paradigm of imaging with ultrasound alone, an approach with many proponents [22,23,24,25]. Our findings indicate that near-occlusions will be systematically missed if imaging with ultrasound alone. Given the very low risk of CTA and the potential to alter management, we recommend routine use of CTA in all cases with suspected carotid stenosis on carotid ultrasound (PSV > 125 cm/s) in order to distinguish near-occlusion from conventional stenosis. We do not propose that CTA replace ultrasound, rather that it complements it, particularly when surgery is being considered. In any situation where CTA is performed, it is important to note that while near-occlusion with full collapse is usually immediately apparent, near-occlusion without full collapse is easily overlooked without attention to detail. Readers of CT angiograms must be systematic and specifically evaluate for the presence of near-occlusion. MRA may be chosen as a follow-up diagnostic modality; however, the diagnostic accuracy of MRA for near-occlusion has not yet been sufficiently established [1]. Combined carotid and transcranial ultrasound may provide additional diagnostic information for near occlusion, particularly regarding intracranial collateral circulation, and remains an area for further study.
This study has limitations. The case material is retrospective. Data were obtained from pre-existing databases with differences introducing possible selection bias, including differing database patient populations and different institutional utilization of CTA. Bias may arise if only carotid stenosis cases were examined with both ultrasound and CTA, potentially misrepresenting the frequency of high-velocity near-occlusion, as cases with low PSV or occlusion on ultrasound may be over or under sampled. Selection bias may also account for the varying prevalence of near-occlusions amongst all stenosis cases between ultrasound laboratories, which in turn may influence calculations of predictive value and number of positive cases. Patients from the two Sunnybrook laboratories were from all-comers referred for carotid ultrasound, whereas those from Umeå were symptomatic patients referred for consideration of carotid endarterectomy. The 8-year period from which data were collected at different ultrasound laboratories has the potential to affect ultrasound examination quality and interpretation due to difference in sonographer training and experience as well as advancement in technology. Despite all of these factors with the potential to introduce bias, the proportion of near-occlusions that had high velocity in the ICA stenosis was similar in all three ultrasound laboratories. At Umeå, most CTA images were only available as 2-mm reformats rather than source images and did not have delayed imaging. With universal use of delayed CTA, a few more very slow-flow near-occlusion with full collapse cases may have been detected, but as it seems likely that these would be at high risk of being misdiagnosed as occluded on ultrasound, their inclusion would not refute the findings of the study. There were many exclusion criteria to limit potentially confounding ultrasound results. The applicability of our findings may be reduced or limited when applied to patients meeting one or more of the exclusion criteria. Our findings do however remain robust in the setting of contralateral ICA stenosis. The sample size was small and multiple comparisons undertaken, with the potential for single cases to affect parameter performance and threshold values. Although a newly designed study could produce thresholds less affected by outliers, it seems unlikely that sensitivities and specificities would significantly change or alter the conclusion. Given these limitations, our findings should be considered preliminary.
Conclusion
Most near-occlusions have high PSV in the ICA stenosis on ultrasound, particularly those without full collapse. High-velocity near-occlusions seem to have higher flow velocities in the ICA stenosis and lower flow velocities in the CCA than conventional stenoses. However, due to significant overlap, no ultrasound parameter seems to be sufficiently sensitive and specific to be clinically useful. Since distinguishing near-occlusion from conventional stenosis seems desirable, our findings question the paradigm of ultrasound alone for preoperative stenosis imaging evaluation.
Abbreviations
- ICA:
-
Internal carotid artery
- NASCET:
-
North American Symptomatic Carotid Endarterectomy Trial
- ECST:
-
European Carotid Surgery Trial
- PSV:
-
Peak systolic velocity
- CCA:
-
Common carotid artery
- ANSYSCAP:
-
Additional Neurological Symptoms before Surgery of the Carotid Arteries
- EDV:
-
End diastolic velocity
- PI:
-
Pulsatility index
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
References
Johansson E, Fox AJ (2016) Carotid near-occlusion: a comprehensive review, part 1-definition, terminology, and diagnosis. AJNR Am J Neuroradiol 37:2–10
Fox AJ, Eliasziw M, Rothwell PM, Schmidt MH, Warlow CP, Barnett HJ (2005) Identification, prognosis, and management of patients with carotid artery near occlusion. AJNR Am J Neuroradiol 26:2086–2094
Fox AJ (1993) How to measure carotid stenosis. Radiology 186:316–318
Bartlett ES, Walters TD, Symons SP, Fox AJ (2006) Diagnosing carotid stenosis near-occlusion by using CT angiography. AJNR Am J Neuroradiol 27:632–637
Borst J, Marquering HA, Kappelhof M et al (2015) Diagnostic accuracy of 4 commercially available semiautomatic packages for carotid artery stenosis measurement on CTA. AJNR Am J Neuroradiol 36:1978–1987
Johansson E, Ohman K, Wester P (2015) Symptomatic carotid near-occlusion with full collapse might cause a very high risk of stroke. J Intern Med 277:615–623
Rothwell PM, Warlow CP (2000) Low risk of ischemic stroke in patients with reduced internal carotid artery lumen diameter distal to severe symptomatic carotid stenosis: cerebral protection due to low poststenotic flow? On behalf of the European Carotid Surgery Trialists’ Collaborative Group. Stroke 31:622–630
Rothwell PM, Eliasziw M, Gutnikov SA et al (2003) Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet 361:107–116
El-Saden SM, Grant EG, Hathout GM, Zimmerman PT, Cohen SN, Baker JD (2001) Imaging of the internal carotid artery: the dilemma of total versus near total occlusion. Radiology 221:301–308
Hetzel A, Eckenweber B, Trummer B, Wernz M, Schumacher M, von Reutern G (1998) Colour-coded duplex sonography of preocclusive carotid stenoses. Eur J Ultrasound 8:183–191
Mansour MA, Mattos MA, Hood DB et al (1995) Detection of total occlusion, string sign, and preocclusive stenosis of the internal carotid artery by color-flow duplex scanning. Am J Surg 170:154–158
Johansson EP, Arnerlov C, Wester P (2013) Risk of recurrent stroke before carotid endarterectomy: the ANSYSCAP study. Int J Stroke 8:220–227
Grant EG, Benson CB, Moneta GL et al (2003) Carotid artery stenosis: gray-scale and Doppler US diagnosis—Society of Radiologists in Ultrasound Consensus Conference. Radiology 229:340–346
Barlinn K, Kolieskova S, Shahripour RB et al (2015) Increased pulsatility of the intracranial blood flow spectral waveform on transcranial Doppler does not point to peripheral arterial disease in stroke patients. J Stroke Cerebrovasc Dis 24:189–195
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Androulakis AE, Labropoulos N, Allan R, Tyllis TK, al-Kutoubi A, Nicolaides AN (1996) The role of common carotid artery end-diastolic velocity in near total or total internal carotid artery occlusion. Eur J Vasc Endovasc Surg 11:140–147
Johansson E, Fox AJ (2016) Carotid near-occlusion: a comprehensive review, part 2-prognosis and treatment, pathophysiology, confusions, and areas for improvement. AJNR Am J Neuroradiol 37:200–204
Wardlaw JM, Chappell FM, Best JJ, Wartolowska K, Berry E (2006) Non-invasive imaging compared with intra-arterial angiography in the diagnosis of symptomatic carotid stenosis: a meta-analysis. Lancet 367:1503–1512
Leclerc X, Godefroy O, Lucas C et al (1999) Internal carotid arterial stenosis: CT angiography with volume rendering. Radiology 210:673–682
von Reutern GM, Goertler MW, Bornstein NM et al (2012) Grading carotid stenosis using ultrasonic methods. Stroke 43:916–921
Spencer MP, Reid JM (1979) Quantitation of carotid stenosis with continuous-wave (C-W) Doppler ultrasound. Stroke 10:326–330
Moore WS (2003) For severe carotid stenosis found on ultrasound, further arterial evaluation is unnecessary. Stroke 34:1816–1817 discussion 1819
Ranaboldo C, Davies J, Chant A (1991) Duplex scanning alone before carotid endarterectomy: a 5-year experience. Eur J Vasc Surg 5:415–419
Loftus IM, McCarthy MJ, Pau H et al (1998) Carotid endarterectomy without angiography does not compromise operative outcome. Eur J Vasc Endovasc Surg 16:489–493
Logason K, Karacagil S, Hardemark HG, Bostrom A, Hellberg A, Ljungman C (2002) Carotid artery endarterectomy solely based on duplex scan findings. Vasc Endovasc Surg 36:9–15
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Swedish Stroke Foundation, the Stroke Foundation of Northern Sweden, the Research Fund for Neurological Diseases at Umea University Hospital and the County of Västerbotten’s Research Fund.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
For this type of retrospective study formal consent is not required.
Rights and permissions
About this article
Cite this article
Khangure, S.R., Benhabib, H., Machnowska, M. et al. Carotid near-occlusion frequently has high peak systolic velocity on Doppler ultrasound. Neuroradiology 60, 17–25 (2018). https://doi.org/10.1007/s00234-017-1938-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-017-1938-4