Abstract
Introduction
Cerebrospinal fluid (CSF) absorption has long been held to predominantly entail drainage into the venous outflow system via the intracranial arachnoid granulations. Newer data suggest pathways involving spinal arachnoid granulations and lymphatic channels may also make substantial contributions to CSF outflow.
Methods
The putative major CSF outflow pathways and their proportionate contribution to CSF absorption were reviewed in this article.
Results
CSF is absorbed and drained in bulk not just through cerebral arachnoid granulations (CAG) but also through spinal arachnoid granulations (SAG) and a lymphatic pathway involving egress through cranial and spinal nerve sheaths. The proportions of CSF that efflux through each of these major pathways have yet to be determined with any certainty in humans, though existing evidence (the majority of which is derived from animal studies) suggests that lymphatic pathways may account for up to 50 % of CSF outflow—presumably leaving the CAG and SAG to process the balance.
Conclusion
Knowledge of the CSF pathways holds implications for our ability to understand, prognose, and even treat diseases related to CSF circulation and so is a matter of considerable relevance to neuroradiology and neurology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Produced predominantly by the ventricular choroid plexus, cerebrospinal fluid (CSF) is a clear, colorless liquid that circulates through the ventricular system and subarachnoid space (SAS) of the central nervous system. CSF crucially offsets the weight of the brain to provide it with neutral buoyancy and also serves an important role in the protection, nourishment, chemical stability, and immune function of the central nervous system as a whole. In light of CSF’s physiological significance, it is intriguing that a comprehensive understanding of the pathways contributing to its outflow from the central nervous system and eventual reabsorption into the bloodstream has remained largely elusive. While current clinical consensus holds that CSF absorption across the cranial arachnoid granulations (CAG) and into the venous sinuses of the brain constitutes the preeminent outflow pathway, this account increasingly seems insufficient to explain the dynamics of the outflow system. Indeed, mounting morphological, physiological, and pathological evidence points toward a decidedly more complex conception of CSF drainage, one in which spinal arachnoid granulations (SAG) and the lymphatic system serve as additional outflow pathways [1, 2]. Further understanding of these pathways and their contributions to CSF outflow would be of great benefit to clinicians, as it may help us better understand the pathogenesis, clinical symptoms, and course of conditions associated with disrupted CSF circulation, as well as potentially provide insights into new approaches to treatment.
Cranial arachnoid granulation pathway
First observed by Pacchioni as early as 1721 [3], CAG are finger-like projections of the arachnoid mater that penetrate the dura and protrude into the lumen of the venous sinuses [4]. CAG can be seen extensively in the converge area of parieto-occipital veins of the superior sagittal sinus but are also present in the converge area of the transverse sinus and other venous sinuses [5]. The origin of human CAG can be traced to the 26th week of embryonic development, when cranial dura in certain places begin to concave toward the venous sinus wall. These concaved dura contain arachnoid cell clusters, which proceed to penetrate the dural fiber and reach subendothelial structures of the venous sinus. In the 35th week, these arachnoid cell clusters form protuberances that partially resemble mature arachnoid granulations in structure and protrude into the lumen of the venous sinus; the number of these protuberances increases in the 39th week, as does the complexity of their internal structure [6]. The resultant CAG are not easily observed until after 18 months of age, though their visibility increases considerably by the age of three. CAG ultimately reach maturity at 4 years of age [7, 8].
An early indication of CAG’s involvement in CSF outflow came in 1914 with Weed’s discovery that injection of Berlin blue-colored gelatine solutions into cadaver specimens’ SAS under low pressure led to the presence of the tracer in specimens’ cerebral venous sinuses [9]. Corroborated by the results of equivalent studies conducted in a range of nonhuman species, this finding led to the proposal that CSF is absorbed into the bloodstream through the CAG. Subsequent work determined that movement of CSF from the SAS into the venous sinuses is dependent on a pressure gradient; more specifically, the pressure in the SAS must outstrip that in the venous sinuses by between 3 and 5 mmHg to ensure normal CSF drainage [5]. By 1983, the application of electron microscopy allowed the close examination of arachnoid granulation ultrastructure [10], with several studies using this technique in conjunction with light microscopy and immunohistochemical methods to confirm the similarities between human arachnoid granulations and those of other species [11–13]. Such work established that human arachnoid granulations are composed of four principal parts: an innermost central core, which—in continuity with the SAS—contains a network of arachnoid cells, fibroblasts, and connective tissue fibers and forms the general shape of the granulation; an arachnoid cell layer that is continuous with the arachnoid mater and encompasses the central core; a thin fibrous capsule, comprising an outer layer of endothelial cells (containing many micropinocytotic vesicles), a middle layer of fibrocytes, collagen fibers, and elastins, and an innermost layer of electron-dense cells, and encasing all but the apical portion of the granulation; and a cap cell layer, a thickened cluster of polygonal arachnoid cells present at the apical portion of the granulation that directly abuts either the sinus or adjacent extracellular cisterns. This knowledge of AG ultrastructure has also informed our understanding of arachnoid granulations’ function. Most work in nonhuman animals—whose arachnoid granulations appear to be invested with an endothelial lining that is continuous with the sinus endothelium—has led to proposals that CSF absorption into the sinus is mediated by passive transport (via intercellular cisterns or tubule-like structures) [14, 15] or active transport (via endothelial pinocytosis or transcellular vacuoles) [16]. In humans, however, these endothelial cells are not always present, with the arachnoid cells of the cap cell layer directly abutting the lumen of the venous sinus instead [11]. Accordingly, the cap cell layer is likely a critical structure for human CSF absorption [12]; indeed, several studies implicate it as the primary site for CSF transport via transcellular vacuoles and intercellular cisterns, transport methods that may be the preeminent means of absorption (compared to endothelial pinocytosis) in humans [10, 17, 18].
Spinal arachnoid granulation pathway
Spinal arachnoid granulations (SAG) were observed as early as 1923, though their physiological function was initially largely unknown [5]. Initial support for their role in CSF outflow came in 1948, when Brierley and Fieled injected India ink into the CSF of rabbits and found the ink 9 h later in the epidural space of the spinal canal as well as some lymph nodes there [19]. Subsequently, a number of experiments approaching the issue from both anatomical and physiological perspectives have been performed in various species, confirming the existence of a SAG pathway for CSF outflow.
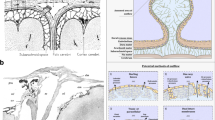
Most often seen around the dorsal nerve root, SAG structurally resemble CAG and can be grouped into three categories based on their positional relationship with dura [20–22] (Figs. 1 and 2) slightly protruding, such that the arachnoid granulation is completely inside the nerve root and does not break into dura; protruding within the dura, such that the arachnoid granulation penetrates into the dura but not beyond it; and protruding through the dura, such that the arachnoid granulation completely penetrates the dura and protrudes into the epidural space. There is some evidence (e.g., Welch’s work in monkeys) that the majority of SAG are of the third category, protruding into the epidural space [20]. These SAG can be further grouped into two subtypes based on their relationship with the epidural veins, as some directly contact a vein while others do not [20–22]. Kido’s research in human cadavers indicated that SAG are located predominantly on thoracic spinal nerve roots and that there are on average two arachnoid granulations for each nerve root [22]. Work in monkeys yielded similar results [23]. More recently, however, Tubbs reported a higher density of human SAG in the lumbar spinal nerve roots than in the thoracic spinal nerve roots and also showed that SAG are not present at every spinal nerve dorsal root [24].
The classification of spinal arachnoid granulations (SAG). Type 1 SAG (1): slightly protruding, which is completely inside the subdural space (7) and does not break into dura (6); type 2 SAG (2): protruding within the dura, which penetrates into the dura but not beyond it; type 3 SAG (3a and 3b): protruding through the dura, which completely penetrates the dura and protrudes into the epidural space (5). This type of SAG can be further grouped into two subtypes based on their relationship with the epidural veins (4), as some directly contact with a vein (3b) while others do not (3a)
Though research in this area has led to considerable support for the existence of a SAG outflow pathway, it has also engendered some dispute over whether this pathway involves CSF absorption into the venous system directly or by way of the lymphatic system. In their experiments with monkeys, for example, Welch et al. observed only 5 out of the 32 nerve roots (16 %) apparently associated with SAG had direct contact with surrounding veins—a fact that would suggest a key role for lymphatic absorption [20]. Contrastingly, however, Tubbs et al. found that all the nerve roots associated with SAG in humans were closely connected with surrounding veins and, moreover, that the number of SAG associated with each nerve root was positively correlated with the diameter of the surrounding veins [24]. Further support for the idea that CSF absorbed through SAG can flow directly into the venous system came from a 2003 study by Seyfert et al., in which researchers injected iopamidol into patients’ SAS via lumbar puncture for myelography and took peripheral venous blood samples at regular intervals to determine the blood iodine concentration [25]. Showing that iodine could be detected in peripheral veins 30 min post-contrast medium injection, the results indirectly confirmed that CSF could be absorbed into the venous system through SAG, since it would normally take at least 60 min for CSF to reach peripheral veins through CAG absorption. Seyfert et al. also found that venous iodine concentrations in peripheral veins were directly related to the radiculographic prominence of the associated spinal nerve root sleeve, a finding that proves consistent with the aforementioned indication that SAG are mainly distributed around spinal dorsal nerve roots [25]. Biceroglu et al. provided more direct evidence for SAG-mediated CSF outflow into venous blood with a 2012 study in which rabbits were injected via direct puncture with the contrast agent Magnevist for MR cisternography [26]. Employing MRI at various post-injection intervals to monitor the flow direction of the contrast agent, Biceroglu et al. found that it drained into venous plexus around the spinal cord directly through SAS [26]. There is, nevertheless, evidence for involvement of the lymphatic system in spinal CSF absorption too, as will be discussed in the next section [27].
Lymphatic system pathway
In 1876, Key discovered that tracer injected into the CSF could be subsequently seen in both arachnoid granulations and peripheral lymph [28]. It was not until more recently, however, that the importance of the lymphatic system to CSF drainage became apparent [1, 3, 5, 8, 29], with a host of studies (the main findings of which are summarized by Table 1) providing strong and varied support for the existence and physiological relevance of a lymphatic outflow pathway [19, 23, 28, 30–42]. More specifically, they demonstrated that CSF could seep out of the cranium through the perineural space of the first, second, fifth, and eighth cranial nerves; enter the lymphatic system around the end of nerve sheath; and finally drain into cervical lymph nodes. Of these drainage routes, the most commonly observed involves CSF flowing along the perineural space of the olfactory nerve (which leaves the brain through foramina of the cribriform plate) into nasal mucosa in the roof of the nasal cavity, whereupon it is absorbed through the underlying submucosal lymphatic system and ultimately drained to the regional lymph nodes that serve the nasopharynx. This is unsurprising given that the endothelial layer of the olfactory nerve sheath becomes increasingly thinner in the nasal mucosa, a factor that would allow CSF easy access to the surrounding extracellular matrix and—subsequently—the widely distributed submucosal lymphatic capillaries. A series of experiments by Mollanji et al. in sheep confirmed the physiological functionality of this outflow route by demonstrating that occlusion of the cribriform plate with bone wax or tissue glue elevated intracranial pressure, increased CSF outflow resistance, and decreased CSF absorption [37–39]. The existence of the nasal submucosal lymphatic system drainage pathway in humans, meanwhile, was suggested by Lowhagen’s postmortem detection of red blood cells in the olfactory nerves of humans following subarachnoid hemorrhage [43] but more pointedly illustrated by Johnston et al. in 2004 [41]. Injecting the silicone compound Microfil into the CSF of human cadaver specimens within 7 h of death (as well as six other species), Johnston observed that the Microfil became distributed across the submucosal lymphatic system [41]. Jackson et al. proposed two models for the precise anatomical relationship between SAS surrounding the olfactory nerve and the lymphatic vessels: an “open” model and a “closed” model [44]. Per the open model, CSF would freely drain into the extracellular matrix outside of the nerve sheath through an open-ended perineurial cuff before finally being absorbed directly by surrounding lymphatic capillaries. Contrastingly, the closed model holds that the end part of the nerve sheath is a closed blind end, requiring CSF to be absorbed by the surrounding lymphatic vessels via pinocytosis. There is some indirect evidence to suggest that the closed model best describes lymphatic submucosal drainage in humans (and that the state of affairs described by the open model may predominate in some nonhuman species) [41], although further work is required to validate this notion.
Research has also revealed that CSF can drain through the spinal nerve sheath (or perhaps alternatively through SAG) into the epidural lymphatic system [23, 27]. Miura et al., for instance, studied lymphatic drainage of CSF in monkeys by injecting ultrafine carbon particles into the SAS and differentiating lymphatics from blood capillaries using the 5′-nucleotidase (5′-Nase)-alkaline phosphatase (ALPase) double staining method. Subsequently, carbon-filled lymphatics and lymph nodes were prevalently seen in cervical and thoracic epidural connective tissue (and also to a lesser extent in lumbosacral regions) surrounding spinal nerve roots [23]. This spinal lymphatic pathway appears to be a crucial compensatory outflow route in conditions such as hydrocephalus. In a ferritin tracer study of rats with kaolin-induced CSF outflow obstruction, Voelz found that significant proportions of CSF were absorbed along spinal nerves into extradural lymphatic vessels, having egressed from the central canal through ruptured ependyma and dorsal columns [45]. Similar results following kaolin-induced obstruction were obtained by other authors using radiological and histological methods to track CSF outflow [46, 47]. Indeed, the lymphatic outflow pathway as a whole may play a compensatory role; as evidenced by Brinker, elevated intracranial pressure caused by experimentally induced subarachnoid hemorrhage in cats enhanced CSF drainage along the optic nerves into orbital and cervical lymph nodes [48]. Ultimately, the focus of this section is the pathway of CSF outflow from the SAS to the outside lymphatic system rather than a review of the paravascular pathway named for the glymphatic system that facilitates the CSF flow through the brain parenchyma and the clearance of interstitial solutes [49].
Before moving on, it is worth noting that other CSF drainage pathways have been proposed beyond those discussed above; these include routes putatively involving the arachnoid mater, ependyma, cerebral capillary, and choroid plexus among others. Outside of some observations made in subjects suffering abnormal physiological states, however, little evidence has arisen to suggest these pathways play any meaningful role in regular CSF absorption [1, 5]. Nonetheless, it is possible they play minor compensatory roles.
Volumetric analysis of CSF outflow
While the aforedescribed research has indicated that CSF can drain through cranial arachnoid granulations, spinal arachnoid granulations, and the lymphatic system, the proportion of outflow handled by each pathway has not yet been conclusively determined. In addition, it is still somewhat uncertain whether these proportions remain consistent across species, across prenatal and postnatal periods and childhood and adulthood, and across healthy and pathological states.
Nonetheless, progress has been made in this sphere. Injecting radioiodinated albumin into the lateral ventricles of anesthetized rabbits and cats and determining its distribution via radioactivity gradients, Bradbury et al. found that 30 % (in the case of rabbits) and 15 % (in the case of cats) of the animals’ CSF appeared to be absorbed through cervical lymph nodes [50]. Meanwhile, a series of comparable studies conducted by Boulton et al. in sheep indicated that 40–48 % of the total CSF volume was absorbed through the extracranial lymphatic system [35, 51, 52]. Boulton et al. also noted that the absorption ability of AG and the lymphatic system was 2.7 and 3.9 times greater, respectively, with elevated intracranial pressure. Yet another experiment by Boulton—this one conducted in rats and involving ligation of the lymph nodes and radioiodinated albumin injection—suggested that approximately 50 % of CSF drained through the lymphatic system while the other half was absorbed through cranial and spinal arachnoid granulations [53]. Since CSF production is likely to be proportional to brain volume and lymphatic drainage is supposed to scale to the cross-sectional area of the lymphatics (and, additionally, there is a vast difference in the brain volume of animals and humans) [23, 54], the proportions of CSF that efflux through each of these major pathways are still indeterminate in humans.
Because of the large change in brain volume from newborn to adult, extra drainage may be required to support brain function. Other studies have lent insight into how CSF outflow may change throughout development. One 2006 experiment utilized Evans blue as a tracer to observe CSF drainage through the lymphatic system in embryonic and postnatal pigs and rats finding that lymphatic outflow could not be observed in rats until 1 week after birth, but was detectable in pigs in as early as the 92nd day of the embryonic period [55]. Given that CAG are scarcely visible in fetal and newborn sheep [36] and are not easily observed in humans until 18 months after birth [7], it might be speculated that—in certain animals at least—the lymphatic pathway plays an especially important role in CSF drainage during embryonic and early postnatal development but takes a proverbial backseat as the arachnoid granulations mature and become better capable of supporting CSF absorption [1, 29]. Indeed, research conducted by Mollanji and Papaiconomou in sheep found that CSF’s absorptive parameters remained steady from embryonic and neonatal development through to adulthood regardless of the quantity and maturity of arachnoid granulations, buoying the idea that other outflow pathways compensate the arachnoid granulation pathways throughout development [36, 56] (and perhaps again in old age as the arachnoid granulations degenerate) [54]. This result was corroborated by Jones’ work in mice which showed that there was no positive pressure difference between CSF and the venous sinuses during the neonatal period corresponding to a high degree of outflow resistance which gradually decreased with age and the maturity of the arachnoid granulations [57]. More direct support for this idea has been supplied by Nagra’s finding that the amount of CSF absorbed through the lymphatic system in rats decreased each year as they aged [58].
Some headway has also been made in determining the proportion of CSF absorbed by the spinal pathway. Marmarou’s 1975 study, for instance, employed dilation balloons to isolate cerebral and spinal SAS in cats, with analysis of the CSF pressure response indicating that approximately 16 % of CSF was absorbed through spinal mechanisms [59]. A later experiment conducted by Bozanovic-Sosic in sheep reached somewhat similar conclusions; separating cerebral and spinal compartments with an extradural ligature, this utilized three different methods (introduction of different radiolabeled human serum albumins into each compartment via perfusion system, introduction of said human serum albumins via bolus injection, or injection of artificial CSF into each compartment via catheter) to parse CSF outflow and found spinal absorption accounted for between 12 and 25 % of overall drainage depending on the method used [27]. Perhaps most informative is a human study conducted by Edsbagge et al., wherein researchers injected 99mTc-DTPA into healthy young people’s SAS via lumbar puncture and examined spinal CSF absorption using radionuclide cisternography [60]. This work calculated that spinal CSF absorption accounted for roughly 20 % of total outflow and also indicated the rate of this absorption ranged from 0.11 to 0.23 ml/min. Interestingly, when spinal CSF absorption was considered in relation to physical activity, it was found that this rate was higher in active compared to resting individuals. It was hypothesized this might be due to a greater transarachnoid granulation pressure gradient in the upright position compared to the prone position [60].
Conclusion
As discussed in this review, the body of evidence in this field indicates that CSF is absorbed and drained in bulk not just through cerebral arachnoid granulations but also through spinal arachnoid granulations and a lymphatic pathway involving egress through cranial (particularly the olfactory nerve) and spinal nerve sheaths (Fig. 3) [61]. The proportions of CSF that efflux through each of these major pathways have yet to be determined with any certainty in humans, though existing evidence (the majority of which is derived from animal studies) suggests that lymphatic pathways may account for up to 50 % of CSF outflow—presumably leaving the CAG and SAG to process the balance. Notably, the development of the lymphatic system pathway seems to precede that of the arachnoid granulation pathways, and there are indications that the former pathway holds particular importance in the drainage of CSF during embryonic development and infancy. The lymphatic pathways also appear to play a compensatory role during pathological disturbances of CSF outflow such as hydrocephalus. Meanwhile, the total proportion of CSF handled by spinal pathways as opposed to cranial ones appears to lie between 15 and 25 %, and it too may have compensatory capacities. Indeed, outflow through both arachnoid granulations and lymphatic vessels has been shown to be pressure dependent [52].
Cerebrospinal fluid (CSF) secretion and absorption schematic diagram. CSF is secreted by the choroid plexus. It circulates rostrocaudally inside the ventricles and drains into the cerebellomedullary cistern (cisterna magna) through the median aperture of the fourth ventricle. CSF circulates in cranial and spinal subarachnoid spaces. It is absorbed and drained in bulk not just through cerebral arachnoid granulations but also through spinal arachnoid granulations and a lymphatic pathway involving egress through cranial (particularly the olfactory nerve) and spinal nerve sheaths. In the spinal subarachnoid space, the remaining CSF which is not absorbed by spinal arachnoid granulations or spinal nerve sheaths circulates rostrally toward the cranial subarachnoid space (reproduced with permission from [61])
A thorough anatomical and physiological understanding of the various CSF outflow pathways is inherently linked to our ability to analyze the mechanism of diseases and conditions related to disturbances in CSF circulation and consequently to make prognoses about said diseases. A clear understanding of the lymphatic pathways, and their potential for compensatory activity for instance, makes it far easier to explain how, in some animal models for hydrocephalus, intracranial pressure can automatically revert to normal levels after modeling [45]. Similarly, knowledge of the existence of SAG near the spinal nerve sheath can help inform our understanding of why some spinal cysts often occur near the spinal nerve sheath—local arachnoid granulation proliferation, when stimulated by trauma or inflammation, can enwrap CSF and lead to the formation of a cyst [62]. And an understanding of the critical nasal submucosal lymphatic drainage system might help explain why olfactory bulb dysplasia can be complicated with hydrocephalus [29, 63].
Though much has been learned about the pathways contributing to CSF outflow, further research is required to clarify the anatomical details of CSF absorption, the regulatory mechanisms that govern CSF absorption, and the proportion of CSF outflow that different pathways process at different stages of development and in various physiological states. Previous studies have—often by necessity—focused more on animals and human cadaver specimens at the expense of noninvasive, in vivo work with human subjects that might better tap CSF dynamics. Now, however, with the growing availability of new imaging technologies [42, 64–66], it is increasingly straightforward to noninvasively and dynamically track the flow direction of CSF; one hopes this development will enable neuroradiologists to make even greater contributions to future research in this field.
Key learning points
-
A thorough anatomical and physiological understanding of the various CSF outflow pathways is inherently linked to our ability to analyze the mechanism of diseases and conditions related to disturbances in CSF circulation and consequently to make prognoses about said diseases.
-
A clear understanding of the lymphatic pathways, and their potential for compensatory activity for instance, is important [45].
-
Knowledge of the existence of SAG near the spinal nerve sheath can help inform our understanding of why some spinal cysts often occur near the spinal nerve sheath—local arachnoid granulation proliferation, when stimulated by trauma or inflammation, can enwrap CSF and lead to the formation of a cyst [61].
-
And an understanding of the critical nasal submucosal lymphatic drainage system might help explain why olfactory bulb dysplasia can be complicated with hydrocephalus [29, 62].
Abbreviations
- CSF:
-
Cerebrospinal fluid
- CAG:
-
Cranial arachnoid granulations
- SAG:
-
Spinal arachnoid granulations
- SAS:
-
Subarachnoid space
References
Kapoor KG, Katz SE, Grzybowski DM, Lubow M (2008) Cerebrospinal fluid outflow: an evolving perspective. Brain Res Bull 77:327–334
Oreskovic D, Klarica M (2010) The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain Res Rev 64:241–262
Brunori A, Vagnozzi R, Giuffre R (1993) Antonio Pacchioni (1665–1726): early studies of the dura mater. J Neurosurg 78:515–518
Galarza M (2002) Evidence of the subcommissural organ in humans and its association with hydrocephalus. Neurosurg Rev 25:205–215
Pollay M (2010) The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res 7:9
Gomez DG, DiBenedetto AT, Pavese AM, Firpo A, Hershan DB, Potts DG (1982) Development of arachnoid villi and granulations in man. Acta Anat (Basel) 111:247–258
le Gros Clark WE (1920) On the pacchionian bodies. J Anat 55:40–48
Johnston M, Papaiconomou C (2002) Cerebrospinal fluid transport: a lymphatic perspective. News Physiol Sci 17:227–230
Weed LH (1914) Studies on cerebro-spinal fluid. No. II: the theories of drainage of cerebro-spinal fluid with an analysis of the methods of investigation. J Med Res 31:21–49
D’Avella D, Cicciarello R, Albiero F, Andrioli G (1983) Scanning electron microscope study of human arachnoid villi. J Neurosurg 59:620–626
Yamashima T (1986) Ultrastructural study of the final cerebrospinal fluid pathway in human arachnoid villi. Brain Res 384:68–76
Kida S, Yamashima T, Kubota T, Ito H, Yamamoto S (1988) A light and electron microscopic and immunohistochemical study of human arachnoid villi. J Neurosurg 69:429–435
Ohta K, Inokuchi T, Hayashida Y, Mizukami T, Yoshida T, Kawahara T (2002) Regional diminution of von Willebrand factor expression on the endothelial covering arachnoid granulations of human, monkey and dog brain. Kurume Med J 49:177–183
Welch K, Pollay M (1961) Perfusion of particles through arachnoid villi of the monkey. Am J Physiol 201:651–654
Jayatilaka DP (1965) Arachnoid granulations in sheep. J Anat 99:315–327
Tripathi RC (1977) The functional morphology of the outflow systems of ocular and cerebrospinal fluids. Exp Eye Res 25(Suppl):65–116
Grzybowski DM, Holman DW, Katz SE, Lubow M (2006) In vitro model of cerebrospinal fluid outflow through human arachnoid granulations. Invest Ophthalmol Vis Sci 47:3664–3672
Glimcher SA, Holman DW, Lubow M, Grzybowski DM (2008) Ex vivo model of cerebrospinal fluid outflow across human arachnoid granulations. Invest Ophthalmol Vis Sci 49:4721–4728
Brierley JB, Field EJ (1948) The connexions of the spinal sub-arachnoid space with the lymphatic system. J Anat 82:153–166
Welch K, Pollay M (1963) The spinal arachnoid villi of the monkeys Cercopithecus aethiops sabaeus and Macaca irus. Anat Rec 145:43–48
Shantha TR, Evans JA (1972) The relationship of epidural anesthesia to neural membranes and arachnoid villi. Anesthesiology 37:543–557
Kido DK, Gomez DG, Pavese AM Jr, Potts DG (1976) Human spinal arachnoid villi and granulations. Neuroradiology 11:221–228
Miura M, Kato S, von Ludinghausen M (1998) Lymphatic drainage of the cerebrospinal fluid from monkey spinal meninges with special reference to the distribution of the epidural lymphatics. Arch Histol Cytol 61:277–286
Tubbs RS, Hansasuta A, Stetler W, Kelly DR, Blevins D, Humphrey R, Chua GD, Shoja MM, Loukas M, Oakes WJ (2007) Human spinal arachnoid villi revisited: immunohistological study and review of the literature. J Neurosurg Spine 7:328–331
Seyfert S, Koch HC, Kunzmann V (2003) Conditions of iodine contrast transfer from lumbosacral CSF to blood. J Neurol Sci 206:85–90
Biceroglu H, Albayram S, Ogullar S, Hasiloglu ZI, Selcuk H, Yuksel O, Karaaslan B, Yildiz C, Kiris A (2012) Direct venous spinal reabsorption of cerebrospinal fluid: a new concept with serial magnetic resonance cisternography in rabbits. J Neurosurg Spine 16:394–401
Bozanovic-Sosic R, Mollanji R, Johnston MG (2001) Spinal and cranial contributions to total cerebrospinal fluid transport. Am J Physiol Regul Integr Comp Physiol 281:R909–916
Kelkenberg U, von Rautenfeld DB, Brinker T, Hans VH (2001) Chicken arachnoid granulations: a new model for cerebrospinal fluid absorption in man. Neuroreport 12:553–557
Koh L, Zakharov A, Johnston M (2005) Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res 2:6
Svane-Knudsen V (1958) Resorption of the cerebro-spinal fluid in guinea-pig; an experimental study. Acta Otolaryngol 49:240–251
Czerniawska A (1970) Experimental investigations on the penetration of 198Au from nasal mucous membrane into cerebrospinal fluid. Acta Otolaryngol 70:58–61
Arnold W, Nitze HR, Ritter R, von Ilberg C, Ganzer U (1972) Qualitative study of the connections of the subarachnoid space with the lymphatic system of the head and neck. Acta Otolaryngol 74:411–424
Pile-Spellman JM, McKusick KA, Strauss HW, Cooney J, Taveras JM (1984) Experimental in vivo imaging of the cranial perineural lymphatic pathway. AJNR Am J Neuroradiol 5:539–545
Kida S, Pantazis A, Weller RO (1993) CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol 19:480–488
Boulton M, Young A, Hay J, Armstrong D, Flessner M, Schwartz M, Johnston M (1996) Drainage of CSF through lymphatic pathways and arachnoid villi in sheep: measurement of 125I-albumin clearance. Neuropathol Appl Neurobiol 22:325–333
Mollanji R, Papaiconomou C, Boulton M, Midha R, Johnston M (2001) Comparison of cerebrospinal fluid transport in fetal and adult sheep. Am J Physiol Regul Integr Comp Physiol 281:R1215–1223
Mollanji R, Bozanovic-Sosic R, Silver I, Li B, Kim C, Midha R, Johnston M (2001) Intracranial pressure accommodation is impaired by blocking pathways leading to extracranial lymphatics. Am J Physiol Regul Integr Comp Physiol 280:R1573–1581
Mollanji R, Bozanovic-Sosic R, Zakharov A, Makarian L, Johnston MG (2002) Blocking cerebrospinal fluid absorption through the cribriform plate increases resting intracranial pressure. Am J Physiol Regul Integr Comp Physiol 282:R1593–1599
Silver I, Kim C, Mollanji R, Johnston M (2002) Cerebrospinal fluid outflow resistance in sheep: impact of blocking cerebrospinal fluid transport through the cribriform plate. Neuropathol Appl Neurobiol 28:67–74
Zakharov A, Papaiconomou C, Djenic J, Midha R, Johnston M (2003) Lymphatic cerebrospinal fluid absorption pathways in neonatal sheep revealed by subarachnoid injection of Microfil. Neuropathol Appl Neurobiol 29:563–573
Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D (2004) Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res 1:2
Mathieu E, Gupta N, Macdonald RL, Ai J, Yucel YH (2013) In vivo imaging of lymphatic drainage of cerebrospinal fluid in mouse. Fluids Barriers CNS 10:35
Lowhagen P, Johansson BB, Nordborg C (1994) The nasal route of cerebrospinal fluid drainage in man. A light-microscope study. Neuropathol Appl Neurobiol 20:543–550
Jackson RT, Tigges J, Arnold W (1979) Subarachnoid space of the CNS, nasal mucosa, and lymphatic system. Arch Otolaryngol 105:180–184
Voelz K, Kondziella D, von Rautenfeld DB, Brinker T, Ludemann W (2007) A ferritin tracer study of compensatory spinal CSF outflow pathways in kaolin-induced hydrocephalus. Acta Neuropathol 113:569–575
Faulhauer K, Donauer E (1985) Experimental hydrocephalus and hydrosyringomyelia in the cat. Radiological findings Acta Neurochir (Wien) 74:72–80
Luedemann W, Kondziella D, Tienken K, Klinge P, Brinker T, Berens von Rautenfeld D (2002) Spinal cerebrospinal fluid pathways and their significance for the compensation of kaolin-hydrocephalus. Acta Neurochir Suppl 81:271–273
Brinker T, Ludemann W, von Rautenfeld DB, Brassel F, Becker H, Samii M (1997) Breakdown of the meningeal barrier surrounding the intraorbital optic nerve after experimental subarachnoid hemorrhage. Am J Ophthalmol 124:373–380
Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H (2013) Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 123:1299–1309
Bradbury MW, Cole DF (1980) The role of the lymphatic system in drainage of cerebrospinal fluid and aqueous humour. J Physiol 299:353–365
Boulton M, Flessner M, Armstrong D, Hay J, Johnston M (1998) Determination of volumetric cerebrospinal fluid absorption into extracranial lymphatics in sheep. Am J Physiol 274:R88–96
Boulton M, Armstrong D, Flessner M, Hay J, Szalai JP, Johnston M (1998) Raised intracranial pressure increases CSF drainage through arachnoid villi and extracranial lymphatics. Am J Physiol 275:R889–896
Boulton M, Flessner M, Armstrong D, Mohamed R, Hay J, Johnston M (1999) Contribution of extracranial lymphatics and arachnoid villi to the clearance of a CSF tracer in the rat. Am J Physiol 276:R818–823
Johanson CE, Duncan JA 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD (2008) Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res 5:10
Koh L, Zakharov A, Nagra G, Armstrong D, Friendship R, Johnston M (2006) Development of cerebrospinal fluid absorption sites in the pig and rat: connections between the subarachnoid space and lymphatic vessels in the olfactory turbinates. Anat Embryol (Berl) 211:335–344
Papaiconomou C, Bozanovic-Sosic R, Zakharov A, Johnston M (2002) Does neonatal cerebrospinal fluid absorption occur via arachnoid projections or extracranial lymphatics? Am J Physiol Regul Integr Comp Physiol 283:R869–876
Jones HC (1985) Cerebrospinal fluid pressure and resistance to absorption during development in normal and hydrocephalic mutant mice. Exp Neurol 90:162–172
Nagra G, Johnston MG (2007) Impact of ageing on lymphatic cerebrospinal fluid absorption in the rat. Neuropathol Appl Neurobiol 33:684–691
Marmarou A, Shulman K, LaMorgese J (1975) Compartmental analysis of compliance and outflow resistance of the cerebrospinal fluid system. J Neurosurg 43:523–534
Edsbagge M, Tisell M, Jacobsson L, Wikkelso C (2004) Spinal CSF absorption in healthy individuals. Am J Physiol Regul Integr Comp Physiol 287:R1450–1455
Sakka L, Coll G, Chazal J (2011) Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol, Head Neck Dis 128:309–316
Rexed B (1947) Arachnoidal proliferations with cyst formation in human spinal nerve roots at their entry into the intervertebral foramina; preliminary report. J Neurosurg 4:414–421
Naruse I, Ueta E (2002) Hydrocephalus manifestation in the genetic polydactyly/arhinencephaly mouse (Pdn/Pdn). Congenit Anom (Kyoto) 42:27–31
Linninger AA, Sweetman B, Penn R (2009) Normal and hydrocephalic brain dynamics: the role of reduced cerebrospinal fluid reabsorption in ventricular enlargement. Ann Biomed Eng 37:1434–1447
Calcagni ML, Lavalle M, Mangiola A, Indovina L, Leccisotti L, De Bonis P, Marra C, Pelliccioni A, Anile C, Giordano A (2012) Early evaluation of cerebral metabolic rate of glucose (CMRglu) with 18F-FDG PET/CT and clinical assessment in idiopathic normal pressure hydrocephalus (INPH) patients before and after ventricular shunt placement: preliminary experience. Eur J Nucl Med Mol Imaging 39:236–241
Reiss-Zimmermann M, Scheel M, Dengl M, Preuss M, Fritzsch D, Hoffmann KT (2013) The influence of lumbar spinal drainage on diffusion parameters in patients with suspected normal pressure hydrocephalus using 3T MRI. Acta Radiol 55:622–630
Ethical standards and patient consent
We declare that this manuscript does not contain clinical studies, patient data or animal care.
Conflict of interest
We declare that we have no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, L., Elias, G., Yostos, M.P. et al. Pathways of cerebrospinal fluid outflow: a deeper understanding of resorption. Neuroradiology 57, 139–147 (2015). https://doi.org/10.1007/s00234-014-1461-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-014-1461-9