Abstract
Introduction
This study aimed to relate growth of the infarct core with time to recanalization in patients receiving mechanical recanalization in whom the time of recanalization is known.
Methods
We analyzed data from patients with anterior circulation acute ischemic stroke who underwent mechanical recanalization. Demographic and angiographic characteristics, initial apparent diffusion coefficient (ADC) infarct volume, time-to-peak defect volume, revascularization grade, 24–48 h nonenhanced computed tomography (CT) infarct volume, symptom onset to recanalization time, diffusion-weighted imaging to recanalization time, and discharge National Institutes of Health Stroke Scale (NIHSS) and modified Rankin Scale (mRS) scores were compared between minimal and substantial infarct growth groups. Substantial infarct growth was defined as an increase of infarct volume >10 cm3 assessed by subtracting initial ADC infarct core volume from infarct volume at 24–48 h CT.
Results
Of 25 patients, 9 had minimal infarct growth (median 0 cm3, interquartile range (IQR) −3 to 5 cm3) and 16 had substantial infarct growth (median 103 cm3, IQR 48–132 cm3). Patients with minimal infarct growth had a median time from symptom onset to recanalization of 329 min (IQR 314–412 min) and a median time from imaging to recanalization of 231 min (IQR 198–309 min). On univariate analysis, minimal infarct growth was related to male gender (p = 0.04), smaller initial ADC volume (p = 0.04), higher recanalization grade (p < 0.001), and lower discharge NIHSS (p = 0.04) and mRS grades (p = 0.04).
Conclusion
There was no or minimal infarct core growth in at least one third of patients despite an exceptionally long median time from magnetic resonance imaging to recanalization of almost 4 h.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Treatment of acute ischemic stroke aims at fast vessel reperfusion to salvage penumbral tissue. Successful reperfusion in acute ischemic stroke therapy is strongly associated with good outcome [1]. The potentially salvageable tissue, the penumbra, has been estimated by the perfusion/diffusion mismatch on magnetic resonance imaging (MRI) [2–5]. In patients who suffer from large artery occlusion, a perfusion/diffusion mismatch has been observed in a large subset of patients even beyond 9 h after stroke onset [6]. On the other hand, the time window for evidence-based treatment of acute ischemic stroke in the anterior circulation, intravenous thrombolysis, is 4.5 h after stroke onset, and a continuous decline of the number of patients who might benefit from treatment has been postulated [7]. However, there is evidence that penumbral tissue may remain stable for certain time periods [6, 8], but the exact correlation with reperfusion time in patients treated with endovascular reperfusion therapy has yet not been performed.
For this purpose, we studied a population of acute ischemic stroke patients suffering from middle cerebral artery occlusion that had been subjected to endovascular reperfusion therapy so that time intervals from initial MRI to reperfusion and final infarct growth could be assessed to identify and characterize the subgroup of patients with stable infarct core.
Materials and methods
Patients with acute ischemic stroke in the anterior circulation admitted to the Stroke Unit of the University Hospital in Eppendorf, Hamburg (certified by the German Stroke Society) between January 2009 and June 2012 were retrospectively assessed. Ethical approval for this type of study was not warranted by our institution. During the study time period, large vessel occlusions were not prioritized for endovascular treatment unless no response or secondary deterioration after intravenous tissue plasminogen activator (iv tPA) therapy had been documented. A neurologist examined all patients immediately after admission to the emergency room, and the neurological deficit was scored using the National Institutes of Health Stroke Scale (NIHSS). Demographics and clinical data were recorded including age, sex, premedication, time of symptom onset, coronary artery disease, atrial fibrillation, hypertension, diabetes, current smoking, hypercholesterolemia according to history of current lipid values, and history of transient ischemic attack or ischemic stroke.
Inclusion criteria for the study were as follows: (1) acute neurological symptoms corresponding to the findings of (2) acute stroke MRI documenting proximal vessel occlusion; (3) diffusion-weighted imaging (DWI) and first pass dynamic susceptibility contrast-enhanced MR-perfusion imaging (MR-perfusion); (4) NIHSS score ≥4 or aphasia; (5) endovascular reperfusion therapy of middle cerebral artery (MCA) occlusion with or without internal carotid artery (ICA) occlusion during the first 8 h after symptom onset; (6) follow-up cranial computed tomography (CT) 24–48 h after stroke onset; and (7) sufficient image quality of MRI and CT scans for analysis and area measurements regarding DWI, MR-perfusion lesion volumes, and final infarct size at CT.
Exclusion criteria were the following: (1) no cerebral artery occlusion at digital subtraction angiography (DSA) and (2) intracerebral hemorrhage on the 24–48-h follow-up CT with blood occupying >30 % of infarct area and substantial mass effect, i.e., a PH2 ECASS class hemorrhage [9, 10].
MRI protocol and bridging therapy
MRI examinations were performed at 1.5 and 3 T scanners (Magnetom Avanto and Magnetom Symphony, Siemens, Erlangen, Germany). Examination protocol included axial DWI, MR-perfusion, 3D time of flight (TOF)-MR angiography, and fluid-attenuated inversion recovery (FLAIR) sequences. During the acquisition of the MR-perfusion sequence 15 ml of 0.5 mmol/ml gadopentate dimeglumine (Magnograf, Marotrast, Jena, Germany) was injected intravenously with a flow rate of 3–4 ml/s.
If patients fulfilled inclusion criteria for intravenous thrombolysis, tPA (Actilyse, Boehringer, Ingelheim, Germany) was given immediately after termination of the MR examination. Patients were given a standard dose of 0.9 mg-tPA/kg body weight. Following iv tPA administration, the patients were closely monitored. If there was evidence of no clinical improvement, clinical deterioration, or no vessel reperfusion, the indication for endovascular therapy was discussed with the attending neurologist and neuroradiologist.
Infarct volume calculation
On the initial apparent diffusion coefficient (ADC) map, the infarct core volume was determined by manual segmentation of the low signal ischemic lesion, while on the MR-perfusion images, the hypoperfused tissue was determined as the maximum volume of circulatory dysfunction on a section-by-section basis [11]. All segmentations were done by one of the authors (S.F.) using the Analyze 9.0 software package (Analyze Direct, Overland Park, KS, USA). The absolute volume difference of DWI and MR-perfusion lesion volumes was calculated and used as a surrogate of penumbral tissue. The final infarct volume was segmented on CT studies of the brain performed 24–48 h after the onset of symptoms. Increase of infarct volume was calculated as the difference between the infarct volume at CT and the initial ADC infarct core volume. According to the volume of infarct growth, the patient population was divided into a group with minimal infarct growth (<10 cm3) and a group with substantial infarct growth (>10 cm3).
Angiography
All patients were subjected to general anesthesia before undergoing endovascular therapy. DSA was performed on a biplane angiography system (Allura Xper FD, Philips, Best, The Netherlands). Topographically, the occlusions were divided into carotid T occlusion, tandem (distinct ICA and MCA occlusions), and M1 and M2 occlusions.
The pial collateral circulation was scored in a standard anteroposterior view of an internal carotid injection angiography according to the grading scheme adapted from Christoforidis et al. [12]: grade 1 if retrograde filling via leptomeningeal collaterals reconstituted the distal M1 segment; grade 2 if collaterals reconstituted the M2 segment; grade 3 if collaterals reconstituted vessels up to the level of M3; and grade 4 if collaterals reconstituted vessels up to the level of M4 segments.
Mechanical thrombectomy
General anesthesia was induced in the angiography suite by using a standardized protocol. After a diagnostic series of both ICAs to document collateral flow, a 6–8-F guiding catheter with or without an occluding balloon was inserted into the proximal ICA, and a microcatheter (2.3 F Prowler Select Plus, Codman Endovascular, MA, USA; 2.8 F Rebar, Covidien, Mansfield, MA, USA) was used to pass the thrombus in the occluded vessel. If a tandem occlusion was present, the proximal ICA stenosis was stented and dilated either before or after treatment of the distal occlusion. Large intraluminal thrombus in the ICA was aspirated through a large bore 5.2-F distal access catheter (DAC; Concentric Medical, Mountain View, CA, USA) before or after stenting of the proximal ICA.
The techniques used for thrombectomy included (1) thromboaspiration and 2) clot entrapment and retrieval with a stentriever or another clot-engaging device. These techniques were applied separately or in combination. Thromboaspiration was performed with the Penumbra thromboaspiration system (Penumbra, Alameda, CA, USA). Stentrievers used included the Solitaire (Covidien) and the Revive (Codman Endovascular) systems. Rarely the Phenox clot retriever system (Phenox, Bochum, Germany) was used. Usually thrombectomy was done under flow blockage with temporary balloon inflation of the guiding catheter and simultaneous aspiration. The number of retrieval attempts for retriever systems was recorded. In one patient only, thrombus disruption with the microguidewire was attempted. Additional intra-arterial thrombolysis with 20–40 mg of tPA was administered at the discretion of the interventionalist. In the case of a persistent significant intracranial stenosis, intracranial stenting was performed and double anti-aggregation therapy was initiated with intravenous application of 300–500 mg aspirin and of 300 mg clopidogrel via the gastric tube. Patients were transferred to the ICU for at least 24 h and were subjected to a control CT within 24–48 h after the DSA.
Reperfusion was documented by angiography and was graded according to the modified thrombolysis in cerebral infarction (TICI) grading system [13]: grade 0, no reperfusion; grade 1, flow beyond occlusion without distal branch reperfusion; grade 2a, reperfusion of less than half of the downstream target arterial territory; grade 2b, reperfusion of more than half, yet incomplete, in the downstream target arterial territory; and grade 3, complete reperfusion of the downstream target arterial territory, including distal branches with slow flow.
The following time intervals were calculated: (1) time to imaging was defined as time from symptom onset to DWI acquisition and (2) time to reperfusion was defined as time from DWI acquisition to sufficient reperfusion at angiography. Discharge NIHSS and modified Rankin Scale (mRS) scores were recorded for every patient.
Statistical analysis
For statistical analysis, patients with minimal infarct growth were identified and compared to the remaining patients showing substantial infarct growth. Statistical analysis was performed by using the SPSS 18 (IBM) statistical software package. We used the Fisher exact test and the Mann–Whitney U test for significance testing for differences between groups for categorical and continuous variables. For multiple group comparison, we used the Kruskal-Wallis one-way analysis by rank tests. p values < 0.05 were considered significant. No comparison of multiple variables was attempted because of the small sample size.
Results
During the study period, 63 patients with anterior cerebral circulation occlusion were referred for endovascular therapy. Of these, 28 patients had an initial MRI investigation, a known time of stroke symptom onset, and a follow-up CT 24–48 h after revascularization therapy. Three patients were excluded from the analysis because of large PH2 bleedings on the 24–48 CT, leaving 25 patients for final analysis. At admission, all patients presented with the typical clinical signs of an ischemic infarct in the right (n = 10) or left (n = 15) MCA territory with contralateral hemiparesis.
The demographics of the patients are shown in Table 1. The median time from symptom onset to DWI was 127 min (interquartile range (IQR) 97–201 min). The median NIHSS at the time of admission was 15 (range 4–23). Following MR imaging, 17 patients (68 %) received iv tPA therapy. Endovascular therapy was performed in all patients and resulted in a TICI reperfusion grade of 0 in six patients (24 %), grade 1 in one patient (4 %), grade 2a in ten patients (40 %), grade 2b in two patients (8 %), and grade 3 in six patients (24 %). Nine patients had minimal (i.e., <10 cm3) infarct growth (median 0 cm3, IQR −3 to 5 cm3), and 16 patients had substantial infarct growth (median 103 cm3, IQR 48–132 cm3).
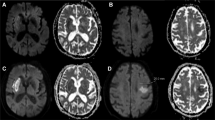
In the group of patients with minimal infarct growth, the median time from DWI imaging to reperfusion was 231 min (IQR 198–309 min), and the median time from symptom onset to reperfusion was 329 min (IQR 314–412 min). The detailed demographic, imaging, and outcome data for these patients are shown in Table 2. In three patients, the volume of the ischemic core had decreased on the 24–48-h CT scan, while in the other six, infarct growth was minimal, not exceeding 7 cm3. Thrombectomy was performed with a stentriever in eight patients while permanent stent placement across a thrombosed M1 stenosis was performed in one patient. The ADC maps and 24–48 h CT scans for patients 3 and 5 with a TICI 3 reperfusion are shown in Fig. 1.
The groups with minimal and substantial infarct growth showed comparable demographics (Table 1), with a higher percentage of male patients in the group with minimal infarct growth (p = 0.04). Though the time from symptom onset to DWI imaging was shorter for the minimal infarct growth group (p = 0.03), there was no statistical difference regarding symptom onset to reperfusion time (p = 0.21) and time from DWI imaging to reperfusion (p = 0.14) (Table 3). The initial ADC volume was larger for the substantial infarct growth group (median 48 cm3) than for the minimal infarct growth group (median = 7 cm3, p = 0.04). There was no statistical difference concerning the percentage of patients receiving iv rtPA prior to angiography (77.8 % in the minimal vs 62.5 % in the substantial infarct growth group, p = 0.04), the site of occlusion (six ICA, one tandem, and two M1 vs three ICA, four tandem, eight M1, and one M2, p = 0.7), the degree of collateral circulation (one M1, five M2, and three M3 vs two M1, nine M2, and five M3, p = 0.8), the incidence of concomitant severe cervical ICA stenosis (two patients vs one patient), and the deployment of a permanent intracranial stent (one vs two patients). Stentrievers were the main thrombectomy devices used in both groups of patients (88.9 % in the minimal and 68.8 % in the substantial infarct growth group, p = 0.29) with a median number of 2 passes (1 to 5). In two patients, only thrombus disruption with the microguidewire was attempted, and in one patient only, an intracranial stent was positioned. The minimal infarct growth group consisted of all six patients with a TICI 3 reperfusion grade and of three patients with a TICI 2a reperfusion grade (Table 2). Discharge NIHSS and mRS were better for the minimal infarct growth group compared to the substantial infarct growth group (median NIHSS 6 vs 13, p = 0.04 and median mRS 3 vs 4, respectively, p = 0.04).
The median volumes of infarct increase showed a significant difference among the TICI 0/1, TICI 2a/2b, and TICI 3 subgroups (p = 0.003) with the volume of infarct increase steadily declining with higher reperfusion grades (Fig. 2).
Discussion
As a main finding, we identified a subgroup of patients with large vessel occlusion, acute ischemic stroke, and reperfusion that showed only minimal infarct growth during the first hours. Median time from imaging to reperfusion was nearly 4 h indicating that the ischemic core might be stable over a period of several hours. Because all patients with TICI 3 reperfusion showed no significant infarct growth, this stability seems to be the rule rather than the exception in large vessel occlusions. A possible explanation would be that in this particular subgroup of patients, once the vessel occlusion has occurred, there is an initial phase of rapid cell death, succeeded by a phase of infarct core stability that may last for several hours and that is stabilized by sufficient collateral blood flow. The differences between the minimal infarct growth group and the substantial infarct growth group included a lower percentage of female patients (p = 0.04), a lower initial ADC volume (p = 0.04), and a higher percentage of TICI grade 3 reperfusions (p < 0.001). A multivariate analysis to sort the important variables was not performed because of the small sample size. However, a larger initial ADC volume could theoretically lead to a larger infarct volume increase as more tissue located at the periphery of the infarct core could be at risk to sustain permanent damage. Remarkably, the size of the hypoperfused tissue did not differ between the minimal and substantial infarct growth groups. On the other hand, the degree of revascularization has been linked to infarct growth and final outcome with significantly smaller final infarct volumes and better clinical outcomes as the TICI grade increases [14, 15]. We could also observe this trend in our study with infarct volume growth becoming significantly smaller as the reperfusion grade increased (Fig. 2). This implies that endovascular stroke therapy should aim at the highest possible grade of revascularization to increase the chances of a potential clinical benefit. However, one should keep in mind the risk of potential complications secondary to excessive endovascular manipulations.
The stability of the size of the infarct core challenges the commonly accepted concept of rapid growth of infarct core in ischemic stroke patients [16]. Penumbral persistence has been suggested by other studies which focused on the evolution of the DWI/MR-perfusion mismatch with time [6, 8]. Gonzalez et al. [8] studied a group of 14 patients with anterior circulation ischemia who presented a stable mismatch of four or more hours; Copen et al. [6] in a series of 109 anterior circulation stroke patients controlled the mismatch after 9 h in 19 patients and observed stability in 13 patients. The comparability of the diffusion and MR-perfusion deficits in stroke patients imaged 3 h after symptom onset relative to patients imaged 3–6 h after onset has also been observed by Fiehler et al. [17]. The stability of the ischemic core beyond 4.5 h after stroke onset, which is the time window of evidence-based acute ischemic stroke treatment, has implications on treatment strategies. The time window might be extended in selected patients, e.g., patients presenting with small DWI lesion volume in combination with large artery occlusion. Selection criteria should be further assessed in larger patient numbers by future studies.
Imaging of the collateral circulation may be used to identify optimal candidates for intervention, and the existence of collateral circulation may be predictive of a large stable mismatch [8, 18]. For anterior circulation strokes, the pial collateral circulation to the MCA territory, primarily from the anterior cerebral artery, is the main determinant of cerebral blood flow impairment and, thus, the rate of neuronal loss [19]. Collateral circulation differs among patients and is a significant predictor of clinical outcome and tissue fate [12, 20–24]. In our study, no differences in collateral status between the minimal infarct growth and the substantial infarct growth groups were found. However, this might be explained by insufficient sample size as well as the absence of a commonly accepted grading system for collateral status.
We used decreased tissue ADC as a surrogate for infarct core. A decrease in ADC indicates the tissue at risk of infarction in acute stroke patients [25] and predicts the infarct core volume [26]. In animal studies of MCA occlusion, the degree of ADC decrease is related to the location and extent of neuronal injury with the most severe histological damage occurring within areas of severest ADC decrease [27]. We also used tissue outcome as a surrogate for stroke outcome, a methodology widely accepted in stroke studies [28, 29]. Only four patients (16 %) had a good clinical outcome with a discharge mRS lower or equal to 2. We attribute this poor result compared to other endovascular therapy series which report good outcome in up to 33 % of patients with ICA occlusion [30] to comorbidities present in our aged patient group and to the use of older revascularization techniques that are no longer used in our department. Additionally, hypotensive episodes during induction of general anesthesia as well as prolonged intubation after the intervention might have influenced patient outcome negatively. The discharge NIHSS and mRS were better for the minimal infarct growth group than for the substantial infarct growth group (p = 0.04 for both parameters). Though the clinical impact of final infarct size strongly depends on anatomical location, a smaller final infarct size should eventually translate into a smaller clinical deficit.
This is a retrospective study on a small patient group that may be subject to bias. Moreover, it extends over a 3-year period during which techniques of revascularization therapy have evolved substantially in the department with a shift from the use of thrombus fragmentation and aspiration devices to the use of stent retrievers as first line devices for endovascular treatment. Also at that time, large vessel occlusions were not prioritized for endovascular treatment unless no response or secondary deterioration after iv rtPA had been documented. This explains the relatively long median time from DWI imaging to final reperfusion. Our reperfusion rate of 72 % (TICI 2a, 2b, and 3) is comparable to the reported rate of 69 % (TIMI 2 and 3) in the prospective SWIFT trial [31].
Conclusion
This retrospective study including patients with exceptionally long times from imaging to reperfusion implies that a subgroup of ischemic stroke patients exists in whom the infarct core and penumbra remain stable for several hours in the acute phase. Nonrecanalizers showed substantial ischemic core growth under the same conditions compared to recanalizers. This observation underlines the possibility of widening the time window for reperfusion therapy in selected patients, i.e., patients with proximal vessel occlusion and small infarct core, but does not justify treatment delay. These results might have an impact on treatment strategies and should be verified in larger prospective studies.
References
Rha JH, Saver JL (2007) The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke; A J Cereb Circ 38(3):967–973. doi:10.1161/01.STR.0000258112.14918.24
Kidwell CS, Alger JR, Saver JL (2003) Beyond mismatch: evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke; A J Cereb Circ 34(11):2729–2735. doi:10.1161/01.STR.0000097608.38779.CC
Hillis AE, Gold L, Kannan V, Cloutman L, Kleinman JT, Newhart M, Heidler-Gary J, Davis C, Aldrich E, Llinas R, Gottesman RF (2008) Site of the ischemic penumbra as a predictor of potential for recovery of functions. Neurology 71(3):184–189. doi:10.1212/01.wnl.0000317091.17339.98
Toth G, Albers GW (2009) Use of MRI to estimate the therapeutic window in acute stroke: is perfusion-weighted imaging/diffusion-weighted imaging mismatch an EPITHET for salvageable ischemic brain tissue? Stroke; A J Cereb Circ 40(1):333–335. doi:10.1161/STROKEAHA.108.525683
Grigoryan M, Tung CE, Albers GW (2011) Role of diffusion and perfusion MRI in selecting patients for reperfusion therapies. Neuroimaging Clin N Am 21(2):247–257. doi:10.1016/j.nic.2011.01.002, ix-x
Copen WA, Rezai Gharai L, Barak ER, Schwamm LH, Wu O, Kamalian S, Gonzalez RG, Schaefer PW (2009) Existence of the diffusion-perfusion mismatch within 24 hours after onset of acute stroke: dependence on proximal arterial occlusion. Radiology 250(3):878–886. doi:10.1148/radiol.2503080811
Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, Albers GW, Kaste M, Marler JR, Hamilton SA, Tilley BC, Davis SM, Donnan GA, Hacke W, Allen K, Mau J, Meier D, del Zoppo G, De Silva DA, Butcher KS, Parsons MW, Barber PA, Levi C, Bladin C, Byrnes G (2010) Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 375(9727):1695–1703. doi:10.1016/S0140-6736(10)60491-6
Gonzalez RG, Hakimelahi R, Schaefer PW, Roccatagliata L, Sorensen AG, Singhal AB (2010) Stability of large diffusion/perfusion mismatch in anterior circulation strokes for 4 or more hours. BMC Neurol 10:13. doi:10.1186/1471-2377-10-13
Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, Boysen G, Bluhmki E, Hoxter G, Mahagne MH et al (1995) Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA: J Am Med Ass 274(13):1017–1025
Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, Schneider D, Diez-Tejedor E, Trouillas P (1998) Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 352(9136):1245–1251
Christensen S, Mouridsen K, Wu O, Hjort N, Karstoft H, Thomalla G, Rother J, Fiehler J, Kucinski T, Ostergaard L (2009) Comparison of 10 perfusion MRI parameters in 97 sub-6-hour stroke patients using voxel-based receiver operating characteristics analysis. Stroke; J Cereb Circ 40(6):2055–2061. doi:10.1161/STROKEAHA.108.546069
Christoforidis GA, Mohammad Y, Kehagias D, Avutu B, Slivka AP (2005) Angiographic assessment of pial collaterals as a prognostic indicator following intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol 26(7):1789–1797
Tomsick T, Broderick J, Carrozella J, Khatri P, Hill M, Palesch Y, Khoury J (2008) Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol 29(3):582–587. doi:10.3174/ajnr.A0843
Almekhlafi MA, Menon BK, Freiheit EA, Demchuk AM, Goyal M (2013) A meta-analysis of observational intra-arterial stroke therapy studies using the Merci device, Penumbra system, and retrievable stents. AJNR Am J Neuroradiol 34(1):140–145. doi:10.3174/ajnr.A3276
Jayaraman MV, Grossberg JA, Meisel KM, Shaikhouni A, Silver B (2012) The clinical and radiographic importance of distinguishing partial from near-complete reperfusion following intra-arterial stroke therapy. AJNR Am J Neuroradiol. doi:10.3174/ajnr.A3278
Saver JL (2006) Time is brain—quantified. Stroke; J Cereb Circ 37(1):263–266. doi:10.1161/01.STR.0000196957.55928.ab
Fiehler J, Kucinski T, Knudsen K, Rosenkranz M, Thomalla G, Weiller C, Rother J, Zeumer H (2004) Are there time-dependent differences in diffusion and perfusion within the first 6 hours after stroke onset? Stroke; A J Cereb Circ 35(9):2099–2104. doi:10.1161/01.STR.0000138450.15078.b5
Liebeskind DS (2005) Collaterals in acute stroke: beyond the clot. Neuroimaging Clin N Am 15(3):553–573. doi:10.1016/j.nic.2005.08.012, x
Liebeskind DS (2003) Collateral circulation. Stroke; J Cereb Circ 34(9):2279–2284. doi:10.1161/01.STR.0000086465.41263.06
Kucinski T, Koch C, Eckert B, Becker V, Kromer H, Heesen C, Grzyska U, Freitag HJ, Rother J, Zeumer H (2003) Collateral circulation is an independent radiological predictor of outcome after thrombolysis in acute ischaemic stroke. Neuroradiology 45(1):11–18. doi:10.1007/s00234-002-0881-0
Tan IY, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, Martin M, Symons SP, Fox AJ, Aviv RI (2009) CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol 30(3):525–531. doi:10.3174/ajnr.A1408
Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, Kim D, Jahan R, Duckwiler GR, Yoon SR, Vinuela F, Liebeskind DS (2008) Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol, Neurosurg, Psychiatry 79(6):625–629. doi:10.1136/jnnp.2007.132100
Ringelstein EB, Biniek R, Weiller C, Ammeling B, Nolte PN, Thron A (1992) Type and extent of hemispheric brain infarctions and clinical outcome in early and delayed middle cerebral artery recanalization. Neurology 42(2):289–298
Takada T, Yasaka M, Minematsu K, Naritomi H, Yamaguchi T (2004) Predictors of clinical outcome in patients receiving local intra-arterial thrombolysis without subsequent symptomatic intracranial hemorrhage against acute middle cerebral artery occlusion. AJNR Am J Neuroradiol 25(10):1796–1801
Fiehler J, Foth M, Kucinski T, Knab R, von Bezold M, Weiller C, Zeumer H, Rother J (2002) Severe ADC decreases do not predict irreversible tissue damage in humans. Stroke; J Cereb Circ 33(1):79–86
Ma L, Gao PY, Hu QM, Lin Y, Jing LN, Xue J, Wang XC, Chen ZJ, Wang YL, Liao XL, Liu ML, Chen WJ (2010) Prediction of infarct core and salvageable ischemic tissue volumes by analyzing apparent diffusion coefficient without intravenous contrast material. Acad Radiol 17(12):1506–1517. doi:10.1016/j.acra.2010.07.010
Knight RA, Dereski MO, Helpern JA, Ordidge RJ, Chopp M (1994) Magnetic resonance imaging assessment of evolving focal cerebral ischemia. Comparison with histopathology in rats. Stroke;J Cereb Circ 25(6):1252–1261, discussion 1261–1252
Yoo AJ, Chaudhry ZA, Nogueira RG, Lev MH, Schaefer PW, Schwamm LH, Hirsch JA, Gonzalez RG (2012) Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke; J Cereb Circ 43(5):1323–1330. doi:10.1161/STROKEAHA.111.639401
Lansberg MG, Lee J, Christensen S, Straka M, De Silva DA, Mlynash M, Campbell BC, Bammer R, Olivot JM, Desmond P, Davis SM, Donnan GA, Albers GW (2011) RAPID automated patient selection for reperfusion therapy: a pooled analysis of the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET) and the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) study. Stroke; J Cereb Circ 42(6):1608–1614. doi:10.1161/STROKEAHA.110.609008
Mokin M, Kass-Hout T, Kass-Hout O, Dumont TM, Kan P, Snyder KV, Hopkins LN, Siddiqui AH, Levy EI (2012) Intravenous thrombolysis and endovascular therapy for acute ischemic stroke with internal carotid artery occlusion: a systematic review of clinical outcomes. Stroke; a J Cereb Circ 43(9):2362–2368. doi:10.1161/STROKEAHA.112.655621
Machi P, Costalat V, Lobotesis K, Maldonado IL, Vendrell JF, Riquelme C, Bonafe A (2012) Solitaire FR thrombectomy system: immediate results in 56 consecutive acute ischemic stroke patients. J Neurointerventional Surg 4(1):62–66. doi:10.1136/jnis.2010.004051
Conflict of interest
JF consults for Microvention, Stryker and Codman and speaks for Penumbra, Philips and Covidien.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Finitsis, S., Kemmling, A., Havemeister, S. et al. Stability of ischemic core volume during the initial hours of acute large vessel ischemic stroke in a subgroup of mechanically revascularized patients. Neuroradiology 56, 325–332 (2014). https://doi.org/10.1007/s00234-014-1329-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-014-1329-z