Abstract
Introduction
Intra-arterial (IA) thrombolysis with plasminogen activator is well-known, but the use of IA tirofiban as an adjuvant for IA thrombolysis is not well-known. We investigated the feasibility of IA tirofiban as an adjuvant after unsuccessful IA recanalization with urokinase (UK) for acute ischemic stroke.
Methods
We retrospectively analyzed all 16 consecutive patients (11 men and five women; mean age, 61.3 years; range, 36–85 years) who were treated with IA tirofiban after isolated IA thrombolysis with UK or bridging therapy with systemic recombinant tissue plasminogen activator (rt-PA; 0.6 mg/Kg) and IA UK for acute ischemic stroke. Outcome measures included angiographic recanalization (thrombolysis in cerebral infarction, TICI), symptomatic and asymptomatic intracerebral hemorrhage (ICH), mortality, and functional independence at 3 months (modified Rankin Scale, 0–2).
Results
Among the 16 patients treated with IA tirofiban as an adjuvant, 10 patients had conventional dose (<25 ug/kg, bolus) and six patients had high dose (≥25 ug/kg, bolus) of IA tirofiban after unsuccessful IA thrombolysis whether systemic rt-PA used or not. Successful angiographic recanalization (TICI grade 2b or 3) was achieved in 13 patients (13/16) and a functional independence at 3 months in eight patients (8/16). Three months after therapy, three patients had died. There were two patients of symptomatic ICH and four asymptomatic ICH.
Conclusion
Conventional dose of IA tirofiban as an adjuvant during IA thrombolysis for acute ischemic stroke seems feasible. However, further dose escalation studies should be performed regarding the IA use of tirofiban for acute ischemic stroke.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Since the Prolyse in Acute Cerebral Thromboembolism II (PROACT II) study [1], intra-arterial (IA) therapy with a plasminogen activator has been increasingly used to treat acute ischemic stroke patients with contraindications for intravenous (IV) recombinant tissue plasminogen activator (rt-PA) or those who have not obtained recanalization after IV thrombolytic therapy [2]. Recent IA thrombolysis for acute ischemic stroke has evolved into mechanical disruption such as direct suction of the thrombus or stenting of an obstructed segment [3–5]. However, medical IA thrombolysis should not be excluded as it sometimes has better results. There are still problems such as reocclusion of a revascularized vessel and formation of fresh thrombi, even with mechanical disruption, due to platelet activation [6–10]. There have been many clinical approaches to solving these problems of IA thrombolysis. A combination of thrombolytic therapy with glycoprotein IIb/IIIa inhibitor administration during IA thrombolysis for acute ischemic stroke is one of those clinical approaches [11–15]. Most of these combination therapies are IV use of glycoprotein IIb/IIIa inhibitor [16, 17]. However, there are only a few reports about the use of IA glycoprotein IIb/IIIa inhibitor [11, 15]. Furthermore, experience with IA tirofiban in acute ischemic stroke is very rare. IA use of tirofiban has several advantages compared with IV administration during the IA thrombolysis [18].
We hypothesized IA tirofiban as an adjuvant in the IA thrombolysis may have synergic effect on the clot. So we used IA tirofiban in the IA thrombolysis in selected acute ischemic stroke patients and investigated the feasibility of IA tirofiban as an adjuvant for acute ischemic stroke.
Patients and methods
Patient selection and acute stroke imaging protocol
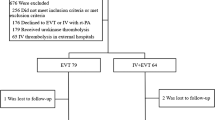
From May 2008 to October 2009, 118 patients were admitted within 6 h after symptom onset for acute ischemic stroke to our hospital. Among these 118 patients, 48 patients underwent IV or IA thrombolytic therapy according to the critical pathway for acute stroke in our hospital. Among these 48 patients, 18 patients were treated only with IV rt-PA (0.9 mg/Kg), eight with both IV rt-PA (0.6 mg/Kg) and IA thrombolysis with urokinase (UK), and 22 with only IA thrombolysis with UK. Of the 30 patients who underwent IA thrombolytic therapy, 16 patients were treated with IA tirofiban (Aggrastat®) as an adjuvant of IA thrombolysis. We evaluated the clinical and radiological information of these 16 patients who received IA tirofiban during IA thrombolysis. We excluded the 14 patients who underwent IA thrombolysis without IA tirofiban therapy. We obtained approval for the retrospective analysis of the patients who received IA tirofiban during IA thrombolysis from our local ethics committee.
When a patient arrived at our hospital within 3 h after symptom onset and there were no contraindications for IV thrombolysis, we initially treated the patient with IV rt-PA (0.6 mg/Kg, 15% of the dose was given as a bolus during 1 min and the remainder was given as a constant infusion during 30 min) after noncontrast brain CT to confirm the absence of hemorrhage, and MRI with MR angiography (MRA) was performed. If the occlusion of the major vessels such as middle cerebral artery (MCA), internal carotid artery (ICA), posterior cerebral artery (PCA), or basilar artery (BA) was suspected, IA thrombolysis was considered. If the branch occlusion such as M2 of the MCA was suspected in MRA or IA approach was impossible, we gave the patient the remaining rt-PA (0.3 mg/Kg). When a patient arrived between 3 and 6 h after symptom onset, we always performed the MRI and MRA without brain CT. After MRI and MRA were performed, a team consisting of an interventional neuroradiologist and stroke neurologist decided whether IA thrombolysis should be performed or not. The other inclusion criteria of IA thrombolysis were those patients (1) whose National Institutes of Health Stroke scale (NIHSS) scores were higher than four points, (2) who underwent initial diffusion-weighted imaging (DWI) and MRA showing the nonvisualization of major intracranial vessels that explained the patient’s clinical symptoms, and (3) who showed a DWI-identified lesion less than 1/2 of the total MCA territory. We also followed the exclusion criteria of thrombolysis used in the PROACT II study.
After bridging IV rt-PA and IA thrombolysis with UK or isolated IA thrombolysis, we infused IA tirofiban with mechanical thrombolysis with microwire if there was no response to IA thrombolysis or suspicion of reocclusion of partially recanalized vessel. The enrolled patients in our study are summarized in Fig. 1.
Angiointerventional procedures
If a patient required IA thrombolysis, we fully explained the procedure to the patient and/or their families and obtained a written informed consent for the therapy. IA thrombolysis was performed under conscious sedation in all patients. After the common femoral artery was punctured, a five or six French-guiding catheter was positioned in either the cervical ICA or the VA. We performed conventional cerebral angiography to identify the occluded segment. The side arm of the guiding catheter was continuously flushed with pressurized and heparinized normal saline. And also a maximum of 3,000 units of heparin (2,000–3,000 units) were administered intravenously. A microcatheter was positioned proximal to the occluded segment. We infused 50,000–100,000 units of UK via microcatheter and traversed the occluded segment with the microwire and microcatheter. And then we performed an angiogram with the microcatheter to confirm the extent of the occluded segment and repeated the same procedure until recanalization was achieved. Attempts were made to disrupt the occluded thrombus with the microwire and induce distal migration (mechanical disruption with microwire) at the same time. The use of IA tirofiban was decided based on the criteria which included (1) reocclusion of partially recanalized vessel and (2) no response to IA UK and mechanical disruption with microwire. If the criteria met, we infused tirofiban just as boluses through a microcatheter. Each bolus consisting of 0.25 mg of tirofiban (mixed with normal saline to make a total of 20 mL of fluid) was infused over 3 min. For a 60 kg patient, this would be approximately 1.39 ug/min/kg in each bolus infusion. Following this, angiogram was performed via the guiding catheter. If there were still remaining occlusions, further tirofiban infusions were carried out as above. Table 1 shows the total amount of Tirofiban used in each of the patients in our study. We did not exceed a total of 2 mg of tirofiban and 400,000 units of UK except in one patient with total BA occlusion. During the procedure, the patient’s neurological status was continuously monitored by the stroke neurologist. After IA thrombolysis, all patients were monitored in the intensive care unit for at least 24 h, and antithrombotics including intravenous tirofiban or anticoagulation therapy were not administered within 24 h following AHA/ASA guideline [19].
Clinical outcome evaluation
We assesed the number of recanalized vessels, symptomatic and asymptomatic ICH, procedural complications, renal insufficiency, death, and the outcome variables including the pre- and postprocedural NIHSS scores, and modified Rankin Scale (mRS) at 3 months. Recanalization status was classified according to the Thrombolysis in cerebral infarction (TICI) classification (grade 0, no perfusion; grade 1, penetration with minimal perfusion; grade 2a, partial filling 2/3 of the entire vascular territory; grade 2b, complete filling, but the filling is slower than normal; grade 3, complete perfusion). Favorable clinical outcome was defined as a decrease of four points on the NIHSS score at 7 days after IA therapy compared with a baseline NIHSS score and 0–2 on the mRS score at 3 months.
Results
The overall clinical outcomes are outlined in Table 1. All patients’ consciousness was variable from alert to stupor according to the involving lesions. Among the 16 patients, 11 were men and five were women (36–85 years of age; mean, 61.3 years). In patients treated with IA tirofiban, IV rt-PA (0.6 mg/kg) was given to eight patients as a bridging therapy. The initial mean NIHSS score of the 16 study patients was 12.9 (median, 14; range, 5–23). Of these patients, 12 had anterior circulation obstruction (ICA, three; MCA, nine) and four patients had posterior circulation obstruction (BA , three; PCA, one). The mechanism of ischemic stroke was determined according to TOAST classification: large artery atherosclerosis in 11 patients, cardioembolism in two, other determined in one, and undetermined in two. CT and MRI were performed in 10 patients, and only MRI in six patients as initial imaging study. The mean interval between the initial brain CT and the subsequent MR was 62.5 min in the 10 patients who were performed both CT and MRI as initial imaging study. The mean interval between stroke onset and initial angiogram was 240 min. Initially, all 16 patients presented complete or near-complete vessel occlusion (TICI grade 0 or 1). Endovascular treatments included IA pharmacological thrombolysis (UK) and mechanical thrombolysis with microwire. Thirteen patients (13/16) achieved partial to complete recanalization (TICI grade 2b or 3). Procedure-related complications did not develop. No myocardial infarction or gastrointestinal hemorrhage occurred after endovascular treatment. Favorable clinical outcome (mRS score ≤2) at 3 months was achieved in eight patients (8/16). Three patients had died (3/16), one patient due to ICH, one severe cerebral edema due to reocclusion leading to herniation, and one undetermined cause leading to shock. Microbleeds (MBs) were observed in four patients on preprocedural gradient-echo MRI in the following locations: left frontal lobe, left cerebellum, pons, and multiple sites including basal ganglia, thalamus, and brainstem. Among these four patients, only one patient developed symptomatic ICH, the other three patients did not develop significant ICH. The total number of patients who developed hemorrhages was six, and among these, symptomatic ICH developed in two patients. Of the two patients who developed symptomatic ICHs, one patient with right MCA territory infarct had fluctuating BP during the procedure and had multiple microbleeds (Fig. 2.) and was treated with 2 mg of IA tirofiban. With the other patient with total BA occlusion and ultimately ICH, there was an aggressive attempt for recanalization despite of the possibility of danger. The mean initial BUN and serum creatinine were 17.5 (10–32.1, SD 5.9) and 1.1 (0.87–1.89, SD 0.2), respectively. Renal function was not aggravated after IA thrombolysis with tirofiban.
Conventional angiography, CT and MRI in a 66-year-old woman admitted due to acute ischemic stroke. She visited hospital with NIHSS 18. The initial angiogram showed occlusion of the right proximal MCA (a, b). Angiogram obtained after IA thrombolysis with 300,000 units of urokinase and 2 mg of tirofiban (total dose), revealed recanalization of the occluded segment (c). During IA thrombolysis, the patient’s BP was high and fluctuating. CT obtained 6 h after IA thrombolysis, showed symptomatic ICH (d). Gradient echo MR image obtained prior to IA thrombolysis, revealed multiple microbleeds in both cerebral hemispheres and chronic hemorrhage in the left basal ganglia (e)
Discussion
Acute ischemic stroke results from thrombotic or embolic occlusion of cerebral vessels which supply blood to the brain [20]. The primary constituents of the initial thrombus are platelets and fibrin [21]. In pharmacologic IA thrombolysis, the role of fibrinolytic agents such as plasminogen activators has been heavily stressed compared with the role of antiplatelet agents. However, after the reporting of reocclusion of recanalized vessels in acute ischemic stroke [6, 7] and adverse effect of fibrinolytic agents such as increasing thrombin activity and platelet activation [10, 22–24], antiplatelet agents such as GP IIb/IIIa inhibitors are receiving attention. Glycoprotein IIb/IIIa receptors on the surface of platelets have a critical role in thrombosis [25]. And Glycoprotein IIb/IIIa inhibitor is a selective and potent antiplatelet [25]. Sometimes, it does not only inhibit the platelet function, but also plays a role in thrombolysis on the proximal clot [26, 27]. To increase the efficacy of IA thrombolysis, there have been several pilot studies of combined thrombolytic therapy with glycoprotein IIb/IIIa inhibitors [11–13, 15]. Among the many kinds of glycoprotein IIb/IIIa inhibitors, abciximab, tirofiban, and eptifibatide are commonly used. Among these three glycoprotein IIb/IIIa inhibitors, only abciximab was investigated with phase III trial in the acute ischemic stroke. Unfortunately, however, the phase III trial of IV abciximab was halted because of increased rate of ICH and lack of efficacy, compared with the placebo arm [28]. Several reports have noted that tirofiban and eptifibatide were feasible for treating acute ischemic stroke [11–13, 29–32]. Considering the point of reversibility of the action on platelets, tirofiban and eptifibatide may be safer than abciximab [25, 30]. Most of these studies about tirofiban and eptifibatide have been IV administration up to date. IA use of glycoprotein IIb/IIIa inhibitor has several advantages compared with IV administration during the IA thrombolysis. First, as direct delivery of a concentrated dose to the target thrombi is possible, we can therefore expect rapid and effective thrombolysis and thus reduce possible hemorrhagic complications. Second, it is possible to adjust the dose according to its response [18].
In our study, the recanalization was achieved in 13 of the 16 patients. Symptomatic ICH and mortality of our study were two of 16 and three of 16 patients, respectively. These results, except symptomatic ICH, appear to be acceptable compared to those of other reported studies [33]. Two symptomatic ICH occurred in the patients who received high dose (≥25 ug/kg) of IA tirofiban. The increased ICH incidences may be due to the use of many kinds of drugs against blood clot such as IV-tPA, heparin, IA UK, and IA tirofiban, although we tried to use as little dose as possible.
There are dose-escalation study regarding IV tirofiban, and study about the safety and effectiveness of high dose IV tirofiban during coronary angioplasty [34, 35]. There, however, have been no dose escalation studies regarding IA tirofiban during IA thrombolysis for acute stroke and we are faced with the task of determining the safe dose of IA tirofiban. We used a high dose of IA tirofiban in six patients in inevitable situation. Among these six patients, one who had fluctuating BP (230–190 mmHg systolic blood pressure) and MBs which was detected on preprocedural MRI, developed symptomatic ICH within 24 h after IA therapy with IA tirofiban. Therefore, physician should be concerned regarding the development of ICH during the use of IA tirofiban, especially in cases with potential signs of hemorrhage such as high and fluctuating BP, and MBs seen on MRI.
Although there were patients who could tolerate high dose of IA tirofiban (Fig. 3), the safety of patients receiving a high dose of IA tirofiban is not guaranteed in acute ischemic stroke considering our study.
MRI and conventional angiography in a 63-year-old man admitted due to suddenly developed altered mentality and left hemiparesis (NIHSS 15). Diffusion weighted image revealed acute infarction in the right thalamus and hippocampus (a). Conventional angiography revealed occlusion of the right PCA (b). During IA thrombolysis, right PCA was partially recanalized (c). However, it was re-occluded immediately after then (d). Angiogram obtained after IA thrombolysis with 300,000 units of urokinase and 2 mg of tirofiban (total dose), revealed recanalization of the re-occluded segment (e). Follow-up angiogram after 1 week revealed patent right PCA even though focal stenosis in the right P2 segment
Our study has several limitations. The number of study patients was small and it was a single-center retrospective noncontrolled case series, and thus it is not possible to draw any conclusions. The selection of patients is biased because the use of tirofiban as an adjuvant was dependent on the discretion of the physician in specific clinical situation. There are many variables that affect the outcome of IA tirofiban during the IA thrombolysis such as IV rt-PA and the dose of UK. We used UK and mechanical maneuver with microwire as main IA thrombolytic method. Recent published article reports that there are good results using the new methods of mechanical thrombectomy such as stent retrievers or the Penumbra system. And we might obtain better results if we used this new mechanical methods and adjuvant tirofiban. However, the use of new mechanical method as endovascular treatment may also lead to disruption of atherosclerotic plaque, endothelial erosion, or distal migration of clot that triggers platelet activation and have danger of reocclusion. Thus, apart from the method of mechanical thrombectomy, adjuvant antiplatelet may be needed for better results of IA thrombolysis [15].
Despite the above limitations, our study showed that conventional dose of IA tirofiban as an adjuvant may be feasible as adjuvant therapy of IA thrombolysis. Further randomized and controlled trials with large patient numbers are needed to evaluate the effectiveness and safety of IA tirofiban as an adjuvant in IA thrombolysis.
References
Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, Pessin M, Ahuja A, Callahan F, Clark WM, Silver F, Rivera F (1999) Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA 282(21):2003–2011
Mandava P, Kent TA (2007) Intra-arterial therapies for acute ischemic stroke. Neurology 68(24):2132–2139
Kang D-H, Hwang Y-H, Kim Y-S (2011) Direct thrombus retrieval using the reperfusion catheter of the penumbra system: forced-suction thrombectomy in acute ischemic stroke. AJNR 32:283–287
Kim SM, Lee DH, Kwon SU, Choi CG, Kim SJ, Suh DC (2011) Treatment of acute ischemic stroke: feasibility of primary or secondary use of a self-expanding stent (Neuroform) during local intra-arterial thrombolysis. Neuroradiology. doi:10.1007/s00234-010-0813-3
Roth C, Papanagiotou P, Behnke S, Walter S, Haass A, Becker C, Fassbender K, Politi M, Korner H, Romann MS, Reith W (2010) Stent-assisted mechanical recanalization for treatment of acute intracerebral artery occlusions. Stroke 41(11):2559–2567
Qureshi AI, Siddiqui AM, Kim SH, Hanel RA, Xavier AR, Kirmani JF, Suri MF, Boulos AS, Hopkins LN (2004) Reocclusion of recanalized arteries during intra-arterial thrombolysis for acute ischemic stroke. AJNR Am J Neuroradiol 25(2):322–328
Janjua N, Alkawi A, Suri MF, Qureshi AI (2008) Impact of arterial reocclusion and distal fragmentation during thrombolysis among patients with acute ischemic stroke. AJNR Am J Neuroradiol 29(2):253–258
Qureshi AI, Hussein HM, Abdelmoula M, Georgiadis AL, Janjua N (2009) Subacute recanalization and reocclusion in patients with acute ischemic stroke following endovascular treatment. Neurocrit Care 10(2):195–203
Hussain MS, Lin R, Moskowitz S, Bain M, Gonugunta V, Rasmussen PA, Masaryk TJ, Horowitz MB, Jovin T, Gupta R (2010) Symptomatic delayed reocclusion after initial successful revascularization in acute ischemic stroke. J Stroke Cerebrovasc Dis 19(1):36–39
Qureshi AI, Luft AR, Sharma M, Guterman LR, Hopkins LN (2000) Prevention and treatment of thromboembolic and ischemic complications associated with endovascular procedures: part I—pathophysiological and pharmacological features. Neurosurgery 46(6):1344–1359
Deshmukh VR, Fiorella DJ, Albuquerque FC, Frey J, Flaster M, Wallace RC, Spetzler RF, McDougall CG (2005) Intra-arterial thrombolysis for acute ischemic stroke: preliminary experience with platelet glycoprotein IIb/IIIa inhibitors as adjunctive therapy. Neurosurgery 56(1):46–54, discussion 54–55
Mangiafico S, Cellerini M, Nencini P, Gensini G, Inzitari D (2005) Intravenous glycoprotein IIb/IIIa inhibitor (tirofiban) followed by intra-arterial urokinase and mechanical thrombolysis in stroke. AJNR Am J Neuroradiol 26(10):2595–2601
Mangiafico S, Cellerini M, Nencini P, Gensini G, Inzitari D (2005) Intravenous tirofiban with intra-arterial urokinase and mechanical thrombolysis in stroke: preliminary experience in 11 cases. Stroke 36(10):2154–2158
Qureshi AI, Suri MF, Ali Z, Ringer AJ, Boulos AS, Nakada MT, Alberico RA, Martin LB, Guterman LR, Hopkins LN (2005) Intraarterial reteplase and intravenous abciximab for treatment of acute ischemic stroke. A preliminary feasibility and safety study in a non-human primate model. Neuroradiology 47(11):845–854
Memon MZ, Natarajan SK, Sharma J, Mathews MS, Synder KV, Siddiqui AH, Hopkins LN, Levy EI (2011) Safety and feasibility of intraarterial eptifibatide as a revascularization tool in acute ischemic stroke. J Neurosurg 114(4):1008–1013
Eckert B, Koch C, Thomalla G, Kucinski T, Grzyska U, Roether J, Alfke K, Jansen O, Zeumer H (2005) Aggressive therapy with intravenous abciximab and intra-arterial rtPA and additional PTA/stenting improves clinical outcome in acute vertebrobasilar occlusion: combined local fibrinolysis and intravenous abciximab in acute vertebrobasilar stroke treatment (FAST): results of a multicenter study. Stroke 36(6):1160–1165
Pfefferkorn T, Mayer TE, Opherk C, Peters N, Straube A, Pfister HW, Holtmannspotter M, Muller-Schunk S, Wiesmann M, Dichgans M (2008) Staged escalation therapy in acute basilar artery occlusion: intravenous thrombolysis and on-demand consecutive endovascular mechanical thrombectomy: preliminary experience in 16 patients. Stroke 39(5):1496–1500
Kwon OK, Lee KJ, Han MH, Oh CW, Han DH, Koh YC (2002) Intraarterially administered abciximab as an adjuvant thrombolytic therapy: report of three cases. AJNR Am J Neuroradiol 23(3):447–451
Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF (2007) Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 38(5):1655–1711
Qureshi AI, Pande RU, Kim SH, Hanel RA, Kirmani JF, Yahia AM (2002) Third generation thrombolytics for the treatment of ischemic stroke. Curr Opin Investig Drugs 3(12):1729–1732
White HD (1997) Unmet therapeutic needs in the management of acute ischemia. Am J Cardiol 80(4A):2B–10B
Kawano K, Aoki I, Aoki N, Homori M, Maki A, Hioki Y, Hasumura Y, Terano A, Arai T, Mizuno H, Ishikawa K (1998) Human platelet activation by thrombolytic agents: effects of tissue-type plasminogen activator and urokinase on platelet surface P-selectin expression. Am Heart J 135(2 Pt 1):268–271
Qureshi AI, Harris-Lane P, Kirmani JF, Janjua N, Divani AA, Mohammad YM, Suarez JI, Montgomery MO (2006) Intra-arterial reteplase and intravenous abciximab in patients with acute ischemic stroke: an open-label, dose-ranging, phase I study. Neurosurgery 59(4):789–796, discussion 796–797
Nordt TK, Moser M, Kohler B, Ruef J, Peter K, Kubler W, Bode C (1998) Augmented platelet aggregation as predictor of reocclusion after thrombolysis in acute myocardial infarction. Thromb Haemost 80(6):881–886
Mandava P, Thiagarajan P, Kent TA (2008) Glycoprotein IIb/IIIa antagonists in acute ischaemic stroke: current status and future directions. Drugs 68(8):1019–1028
Manoharan G, Adgey AA (2004) Considerations in combination therapy: fibrinolytics plus glycoprotein IIb/IIIa receptor inhibitors in acute myocardial infarction. Clin Cardiol 27(7):381–386
Szabo S, Walter T, Etzel D, Ehlers R, Kazmaier S, Beyer ME, Hoffmeister HM (2003) Benefical effects of reteplase in combination with abciximab: platelet/leukocyte interactions and coagulation system. Int J Clin Pharmacol Res 23(2–3):37–40
Adams HP Jr, Effron MB, Torner J, Davalos A, Frayne J, Teal P, Leclerc J, Oemar B, Padgett L, Barnathan ES, Hacke W (2008) Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of an international phase III trial: Abciximab in Emergency Treatment of Stroke Trial (AbESTT-II). Stroke 39(1):87–99
Junghans U, Seitz RJ, Aulich A, Freund HJ, Siebler M (2001) Bleeding risk of tirofiban, a nonpeptide GPIIb/IIIa platelet receptor antagonist in progressive stroke: an open pilot study. Cerebrovasc Dis 12(4):308–312
Philipps J, Thomalla G, Glahn J, Schwarze M, Rother J (2009) Treatment of progressive stroke with tirofiban—experience in 35 patients. Cerebrovasc Dis 28(5):435–438
Pancioli AM, Broderick J, Brott T, Tomsick T, Khoury J, Bean J, del Zoppo G, Kleindorfer D, Woo D, Khatri P, Castaldo J, Frey J, Gebel J Jr, Kasner S, Kidwell C, Kwiatkowski T, Libman R, Mackenzie R, Scott P, Starkman S, Thurman RJ (2008) The combined approach to lysis utilizing eptifibatide and rt-PA in acute ischemic stroke: the CLEAR stroke trial. Stroke 39(12):3268–3276
Qureshi AI, Hussein HM, Janjua N, Harris-Lane P, Ezzeddine MA (2008) Postprocedure intravenous eptifibatide following intra-arterial reteplase in patients with acute ischemic stroke. J Neuroimaging 18(1):50–55
Langer D, Alexander M, Janardhan V, Hartmann M, Jansen O, Sit SP, Yavagal D, Stingele R, DeMuth G, Bose A, Clark W, Lutsep H, Barnwell S, Nesbit G, Egan R, North E, Yanase L, Lowenkopf T, Petersen B, Grunwald IQ, Mayer T, Doerfler A, Struffert T, Engelhorn T, Richter G, Grunwald IQ, Reith W, Berkefeld J, Madison M, Myers M, Goddard J, Lassig J, Lopes D, Shownkeen H, Echiverri H, Nour F, Mazumdar A, Budzik R, Pema P, Frei D, Huddle D, Bellon R, Heck D, Ferguson R, McDougall C, Flaster M, Frey J, Albuquerque F, Malkoff M, Zaidat O, Branca V, Ahktar N, Rymer M, Rai A, Brooks C, Carpenter J, Popovich T, Chaloupka J, Hellinger F, Rasmussen P, Masaryk T, Fiorella D, Woo H, Rudolph S, Spiegel G, Silverman I, Ohki S, Gomes J, Trial PPS (2009) The Penumbra Pivotal Stroke Trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke 40(8):2761–2768
Danzi GB, Capuano C, Sesana M, Baglini R (2004) Safety of a high bolus dose of tirofiban in patients undergoing coronary stent placement. Catheter Cardiovasc Interv 61(2):179–184
Marmur JD, Poludasu S, Agarwal A, Manjappa N, Cavusoglu E (2008) High-dose tirofiban administered as bolus-only during percutaneous coronary intervention. J Invasive Cardiol 20(2):53–58
Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, Schneider D, Diez-Tejedor E, Trouillas P (1998) Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European–Australasian Acute Stroke Study Investigators. Lancet 352(9136):1245–1251
Acknowledgments
We thank Dr. Sarawana CM, Kuala Lumpur Hospital, Malaysia and Bonnie Hami, MA, USA for their English editorial assistance.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kwon, JH., Shin, S.H., Weon, Y.C. et al. Intra-arterial adjuvant tirofiban after unsuccessful intra-arterial thrombolysis of acute ischemic stroke: preliminary experience in 16 patients. Neuroradiology 53, 779–785 (2011). https://doi.org/10.1007/s00234-011-0939-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-011-0939-y